 Found 3060 hits with Last Name = 'doherty' and Initial = 'ga'
Found 3060 hits with Last Name = 'doherty' and Initial = 'ga' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | US20240043404, Example 378
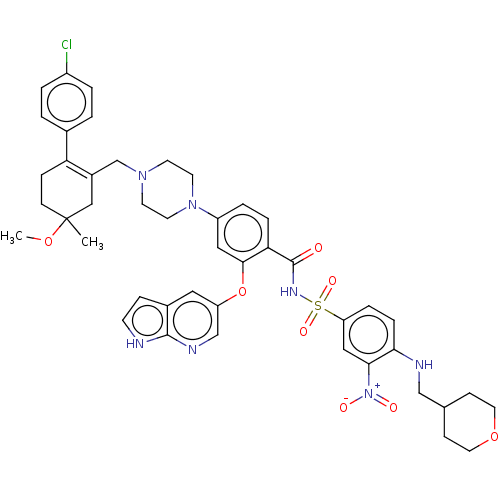
(US10213433, Compound 378 | US11369599, Compound 37...)Show SMILES COC1(C)CCC(=C(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)C1)c1ccc(Cl)cc1 |t:6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM189797
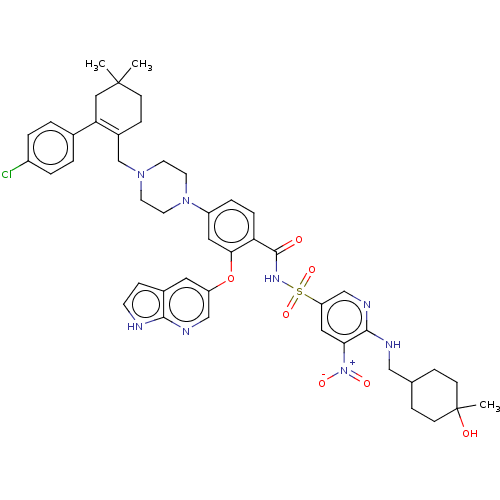
(US11369599, Compound 376 | US9174982, 371 | US9174...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3cnc(NCC4CCC(C)(O)CC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:59,(8.08,-9.44,;7.26,-8.14,;8.8,-8.19,;7.26,-6.6,;5.93,-5.83,;4.59,-6.6,;3.26,-5.83,;3.26,-4.29,;4.59,-3.52,;4.59,-1.98,;3.26,-1.21,;1.92,-1.98,;1.92,-3.52,;3.26,.33,;4.59,1.1,;4.59,2.64,;3.26,3.41,;3.26,4.95,;4.59,5.72,;1.92,5.72,;1.92,7.26,;1.92,8.8,;3.46,7.26,;.38,7.26,;-.39,5.93,;-1.93,5.93,;-2.7,7.26,;-4.24,7.26,;-5.01,8.59,;-6.55,8.59,;-7.32,7.26,;-8.86,7.26,;-9.63,8.59,;-10.99,7.87,;-10.99,9.32,;-8.86,9.93,;-7.32,9.93,;-1.93,8.59,;-.39,8.59,;-2.7,9.93,;-1.93,11.26,;-4.24,9.93,;1.92,2.64,;.59,3.41,;-.74,2.64,;-.74,1.1,;-2.08,.33,;-3.41,1.1,;-4.87,.63,;-5.78,1.87,;-4.87,3.12,;-3.41,2.64,;-2.08,3.41,;1.92,1.1,;4.59,-8.14,;5.93,-8.91,;3.26,-8.91,;3.26,-10.45,;1.92,-11.22,;.59,-10.45,;-.74,-11.22,;.59,-8.91,;1.92,-8.14,)| Show InChI InChI=1S/C46H53ClN8O7S/c1-45(2)14-12-33(39(25-45)31-4-6-34(47)7-5-31)29-53-18-20-54(21-19-53)35-8-9-38(41(23-35)62-36-22-32-13-17-48-42(32)50-27-36)44(56)52-63(60,61)37-24-40(55(58)59)43(51-28-37)49-26-30-10-15-46(3,57)16-11-30/h4-9,13,17,22-24,27-28,30,57H,10-12,14-16,18-21,25-26,29H2,1-3H3,(H,48,50)(H,49,51)(H,52,56) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US9174982 (2015)
BindingDB Entry DOI: 10.7270/Q2RB73D5 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | US20240043404, Example 374

(US10213433, Compound 374 | US11369599, Compound 37...)Show SMILES COCC1CCC(CNc2ccc(cc2[N+]([O-])=O)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cnc3[nH]ccc3c2)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)CC1 |t:49,(-10.07,6.16,;-8.53,6.16,;-7.76,4.83,;-6.22,4.83,;-5.45,3.49,;-3.91,3.49,;-3.14,4.83,;-1.6,4.83,;-.83,6.16,;.71,6.16,;1.48,4.83,;3.02,4.83,;3.79,6.16,;3.02,7.49,;1.48,7.49,;.71,8.83,;1.48,10.16,;-.83,8.83,;5.33,6.16,;5.33,7.7,;6.87,6.16,;5.33,4.62,;6.67,3.85,;8,4.62,;6.67,2.31,;8,1.54,;8,,;6.67,-.77,;5.33,,;5.33,1.54,;4,2.31,;2.67,1.54,;2.67,,;1.33,-.77,;;-1.46,-.48,;-2.37,.77,;-1.46,2.02,;,1.54,;1.33,2.31,;6.67,-2.31,;8,-3.08,;8,-4.62,;6.67,-5.39,;6.67,-6.93,;8,-7.7,;8,-9.24,;9.34,-10.01,;10.67,-9.24,;11.49,-10.55,;12.21,-9.29,;10.67,-7.7,;9.34,-6.93,;6.67,-10.01,;6.67,-11.55,;5.33,-12.32,;4,-11.55,;2.67,-12.32,;4,-10.01,;5.33,-9.24,;5.33,-4.62,;5.33,-3.08,;-3.91,6.16,;-5.45,6.16,)| Show InChI InChI=1S/C48H56ClN7O7S/c1-48(2)18-16-36(42(27-48)34-8-10-37(49)11-9-34)30-54-20-22-55(23-21-54)38-12-14-41(45(25-38)63-39-24-35-17-19-50-46(35)52-29-39)47(57)53-64(60,61)40-13-15-43(44(26-40)56(58)59)51-28-32-4-6-33(7-5-32)31-62-3/h8-15,17,19,24-26,29,32-33,51H,4-7,16,18,20-23,27-28,30-31H2,1-3H3,(H,50,52)(H,53,57) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM144940
