

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16318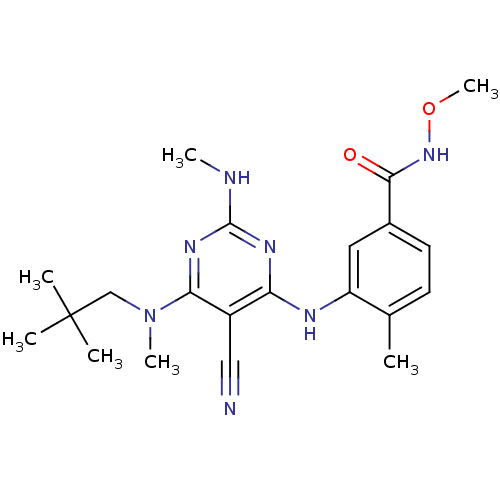 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0470 | -58.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16319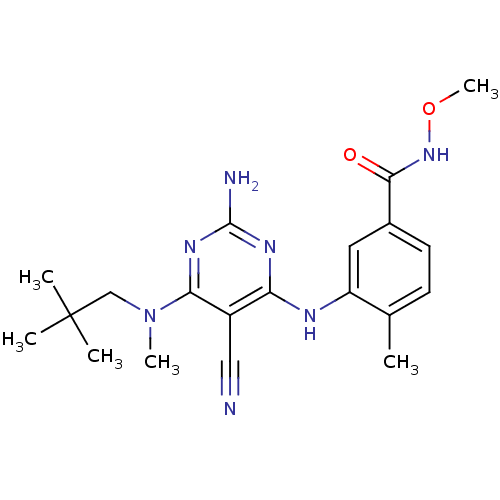 (3-({2-amino-5-cyano-6-[(2,2-dimethylpropyl)(methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160159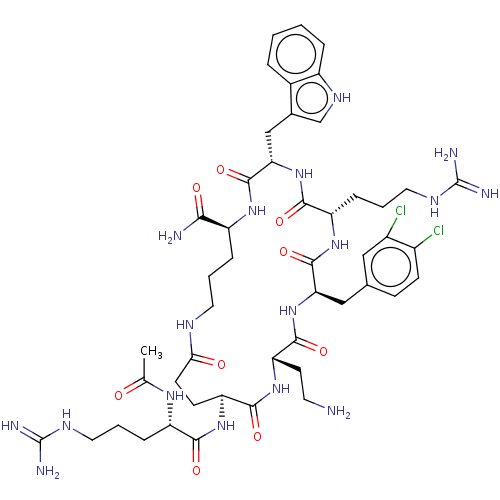 (US9040663, 5) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0550 | -60.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160158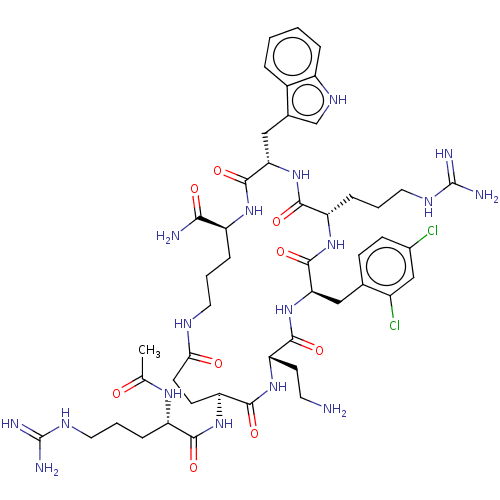 (US9040663, 4) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0550 | -60.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16329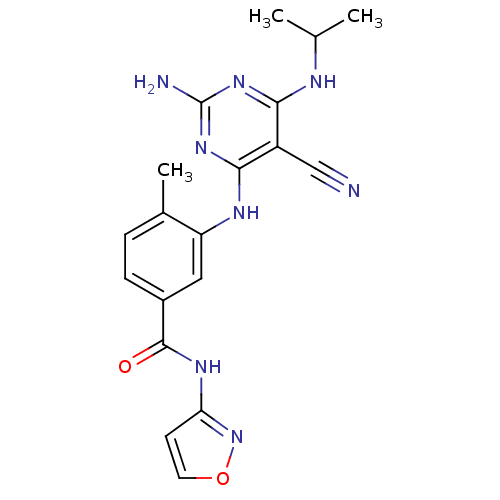 (3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16317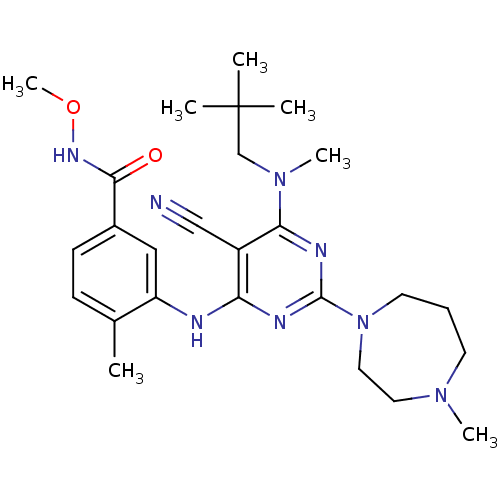 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160174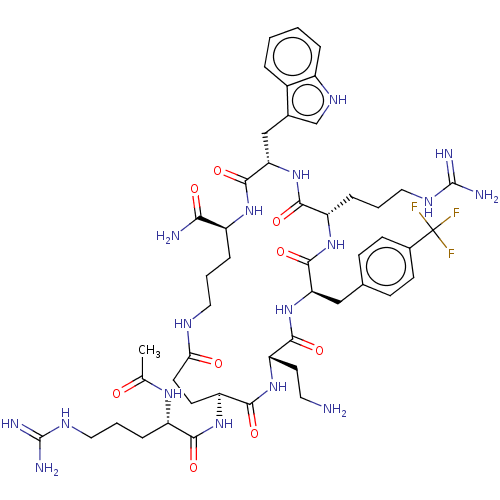 (US9040663, 20) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | -60.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160157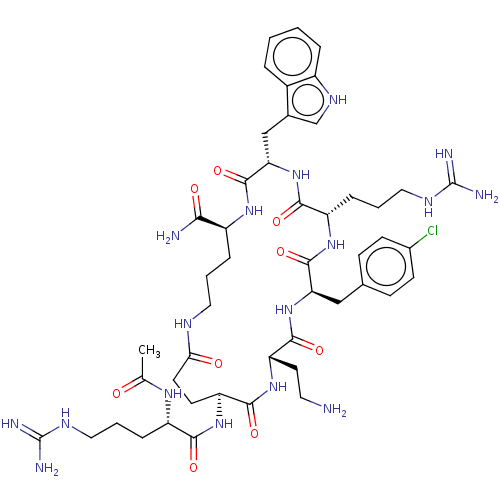 (US9040663, 3) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | -59.