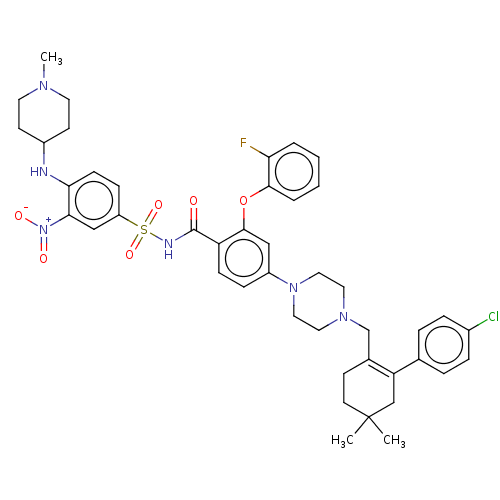
(US8952157, 122 | US9303025, 122)Show SMILES CN1CCC(CC1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1Oc1ccccc1F)N1CCN(CC2=C(CC(C)(C)CC2)c2ccc(Cl)cc2)CC1 |t:46| Show InChI InChI=1S/C44H50ClFN6O6S/c1-44(2)19-16-31(37(28-44)30-8-10-32(45)11-9-30)29-50-22-24-51(25-23-50)34-12-14-36(42(26-34)58-41-7-5-4-6-38(41)46)43(53)48-59(56,57)35-13-15-39(40(27-35)52(54)55)47-33-17-20-49(3)21-18-33/h4-15,26-27,33,47H,16-25,28-29H2,1-3H3,(H,48,53) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US8952157 (2015)
BindingDB Entry DOI: 10.7270/Q2QN65G9 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178562
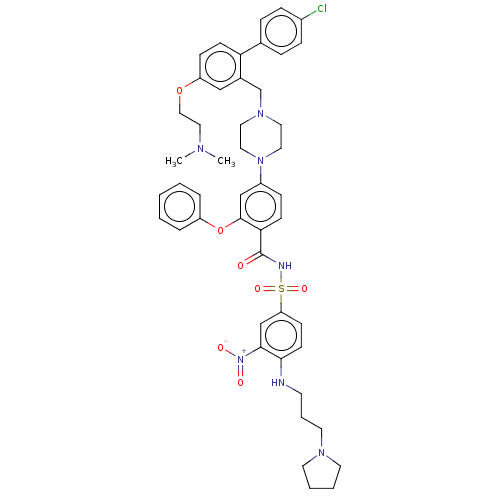
(US9125913, 121)Show SMILES CN(C)CCOc1ccc(c(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCCCN4CCCC4)c(c3)[N+]([O-])=O)c(Oc3ccccc3)c2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C47H54ClN7O7S/c1-51(2)29-30-61-40-16-19-42(35-11-13-37(48)14-12-35)36(31-40)34-53-25-27-54(28-26-53)38-15-18-43(46(32-38)62-39-9-4-3-5-10-39)47(56)50-63(59,60)41-17-20-44(45(33-41)55(57)58)49-21-8-24-52-22-6-7-23-52/h3-5,9-20,31-33,49H,6-8,21-30,34H2,1-2H3,(H,50,56) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | US20240043404, Example 373
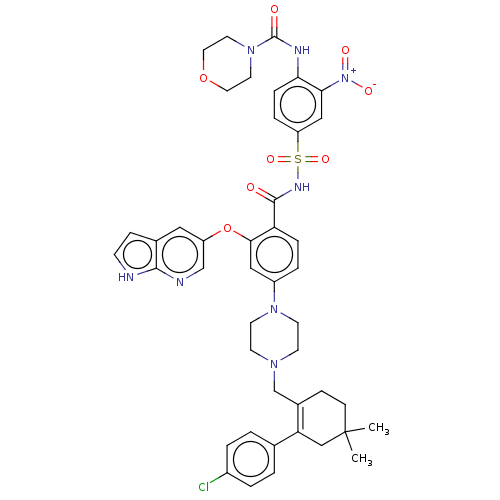
(US10213433, Compound 373 | US11369599, Compound 37...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NC(=O)N4CCOCC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:58| Show InChI InChI=1S/C44H47ClN8O8S/c1-44(2)13-11-31(37(26-44)29-3-5-32(45)6-4-29)28-50-15-17-51(18-16-50)33-7-9-36(40(24-33)61-34-23-30-12-14-46-41(30)47-27-34)42(54)49-62(58,59)35-8-10-38(39(25-35)53(56)57)48-43(55)52-19-21-60-22-20-52/h3-10,12,14,23-25,27H,11,13,15-22,26,28H2,1-2H3,(H,46,47)(H,48,55)(H,49,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | US20240043404, Example 371
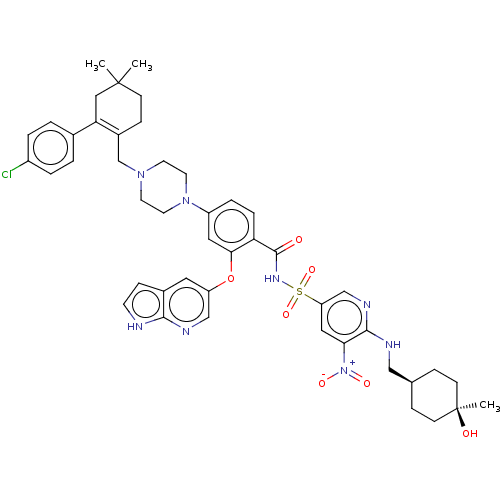
(4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3cnc(NC[C@H]4CC[C@@](C)(O)CC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |wU:32.34,29.29,wD:32.33,c:59,(2.25,13.94,;1.48,12.6,;3.02,12.6,;1.48,11.06,;.15,10.29,;-1.18,11.06,;-2.52,10.29,;-2.52,8.75,;-3.85,7.98,;-3.85,6.44,;-2.52,5.67,;-1.18,6.44,;-1.18,7.98,;-2.52,4.13,;-3.85,3.36,;-3.85,1.82,;-2.52,1.05,;-2.52,-.49,;-3.85,-1.26,;-1.18,-1.26,;-1.18,-2.8,;-2.72,-2.8,;.36,-2.8,;-1.18,-4.34,;-2.52,-5.11,;-2.52,-6.65,;-1.18,-7.42,;-1.18,-8.96,;-2.52,-9.73,;-2.52,-11.27,;-1.18,-12.04,;-1.18,-13.58,;-2.52,-14.35,;-3.29,-15.68,;-1.75,-15.68,;-3.85,-13.58,;-3.85,-12.04,;.15,-6.65,;.15,-5.11,;1.48,-7.42,;2.82,-6.65,;1.48,-8.96,;-1.18,1.82,;.15,1.05,;1.48,1.82,;1.48,3.36,;2.82,4.13,;4.15,3.36,;5.61,3.84,;6.52,2.59,;5.61,1.35,;4.15,1.82,;2.82,1.05,;-1.18,3.36,;-1.18,12.6,;.15,13.37,;-2.52,13.37,;-3.85,12.6,;-5.19,13.37,;-5.19,14.91,;-6.52,15.68,;-3.85,15.68,;-2.52,14.91,)| Show InChI InChI=1S/C46H53ClN8O7S/c1-45(2)14-12-33(39(25-45)31-4-6-34(47)7-5-31)29-53-18-20-54(21-19-53)35-8-9-38(41(23-35)62-36-22-32-13-17-48-42(32)50-27-36)44(56)52-63(60,61)37-24-40(55(58)59)43(51-28-37)49-26-30-10-15-46(3,57)16-11-30/h4-9,13,17,22-24,27-28,30,57H,10-12,14-16,18-21,25-26,29H2,1-3H3,(H,48,50)(H,49,51)(H,52,56)/t30-,46+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM451126