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160175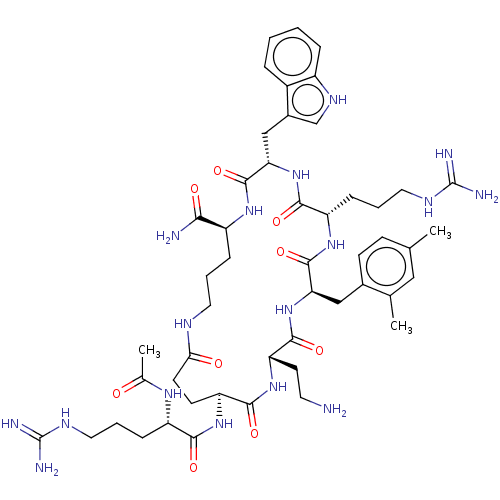 (US9040663, 21) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0850 | -59.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160169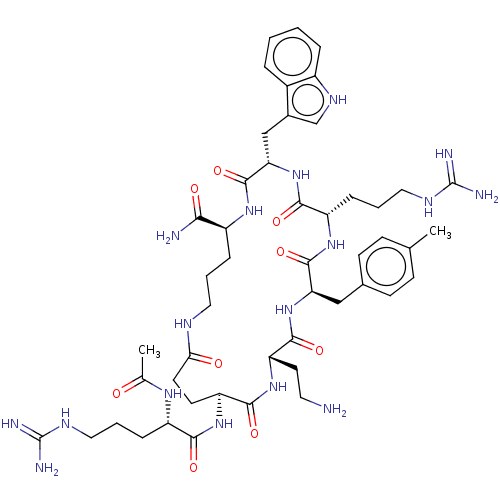 (US9040663, 15) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.145 | -58.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM160170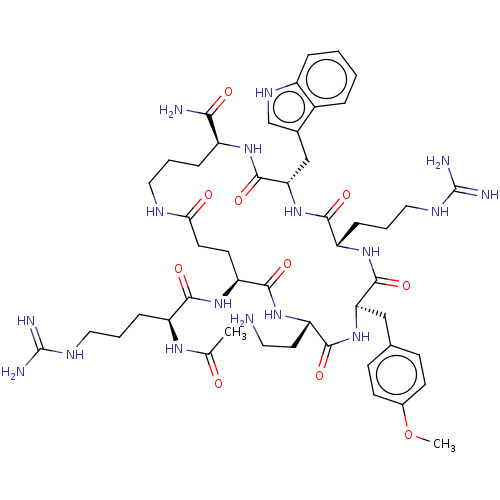 (US9040663, 16) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | -58.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16320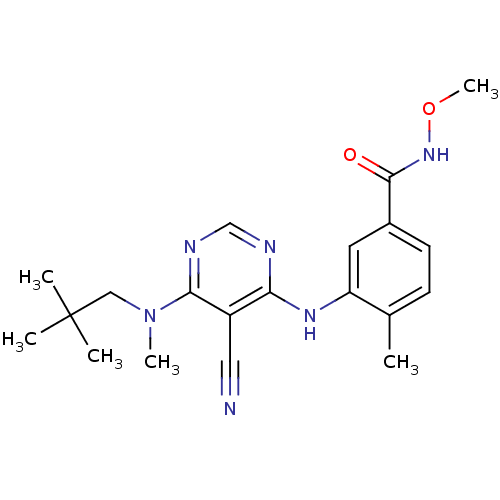 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16330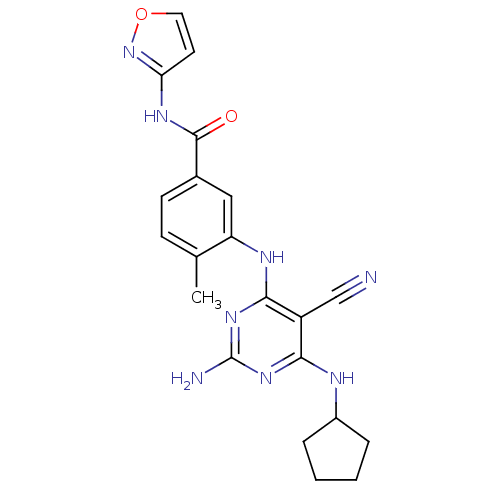 (3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226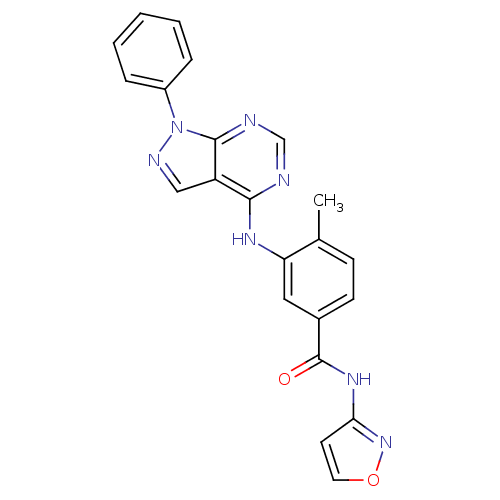 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM160159 (US9040663, 5) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -57.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160165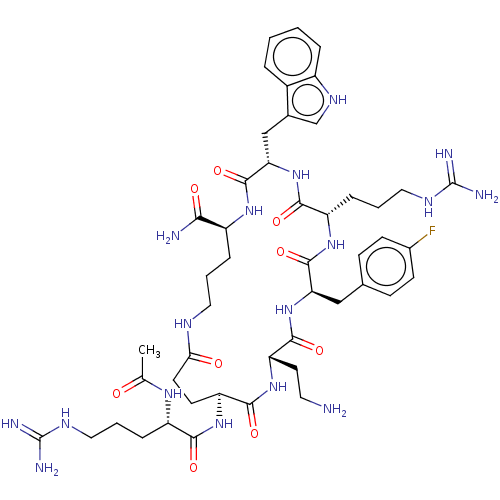 (US9040663, 11) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -57.