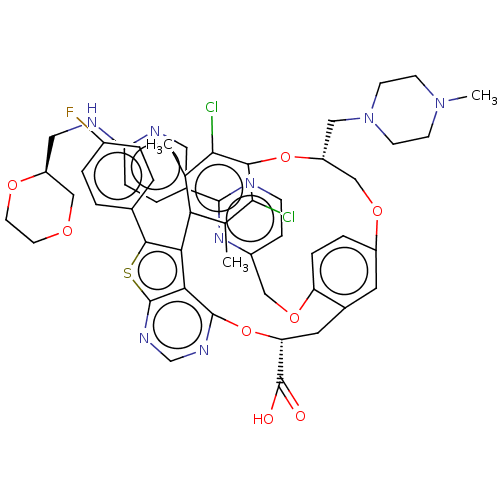
(US10676485, Example 36)Show SMILES CN1CCN(C[C@@H]2COc3ccc(OCc4ccnc(n4)-c4ccc(NC[C@H]5COCCO5)nc4)c(C[C@@H](Oc4ncnc5sc(c(-c6c(C)c(Cl)c(O2)c(Cl)c6C)c45)-c2ccc(F)cc2)C(O)=O)c3)CC1 |r| Show InChI InChI=1S/C52H51Cl2FN8O8S/c1-29-42-30(2)46(54)47(45(29)53)70-38(24-63-16-14-62(3)15-17-63)27-68-36-9-10-39(69-25-35-12-13-56-49(61-35)32-6-11-41(57-22-32)58-23-37-26-66-18-19-67-37)33(20-36)21-40(52(64)65)71-50-44-43(42)48(72-51(44)60-28-59-50)31-4-7-34(55)8-5-31/h4-13,20,22,28,37-38,40H,14-19,21,23-27H2,1-3H3,(H,57,58)(H,64,65)/t37-,38+,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The ability of the exemplary MCL-1 inhibitors of Examples 1 through 151 to bind MCL-1 was demonstrated using the Time Resolved-Fluorescence Resonance... |
US Patent US10676485 (2020)
BindingDB Entry DOI: 10.7270/Q2PZ5CWK |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM144940

(US8952157, 122 | US9303025, 122)Show SMILES CN1CCC(CC1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1Oc1ccccc1F)N1CCN(CC2=C(CC(C)(C)CC2)c2ccc(Cl)cc2)CC1 |t:46| Show InChI InChI=1S/C44H50ClFN6O6S/c1-44(2)19-16-31(37(28-44)30-8-10-32(45)11-9-30)29-50-22-24-51(25-23-50)34-12-14-36(42(26-34)58-41-7-5-4-6-38(41)46)43(53)48-59(56,57)35-13-15-39(40(27-35)52(54)55)47-33-17-20-49(3)21-18-33/h4-15,26-27,33,47H,16-25,28-29H2,1-3H3,(H,48,53) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... |
US Patent US9303025 (2016)
BindingDB Entry DOI: 10.7270/Q2XD10JP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM189804

(US10213433, Compound 378 | US11369599, Compound 37...)Show SMILES COC1(C)CCC(=C(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)C1)c1ccc(Cl)cc1 |t:6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US9174982 (2015)
BindingDB Entry DOI: 10.7270/Q2RB73D5 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM189803
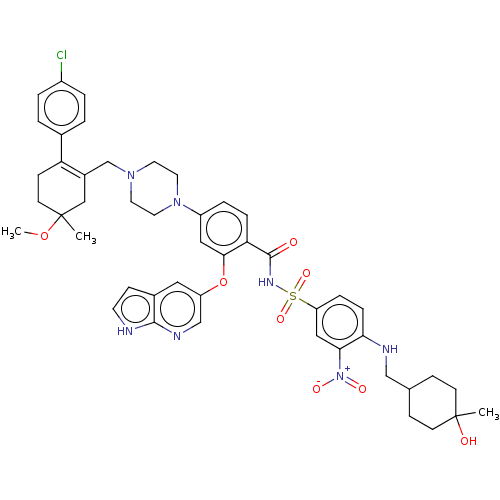
(US11369599, Compound 377 | US9174982, 377)Show SMILES COC1(C)CCC(=C(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCC(C)(O)CC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)C1)c1ccc(Cl)cc1 |t:6,(7.24,-3.72,;7.96,-5.08,;7.15,-6.39,;8.68,-6.33,;7.15,-7.93,;5.81,-8.7,;4.48,-7.93,;4.48,-6.39,;3.14,-5.62,;3.14,-4.08,;4.48,-3.31,;4.48,-1.77,;3.14,-1,;1.81,-1.77,;1.81,-3.31,;3.14,.54,;4.48,1.31,;4.48,2.85,;3.14,3.62,;3.14,5.16,;4.48,5.93,;1.81,5.93,;1.81,7.47,;1.81,9.01,;3.35,7.47,;.27,7.47,;-.5,6.14,;-2.04,6.14,;-2.81,7.47,;-4.35,7.47,;-5.12,6.14,;-6.66,6.14,;-7.43,4.81,;-8.97,4.81,;-9.74,6.14,;-11.1,5.42,;-11.1,6.86,;-8.97,7.47,;-7.43,7.47,;-2.04,8.81,;-.5,8.81,;-2.81,10.14,;-2.04,11.48,;-4.35,10.14,;1.81,2.85,;.48,3.62,;-.86,2.85,;-.86,1.31,;-2.19,.54,;-3.52,1.31,;-4.99,.84,;-5.89,2.08,;-4.99,3.33,;-3.52,2.85,;-2.19,3.62,;1.81,1.31,;5.81,-5.62,;3.14,-8.7,;3.14,-10.24,;1.81,-11.01,;.48,-10.24,;-.86,-11.01,;.48,-8.7,;1.81,-7.93,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US9174982 (2015)
BindingDB Entry DOI: 10.7270/Q2RB73D5 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM189797

(US11369599, Compound 376 | US9174982, 371 | US9174...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3cnc(NCC4CCC(C)(O)CC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:59,(8.08,-9.44,;7.26,-8.14,;8.8,-8.19,;7.26,-6.6,;5.93,-5.83,;4.59,-6.6,;3.26,-5.83,;3.26,-4.29,;4.59,-3.52,;4.59,-1.98,;3.26,-1.21,;1.92,-1.98,;1.92,-3.52,;3.26,.33,;4.59,1.1,;4.59,2.64,;3.26,3.41,;3.26,4.95,;4.59,5.72,;1.92,5.72,;1.92,7.26,;1.92,8.8,;3.46,7.26,;.38,7.26,;-.39,5.93,;-1.93,5.93,;-2.7,7.26,;-4.24,7.26,;-5.01,8.59,;-6.55,8.59,;-7.32,7.26,;-8.86,7.26,;-9.63,8.59,;-10.99,7.87,;-10.99,9.32,;-8.86,9.93,;-7.32,9.93,;-1.93,8.59,;-.39,8.59,;-2.7,9.93,;-1.93,11.26,;-4.24,9.93,;1.92,2.64,;.59,3.41,;-.74,2.64,;-.74,1.1,;-2.08,.33,;-3.41,1.1,;-4.87,.63,;-5.78,1.87,;-4.87,3.12,;-3.41,2.64,;-2.08,3.41,;1.92,1.1,;4.59,-8.14,;5.93,-8.91,;3.26,-8.91,;3.26,-10.45,;1.92,-11.22,;.59,-10.45,;-.74,-11.22,;.59,-8.91,;1.92,-8.14,)| Show InChI InChI=1S/C46H53ClN8O7S/c1-45(2)14-12-33(39(25-45)31-4-6-34(47)7-5-31)29-53-18-20-54(21-19-53)35-8-9-38(41(23-35)62-36-22-32-13-17-48-42(32)50-27-36)44(56)52-63(60,61)37-24-40(55(58)59)43(51-28-37)49-26-30-10-15-46(3,57)16-11-30/h4-9,13,17,22-24,27-28,30,57H,10-12,14-16,18-21,25-26,29H2,1-3H3,(H,48,50)(H,49,51)(H,52,56) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US9174982 (2015)
BindingDB Entry DOI: 10.7270/Q2RB73D5 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM189800

(US10213433, Compound 374 | US11369599, Compound 37...)Show SMILES COCC1CCC(CNc2ccc(cc2[N+]([O-])=O)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cnc3[nH]ccc3c2)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)CC1 |t:49,(-10.07,6.16,;-8.53,6.16,;-7.76,4.83,;-6.22,4.83,;-5.45,3.49,;-3.91,3.49,;-3.14,4.83,;-1.6,4.83,;-.83,6.16,;.71,6.16,;1.48,4.83,;3.02,4.83,;3.79,6.16,;3.02,7.49,;1.48,7.49,;.71,8.83,;1.48,10.16,;-.83,8.83,;5.33,6.16,;5.33,7.7,;6.87,6.16,;5.33,4.62,;6.67,3.85,;8,4.62,;6.67,2.31,;8,1.54,;8,,;6.67,-.77,;5.33,,;5.33,1.54,;4,2.31,;2.67,1.54,;2.67,,;1.33,-.77,;;-1.46,-.48,;-2.37,.77,;-1.46,2.02,;,1.54,;1.33,2.31,;6.67,-2.31,;8,-3.08,;8,-4.62,;6.67,-5.39,;6.67,-6.93,;8,-7.7,;8,-9.24,;9.34,-10.01,;10.67,-9.24,;11.49,-10.55,;12.21,-9.29,;10.67,-7.7,;9.34,-6.93,;6.67,-10.01,;6.67,-11.55,;5.33,-12.32,;4,-11.55,;2.67,-12.32,;4,-10.01,;5.33,-9.24,;5.33,-4.62,;5.33,-3.08,;-3.91,6.16,;-5.45,6.16,)| Show InChI InChI=1S/C48H56ClN7O7S/c1-48(2)18-16-36(42(27-48)34-8-10-37(49)11-9-34)30-54-20-22-55(23-21-54)38-12-14-41(45(25-38)63-39-24-35-17-19-50-46(35)52-29-39)47(57)53-64(60,61)40-13-15-43(44(26-40)56(58)59)51-28-32-4-6-33(7-5-32)31-62-3/h8-15,17,19,24-26,29,32-33,51H,4-7,16,18,20-23,27-28,30-31H2,1-3H3,(H,50,52)(H,53,57) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US9174982 (2015)
BindingDB Entry DOI: 10.7270/Q2RB73D5 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | US20240043404, Example 377
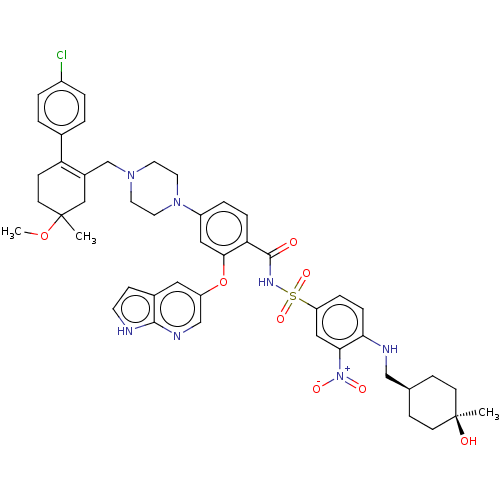