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160208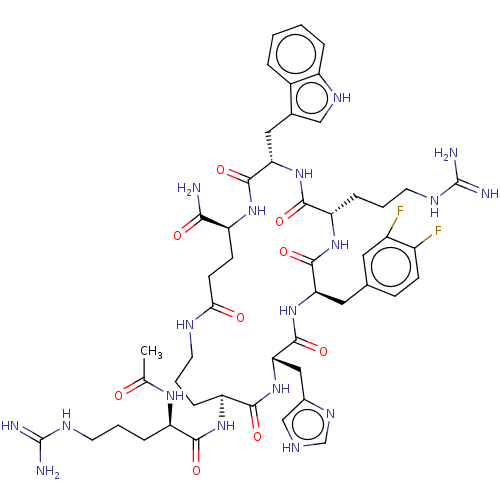 (US9040663, 54) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -57.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160170 (US9040663, 16) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -57.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160180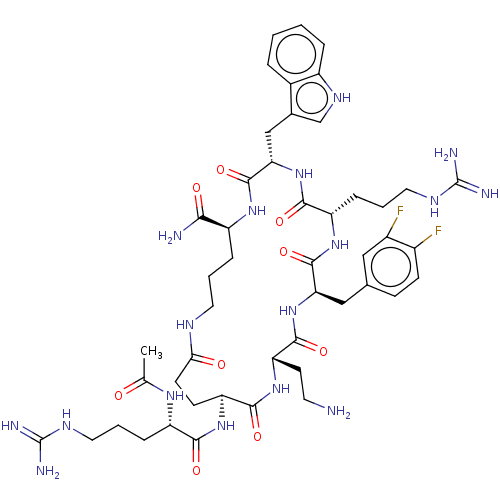 (US9040663, 26) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160201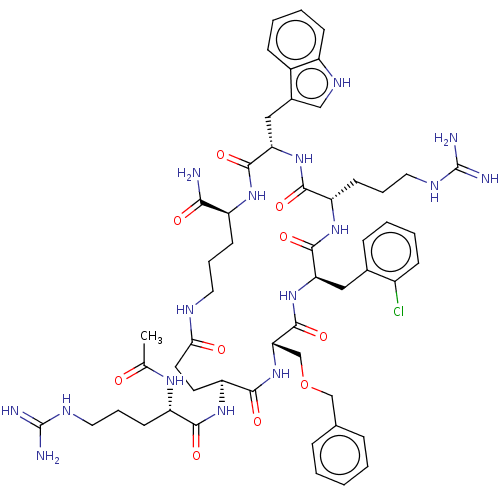 (US9040663, 47) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM160157 (US9040663, 3) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50348414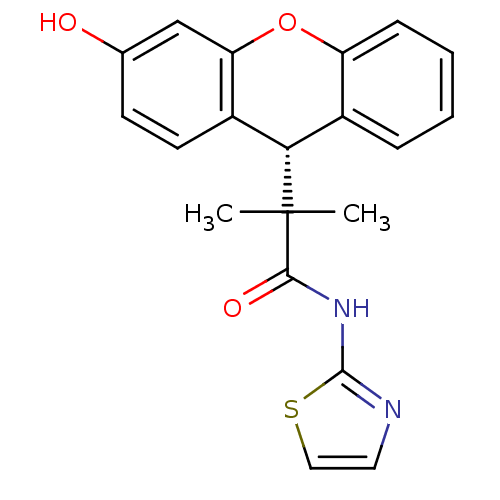 (CHEMBL1800984) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from GRapha by fluorescence polarization assay | J Med Chem 53: 8241-51 (2010) Article DOI: 10.1021/jm100957a BindingDB Entry DOI: 10.7270/Q2HX1DPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160217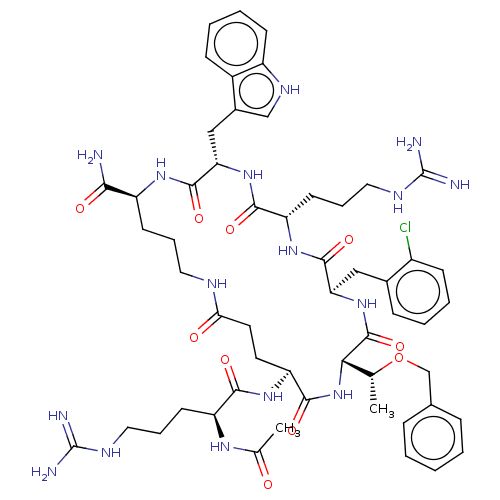 (US9040663, 63) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160173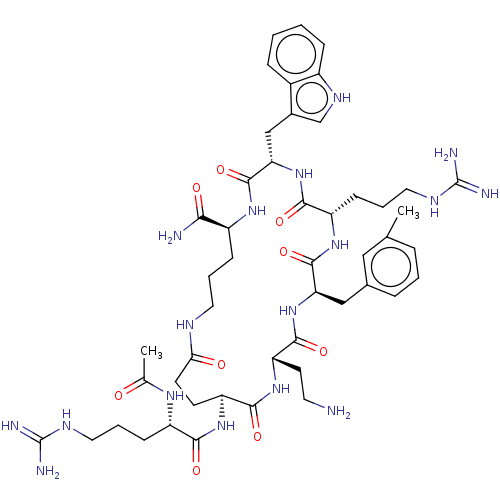 (US9040663, 19) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM135736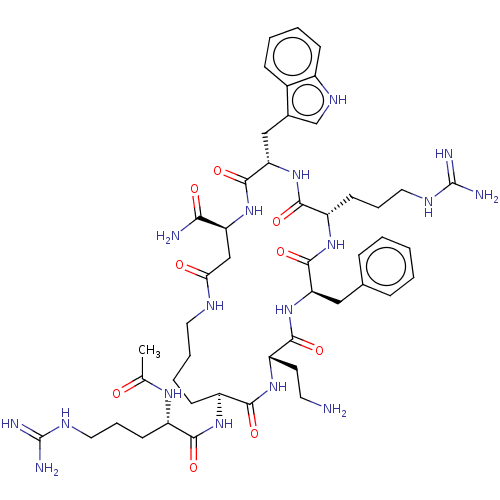 (US10179804, Example 121 | US10632171, Peptide No. ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay is performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMC4-R, hMC3-R, o... | US Patent US8846601 (2014) BindingDB Entry DOI: 10.7270/Q2SF2TWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM135752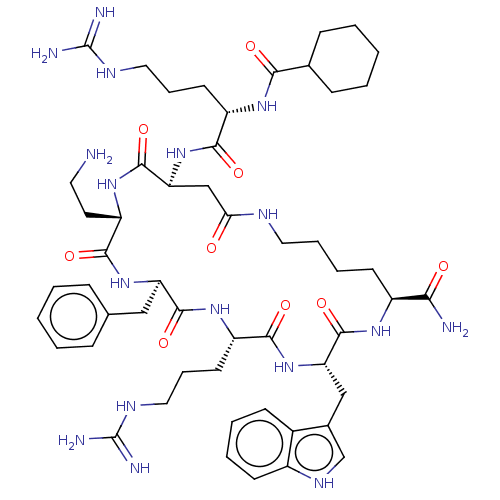 (US10179804, Example 137 | US10632171, Peptide No. ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay is performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMC4-R, hMC3-R, o... | US Patent US8846601 (2014) BindingDB Entry DOI: 10.7270/Q2SF2TWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM135736 (US10179804, Example 121 | US10632171, Peptide No. ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay is performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMC4-R, hMC3-R, o... | US Patent US10632171 (2020) BindingDB Entry DOI: 10.7270/Q2BG2S18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM135752 (US10179804, Example 137 | US10632171, Peptide No. ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay is performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMC4-R, hMC3-R, o... | US Patent US10632171 (2020) BindingDB Entry DOI: 10.7270/Q2BG2S18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM135752 (US10179804, Example 137 | US10632171, Peptide No. ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay is performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMC4-R, hMC3-R, o... | US Patent US9458201 (2016) BindingDB Entry DOI: 10.7270/Q2HT2N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM135752 (US10179804, Example 137 | US10632171, Peptide No. ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palatin Technologies, Inc. US Patent | Assay Description Accumulation of intracellular cAMP was examined as a measure of the ability of the peptides of the present invention to elicit a functional response ... | US Patent US10179804 (2019) BindingDB Entry DOI: 10.7270/Q24T6MFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50348407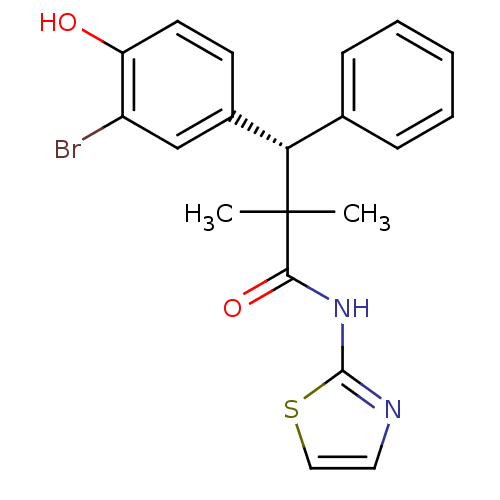 (CHEMBL1801006) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from GRapha by fluorescence polarization assay | J Med Chem 53: 8241-51 (2010) Article DOI: 10.1021/jm100957a BindingDB Entry DOI: 10.7270/Q2HX1DPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50348412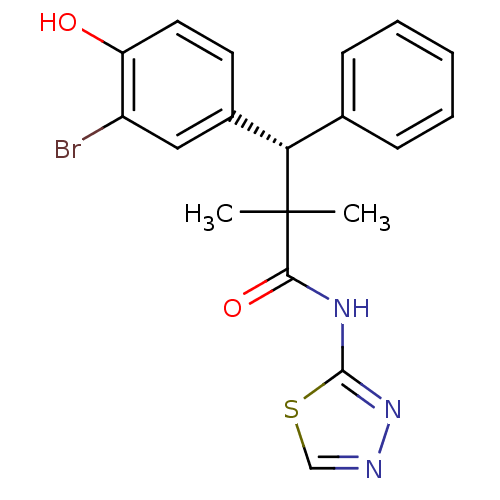 (CHEMBL1800982) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from GRapha by fluorescence polarization assay | J Med Chem 53: 8241-51 (2010) Article DOI: 10.1021/jm100957a BindingDB Entry DOI: 10.7270/Q2HX1DPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM135736 (US10179804, Example 121 | US10632171, Peptide No. ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay is performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMC4-R, hMC3-R, o... | US Patent US9458201 (2016) BindingDB Entry DOI: 10.7270/Q2HT2N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM135736 (US10179804, Example 121 | US10632171, Peptide No. ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palatin Technologies, Inc. US Patent | Assay Description Accumulation of intracellular cAMP was examined as a measure of the ability of the peptides of the present invention to elicit a functional response ... | US Patent US10179804 (2019) BindingDB Entry DOI: 10.7270/Q24T6MFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16325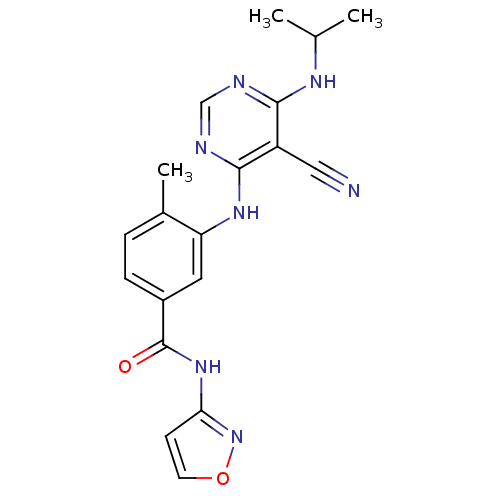 (3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376456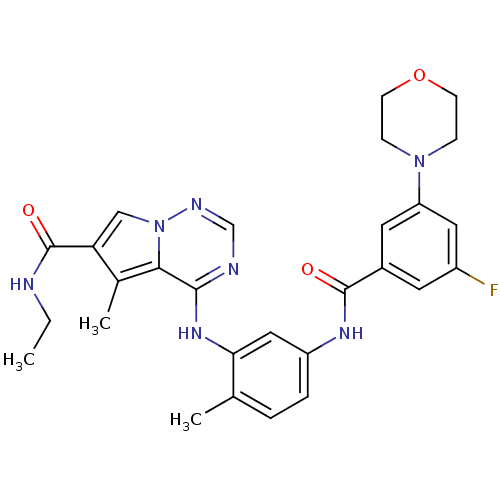 (CHEMBL262592) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16324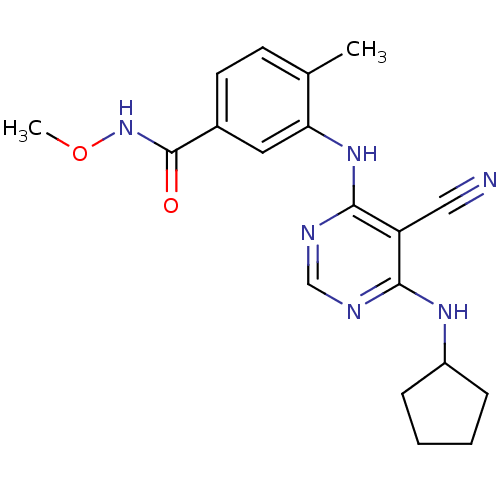 (3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376433 (CHEMBL258895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160178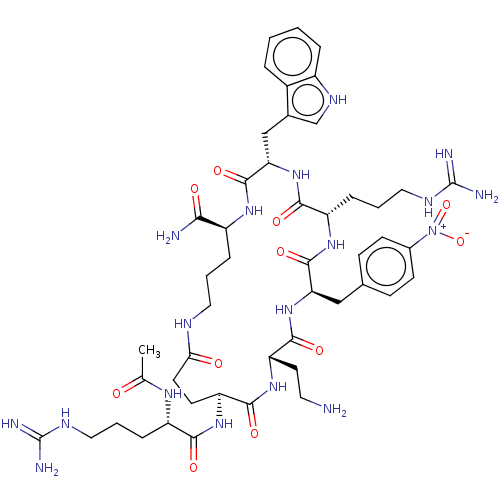 (US9040663, 24) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.450 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376434 (CHEMBL408150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440220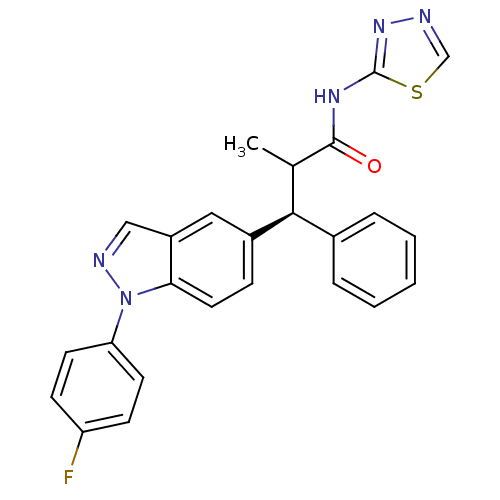 (CHEMBL2426624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5442-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.089 BindingDB Entry DOI: 10.7270/Q29888FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440220 (CHEMBL2426624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5442-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.089 BindingDB Entry DOI: 10.7270/Q29888FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160172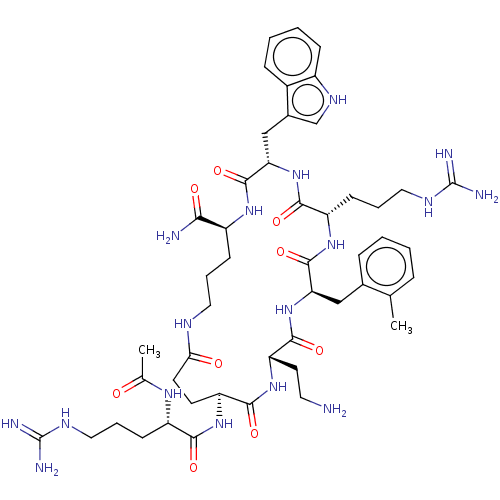 (US9040663, 18) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160183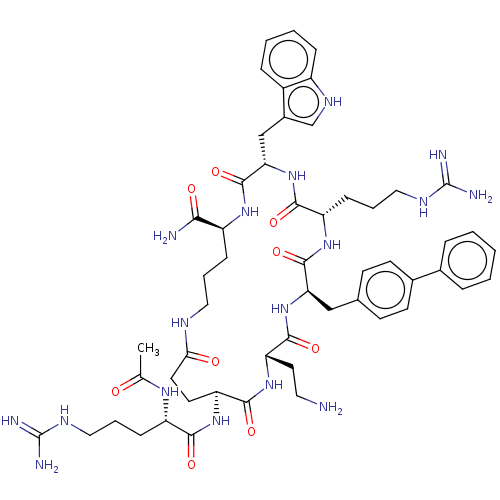 (US9040663, 29) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM160165 (US9040663, 11) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM160158 (US9040663, 4) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160253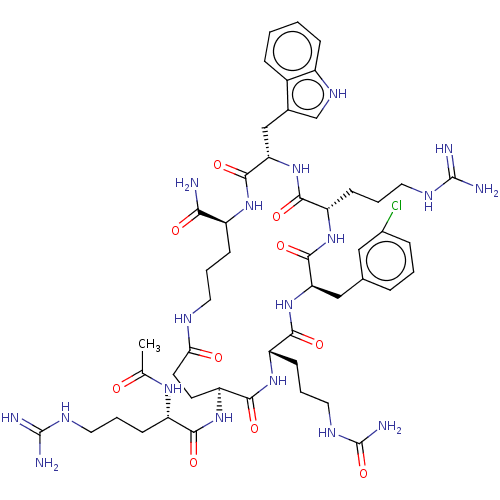 (US9040663, 99) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440105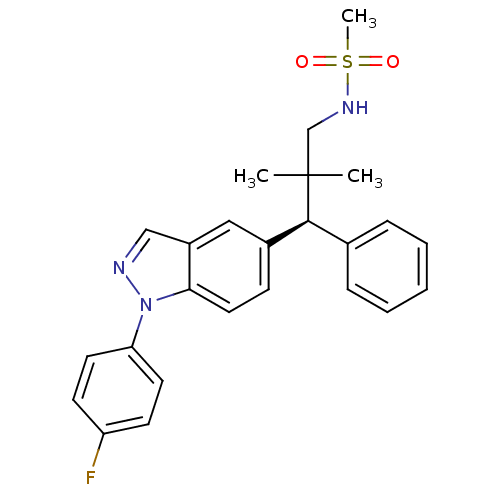 (CHEMBL2426114) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay | Bioorg Med Chem Lett 23: 5448-51 (2013) Article DOI: 10.1016/j.bmcl.2013.06.085 BindingDB Entry DOI: 10.7270/Q22R3T2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376435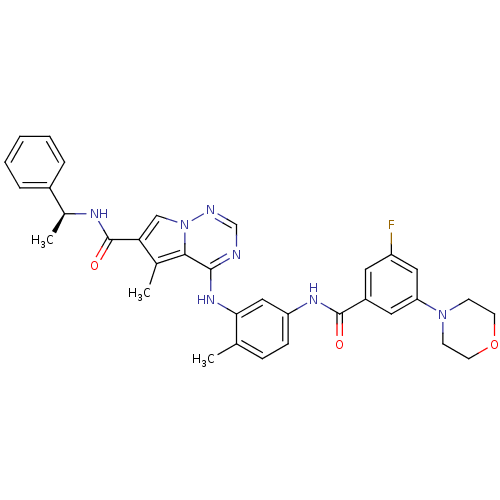 (CHEMBL261845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2581 total ) | Next | Last >> |