(4-(4-{[2-(4-chlorophenyl)-5-methoxy-5-methylcycloh...)Show SMILES COC1(C)CCC(=C(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NC[C@H]4CC[C@@](C)(O)CC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)C1)c1ccc(Cl)cc1 |wU:34.36,31.31,wD:34.35,t:6,(3.79,9.73,;3.02,11.06,;1.48,11.06,;2.25,9.73,;1.48,12.6,;.15,13.37,;-1.18,12.6,;-1.18,11.06,;-2.52,10.29,;-2.52,8.75,;-3.85,7.98,;-3.85,6.44,;-2.52,5.67,;-1.18,6.44,;-1.18,7.98,;-2.52,4.13,;-3.85,3.36,;-3.85,1.82,;-2.52,1.05,;-2.52,-.49,;-3.85,-1.26,;-1.18,-1.26,;-1.18,-2.8,;-2.72,-2.8,;.36,-2.8,;-1.18,-4.34,;-2.52,-5.11,;-2.52,-6.65,;-1.18,-7.42,;-1.18,-8.96,;-2.52,-9.73,;-2.52,-11.27,;-1.18,-12.04,;-1.18,-13.58,;-2.52,-14.35,;-3.29,-15.68,;-1.75,-15.68,;-3.85,-13.58,;-3.85,-12.04,;.15,-6.65,;.15,-5.11,;1.48,-7.42,;2.82,-6.65,;1.48,-8.96,;-1.18,1.82,;.15,1.05,;1.48,1.82,;1.48,3.36,;2.82,4.13,;4.15,3.36,;5.61,3.84,;6.52,2.59,;5.61,1.35,;4.15,1.82,;2.82,1.05,;-1.18,3.36,;.15,10.29,;-2.52,13.37,;-3.85,12.6,;-5.19,13.37,;-5.19,14.91,;-6.52,15.68,;-3.85,15.68,;-2.52,14.91,)| Show InChI InChI=1S/C47H54ClN7O8S/c1-46(57)16-12-31(13-17-46)28-50-41-11-9-38(26-42(41)55(58)59)64(60,61)52-45(56)40-10-8-36(25-43(40)63-37-24-33-15-19-49-44(33)51-29-37)54-22-20-53(21-23-54)30-34-27-47(2,62-3)18-14-39(34)32-4-6-35(48)7-5-32/h4-11,15,19,24-26,29,31,50,57H,12-14,16-18,20-23,27-28,30H2,1-3H3,(H,49,51)(H,52,56)/t31-,46+,47? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM189799

(US10213433, Compound 373 | US11369599, Compound 37...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NC(=O)N4CCOCC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:58| Show InChI InChI=1S/C44H47ClN8O8S/c1-44(2)13-11-31(37(26-44)29-3-5-32(45)6-4-29)28-50-15-17-51(18-16-50)33-7-9-36(40(24-33)61-34-23-30-12-14-46-41(30)47-27-34)42(54)49-62(58,59)35-8-10-38(39(25-35)53(56)57)48-43(55)52-19-21-60-22-20-52/h3-10,12,14,23-25,27H,11,13,15-22,26,28H2,1-2H3,(H,46,47)(H,48,55)(H,49,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US9174982 (2015)
BindingDB Entry DOI: 10.7270/Q2RB73D5 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | US20240043404, Example 376
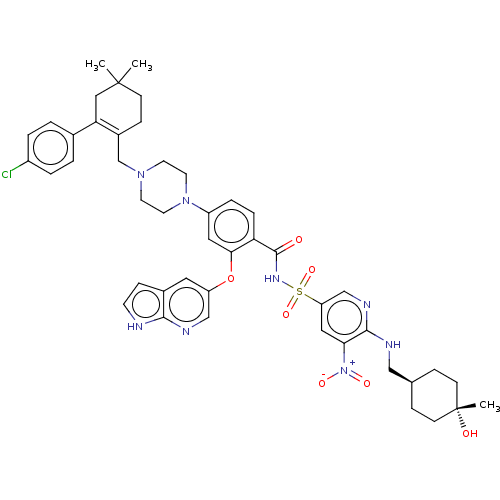
(4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3cnc(NC[C@H]4CC[C@](C)(O)CC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |wU:32.33,29.29,wD:32.34,c:59,(2.25,13.94,;1.48,12.6,;3.02,12.6,;1.48,11.06,;.15,10.29,;-1.18,11.06,;-2.52,10.29,;-2.52,8.75,;-3.85,7.98,;-3.85,6.44,;-2.52,5.67,;-1.18,6.44,;-1.18,7.98,;-2.52,4.13,;-3.85,3.36,;-3.85,1.82,;-2.52,1.05,;-2.52,-.49,;-3.85,-1.26,;-1.18,-1.26,;-1.18,-2.8,;-2.72,-2.8,;.36,-2.8,;-1.18,-4.34,;-2.52,-5.11,;-2.52,-6.65,;-1.18,-7.42,;-1.18,-8.96,;-2.52,-9.73,;-2.52,-11.27,;-1.18,-12.04,;-1.18,-13.58,;-2.52,-14.35,;-3.29,-15.68,;-1.75,-15.68,;-3.85,-13.58,;-3.85,-12.04,;.15,-6.65,;.15,-5.11,;1.48,-7.42,;2.82,-6.65,;1.48,-8.96,;-1.18,1.82,;.15,1.05,;1.48,1.82,;1.48,3.36,;2.82,4.13,;4.15,3.36,;5.61,3.84,;6.52,2.59,;5.61,1.35,;4.15,1.82,;2.82,1.05,;-1.18,3.36,;-1.18,12.6,;.15,13.37,;-2.52,13.37,;-3.85,12.6,;-5.19,13.37,;-5.19,14.91,;-6.52,15.68,;-3.85,15.68,;-2.52,14.91,)| Show InChI InChI=1S/C46H53ClN8O7S/c1-45(2)14-12-33(39(25-45)31-4-6-34(47)7-5-31)29-53-18-20-54(21-19-53)35-8-9-38(41(23-35)62-36-22-32-13-17-48-42(32)50-27-36)44(56)52-63(60,61)37-24-40(55(58)59)43(51-28-37)49-26-30-10-15-46(3,57)16-11-30/h4-9,13,17,22-24,27-28,30,57H,10-12,14-16,18-21,25-26,29H2,1-3H3,(H,48,50)(H,49,51)(H,52,56)/t30-,46- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178843
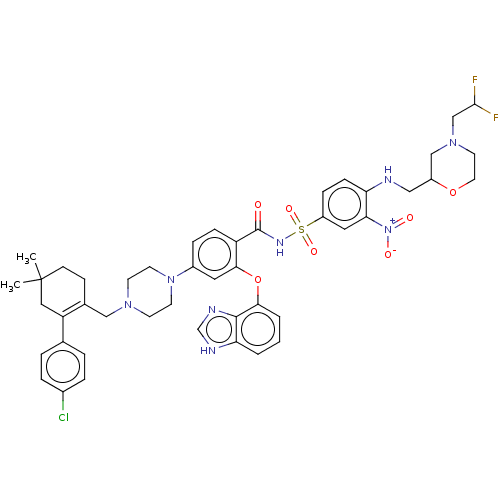
(US9125913, 415)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CN(CC(F)F)CCO4)c(c3)[N+]([O-])=O)c(Oc3cccc4[nH]cnc34)c2)=C(C1)c1ccc(Cl)cc1 |c:61| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178836
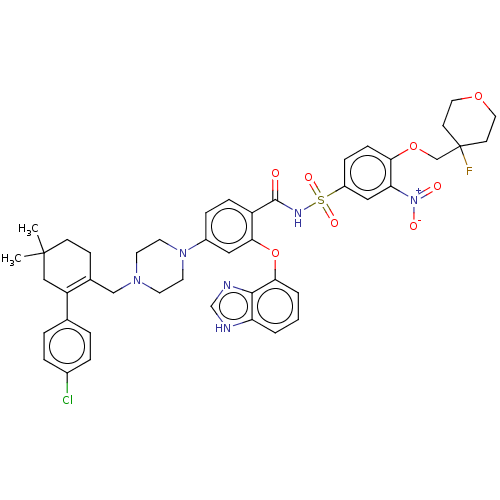
(US9125913, 408)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(OCC4(F)CCOCC4)c(c3)[N+]([O-])=O)c(Oc3cccc4[nH]cnc34)c2)=C(C1)c1ccc(Cl)cc1 |c:58| Show InChI InChI=1S/C45H48ClFN6O8S/c1-44(2)15-14-31(36(26-44)30-6-8-32(46)9-7-30)27-51-18-20-52(21-19-51)33-10-12-35(41(24-33)61-40-5-3-4-37-42(40)49-29-48-37)43(54)50-62(57,58)34-11-13-39(38(25-34)53(55)56)60-28-45(47)16-22-59-23-17-45/h3-13,24-25,29H,14-23,26-28H2,1-2H3,(H,48,49)(H,50,54) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM189567
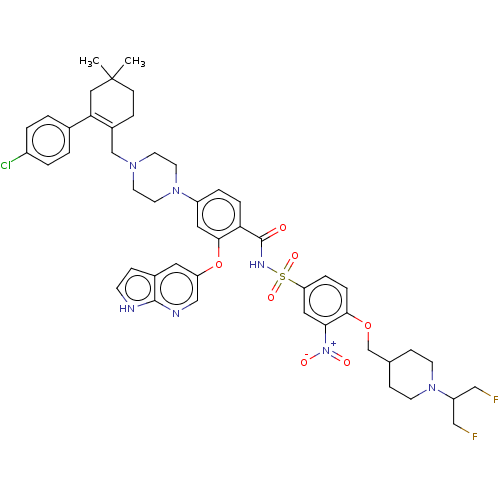
(US10213433, Compound 129 | US11369599, Compound 12...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(OCC4CCN(CC4)C(CF)CF)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:62| Show InChI InChI=1S/C48H54ClF2N7O7S/c1-48(2)15-11-35(42(26-48)33-3-5-36(49)6-4-33)30-55-19-21-57(22-20-55)37-7-9-41(45(24-37)65-39-23-34-12-16-52-46(34)53-29-39)47(59)54-66(62,63)40-8-10-44(43(25-40)58(60)61)64-31-32-13-17-56(18-14-32)38(27-50)28-51/h3-10,12,16,23-25,29,32,38H,11,13-15,17-22,26-28,30-31H2,1-2H3,(H,52,53)(H,54,59) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00200 | -66.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US9174982 (2015)
BindingDB Entry DOI: 10.7270/Q2RB73D5 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | US20240043404, Example 129

(US10213433, Compound 129 | US11369599, Compound 12...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(OCC4CCN(CC4)C(CF)CF)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:62| Show InChI InChI=1S/C48H54ClF2N7O7S/c1-48(2)15-11-35(42(26-48)33-3-5-36(49)6-4-33)30-55-19-21-57(22-20-55)37-7-9-41(45(24-37)65-39-23-34-12-16-52-46(34)53-29-39)47(59)54-66(62,63)40-8-10-44(43(25-40)58(60)61)64-31-32-13-17-56(18-14-32)38(27-50)28-51/h3-10,12,16,23-25,29,32,38H,11,13-15,17-22,26-28,30-31H2,1-2H3,(H,52,53)(H,54,59) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178855
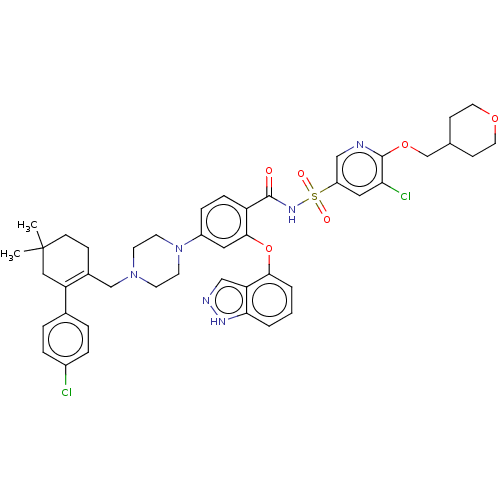
(US9125913, 427)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3cnc(OCC4CCOCC4)c(Cl)c3)c(Oc3cccc4[nH]ncc34)c2)=C(C1)c1ccc(Cl)cc1 |c:55| Show InChI InChI=1S/C44H48Cl2N6O6S/c1-44(2)15-12-31(36(24-44)30-6-8-32(45)9-7-30)27-51-16-18-52(19-17-51)33-10-11-35(41(22-33)58-40-5-3-4-39-37(40)26-48-49-39)42(53)50-59(54,55)34-23-38(46)43(47-25-34)57-28-29-13-20-56-21-14-29/h3-11,22-23,25-26,29H,12-21,24,27-28H2,1-2H3,(H,48,49)(H,50,53) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178831
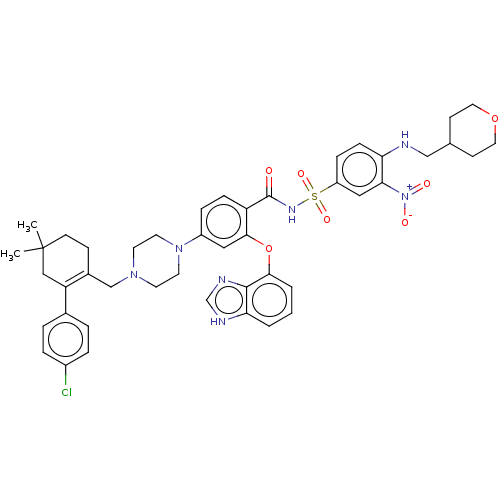
(US9125913, 403)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)[N+]([O-])=O)c(Oc3cccc4[nH]cnc34)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)17-14-32(37(26-45)31-6-8-33(46)9-7-31)28-51-18-20-52(21-19-51)34-10-12-36(42(24-34)60-41-5-3-4-39-43(41)49-29-48-39)44(54)50-61(57,58)35-11-13-38(40(25-35)53(55)56)47-27-30-15-22-59-23-16-30/h3-13,24-25,29-30,47H,14-23,26-28H2,1-2H3,(H,48,49)(H,50,54) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178839
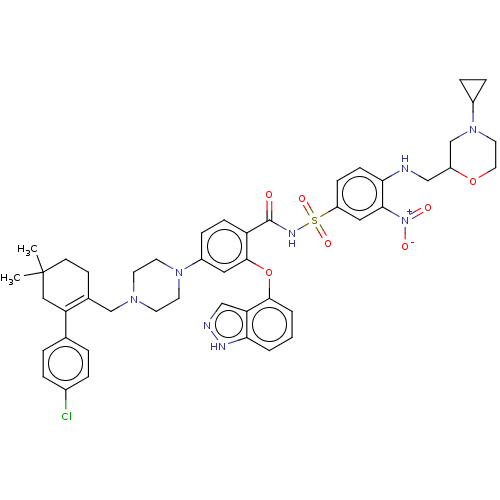
(US9125913, 411)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CN(CCO4)C4CC4)c(c3)[N+]([O-])=O)c(Oc3cccc4[nH]ncc34)c2)=C(C1)c1ccc(Cl)cc1 |c:61| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178856
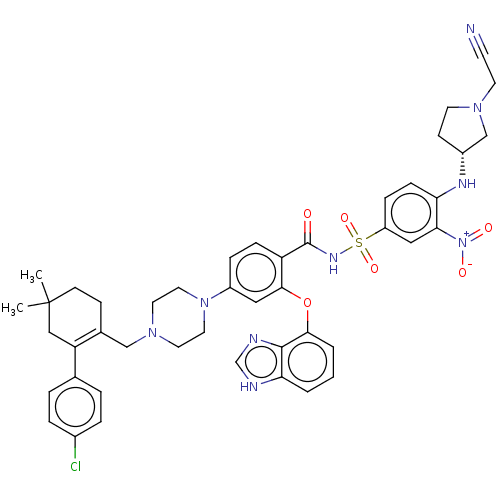
(US9125913, 428)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(N[C@@H]4CCN(CC#N)C4)c(c3)[N+]([O-])=O)c(Oc3cccc4[nH]cnc34)c2)=C(C1)c1ccc(Cl)cc1 |c:58| Show InChI InChI=1S/C45H48ClN9O6S/c1-45(2)16-14-31(37(26-45)30-6-8-32(46)9-7-30)27-53-20-22-54(23-21-53)34-10-12-36(42(24-34)61-41-5-3-4-39-43(41)49-29-48-39)44(56)51-62(59,60)35-11-13-38(40(25-35)55(57)58)50-33-15-18-52(28-33)19-17-47/h3-13,24-25,29,33,50H,14-16,18-23,26-28H2,1-2H3,(H,48,49)(H,51,56)/t33-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178566
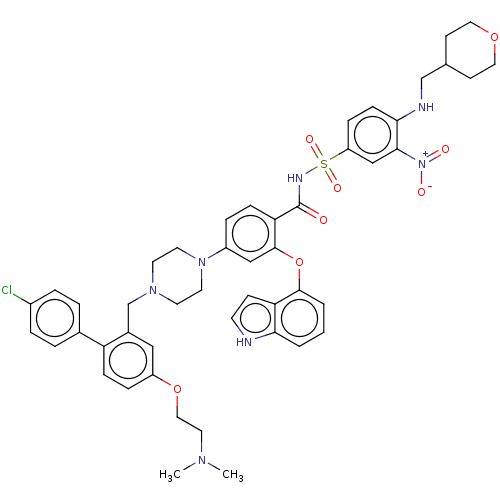
(US9125913, 125)Show SMILES CN(C)CCOc1ccc(c(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)[N+]([O-])=O)c(Oc3cccc4[nH]ccc34)c2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C48H52ClN7O8S/c1-53(2)24-27-63-38-11-14-40(34-6-8-36(49)9-7-34)35(28-38)32-54-20-22-55(23-21-54)37-10-13-42(47(29-37)64-46-5-3-4-43-41(46)16-19-50-43)48(57)52-65(60,61)39-12-15-44(45(30-39)56(58)59)51-31-33-17-25-62-26-18-33/h3-16,19,28-30,33,50-51H,17-18,20-27,31-32H2,1-2H3,(H,52,57) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM451115
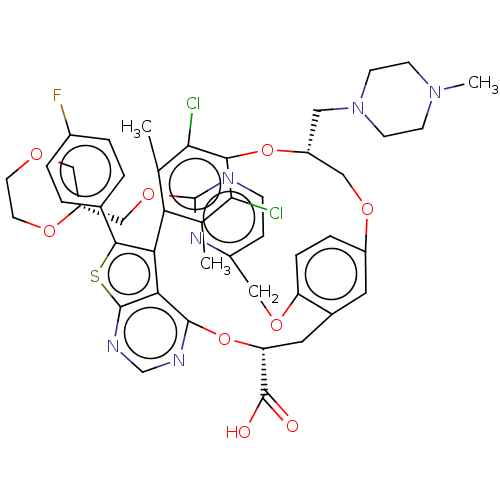
(US10676485, Example 25)Show SMILES CN1CCN(C[C@@H]2COc3ccc(OCc4ccnc(OC[C@H]5COCCO5)n4)c(C[C@@H](Oc4ncnc5sc(c(-c6c(C)c(Cl)c(O2)c(Cl)c6C)c45)-c2ccc(F)cc2)C(O)=O)c3)CC1 |r| Show InChI InChI=1S/C47H47Cl2FN6O9S/c1-26-37-27(2)41(49)42(40(26)48)64-33(20-56-14-12-55(3)13-15-56)23-61-32-8-9-35(62-21-31-10-11-51-47(54-31)63-24-34-22-59-16-17-60-34)29(18-32)19-36(46(57)58)65-44-39-38(37)43(66-45(39)53-25-52-44)28-4-6-30(50)7-5-28/h4-11,18,25,33-34,36H,12-17,19-24H2,1-3H3,(H,57,58)/t33-,34-,36-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The ability of the exemplary MCL-1 inhibitors of Examples 1 through 151 to bind MCL-1 was demonstrated using the Time Resolved-Fluorescence Resonance... |
US Patent US10676485 (2020)
BindingDB Entry DOI: 10.7270/Q2PZ5CWK |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178853
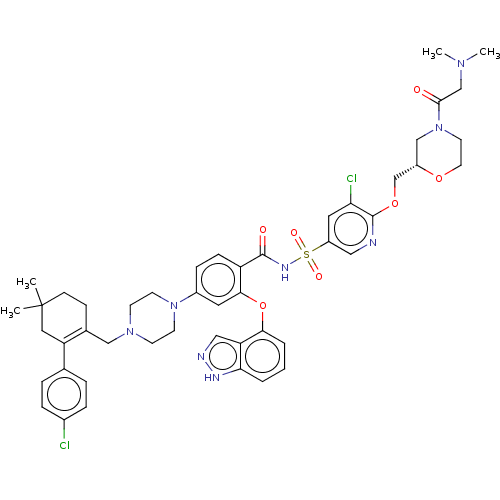
(US9125913, 425)Show SMILES CN(C)CC(=O)N1CCO[C@H](COc2ncc(cc2Cl)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cccc3[nH]ncc23)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)C1 |t:51| Show InChI InChI=1S/C47H54Cl2N8O7S/c1-47(2)15-14-32(38(24-47)31-8-10-33(48)11-9-31)27-55-16-18-56(19-17-55)34-12-13-37(43(22-34)64-42-7-5-6-41-39(42)26-51-52-41)45(59)53-65(60,61)36-23-40(49)46(50-25-36)63-30-35-28-57(20-21-62-35)44(58)29-54(3)4/h5-13,22-23,25-26,35H,14-21,24,27-30H2,1-4H3,(H,51,52)(H,53,59)/t35-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM451129
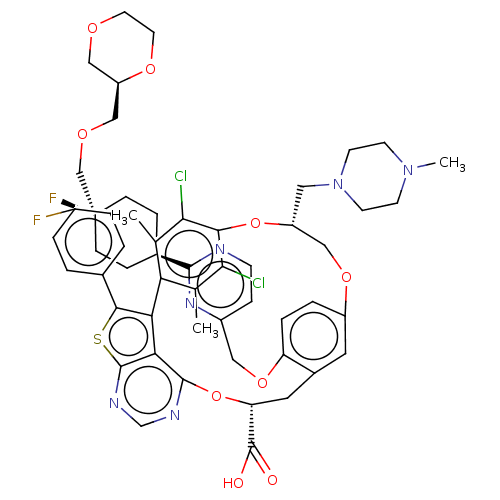
(US10676485, Example 39)Show SMILES CN1CCN(C[C@@H]2COc3ccc(OCc4ccnc(n4)[C@H]4CC[C@](F)(COC[C@H]5COCCO5)CC4)c(C[C@@H](Oc4ncnc5sc(c(-c6c(C)c(Cl)c(O2)c(Cl)c6C)c45)-c2ccc(F)cc2)C(O)=O)c3)CC1 |r,wU:29.29,24.26,6.5,39.75,wD:21.21,24.25,(-15.71,-6.12,;-14.23,-5.72,;-13.83,-4.23,;-12.34,-3.83,;-11.25,-4.92,;-9.76,-4.52,;-9.36,-3.03,;-7.88,-2.63,;-7.48,-1.15,;-5.99,-1.55,;-5.59,-3.03,;-4.1,-3.43,;-3.02,-2.34,;-1.53,-2.74,;.01,-2.74,;.78,-1.41,;.01,-.07,;.78,1.26,;2.32,1.26,;3.09,-.07,;2.32,-1.41,;4.63,-.07,;5.4,-1.41,;6.94,-1.41,;7.71,-.07,;8.48,1.26,;9.05,-.84,;10.38,-.07,;11.71,-.84,;13.05,-.07,;13.05,1.47,;14.38,2.24,;15.71,1.47,;15.71,-.07,;14.38,-.84,;6.94,1.26,;5.4,1.26,;-3.41,-.86,;-2.33,.23,;-2.71,1.94,;-4.2,2.34,;-4.59,3.83,;-3.51,4.92,;-3.9,6.41,;-5.39,6.81,;-6.48,5.72,;-8.02,5.8,;-8.57,4.36,;-7.37,3.39,;-8.14,2.06,;-9.68,2.06,;-10.45,3.39,;-10.45,.72,;-11.99,.72,;-9.68,-.61,;-10.45,-1.94,;-8.14,-.61,;-8.91,-1.94,;-7.37,.72,;-6.04,1.49,;-6.08,4.23,;-10.06,4.76,;-10.46,6.25,;-11.94,6.64,;-13.03,5.56,;-14.52,5.95,;-12.63,4.07,;-11.15,3.67,;-1.62,3.03,;-.13,2.63,;-2.02,4.52,;-4.9,-.46,;-11.65,-6.41,;-13.14,-6.81,)| Show InChI InChI=1S/C54H58Cl2F2N6O9S/c1-31-43-32(2)47(56)48(46(31)55)72-39(24-64-18-16-63(3)17-19-64)28-70-38-8-9-41(71-25-37-12-15-59-50(62-37)34-10-13-54(58,14-11-34)29-68-27-40-26-67-20-21-69-40)35(22-38)23-42(53(65)66)73-51-45-44(43)49(74-52(45)61-30-60-51)33-4-6-36(57)7-5-33/h4-9,12,15,22,30,34,39-40,42H,10-11,13-14,16-21,23-29H2,1-3H3,(H,65,66)/t34-,39-,40-,42-,54+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The ability of the exemplary MCL-1 inhibitors of Examples 1 through 151 to bind MCL-1 was demonstrated using the Time Resolved-Fluorescence Resonance... |
US Patent US10676485 (2020)
BindingDB Entry DOI: 10.7270/Q2PZ5CWK |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178883
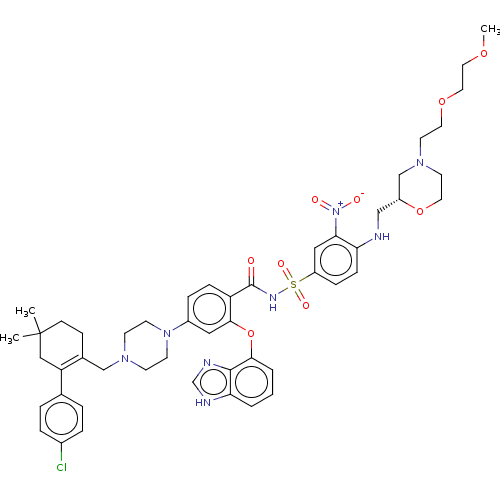
(US9125913, 455)Show SMILES COCCOCCN1CCO[C@H](CNc2ccc(cc2[N+]([O-])=O)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cccc3[nH]cnc23)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)C1 |t:54| Show InChI InChI=1S/C49H59ClN8O9S/c1-49(2)16-15-35(41(29-49)34-7-9-36(50)10-8-34)31-55-17-19-57(20-18-55)37-11-13-40(46(27-37)67-45-6-4-5-43-47(45)53-33-52-43)48(59)54-68(62,63)39-12-14-42(44(28-39)58(60)61)51-30-38-32-56(22-24-66-38)21-23-65-26-25-64-3/h4-14,27-28,33,38,51H,15-26,29-32H2,1-3H3,(H,52,53)(H,54,59)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178846
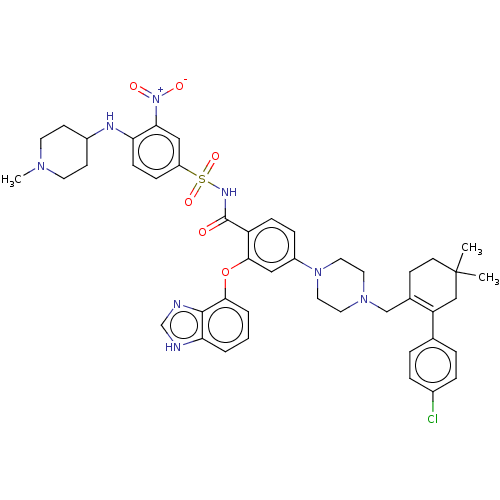
(US9125913, 418)Show SMILES CN1CCC(CC1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1Oc1cccc2[nH]cnc12)N1CCN(CC2=C(CC(C)(C)CC2)c2ccc(Cl)cc2)CC1 |t:49| Show InChI InChI=1S/C45H51ClN8O6S/c1-45(2)18-15-31(37(27-45)30-7-9-32(46)10-8-30)28-52-21-23-53(24-22-52)34-11-13-36(42(25-34)60-41-6-4-5-39-43(41)48-29-47-39)44(55)50-61(58,59)35-12-14-38(40(26-35)54(56)57)49-33-16-19-51(3)20-17-33/h4-14,25-26,29,33,49H,15-24,27-28H2,1-3H3,(H,47,48)(H,50,55) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178876
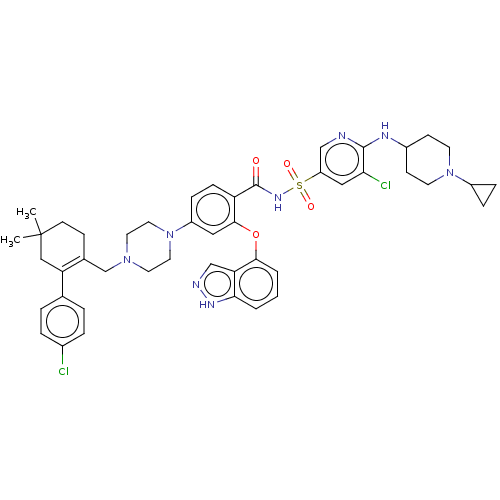
(US9125913, 448)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3cnc(NC4CCN(CC4)C4CC4)c(Cl)c3)c(Oc3cccc4[nH]ncc34)c2)=C(C1)c1ccc(Cl)cc1 |c:58| Show InChI InChI=1S/C46H52Cl2N8O4S/c1-46(2)17-14-31(38(26-46)30-6-8-32(47)9-7-30)29-54-20-22-56(23-21-54)35-12-13-37(43(24-35)60-42-5-3-4-41-39(42)28-50-52-41)45(57)53-61(58,59)36-25-40(48)44(49-27-36)51-33-15-18-55(19-16-33)34-10-11-34/h3-9,12-13,24-25,27-28,33-34H,10-11,14-23,26,29H2,1-2H3,(H,49,51)(H,50,52)(H,53,57) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM145154
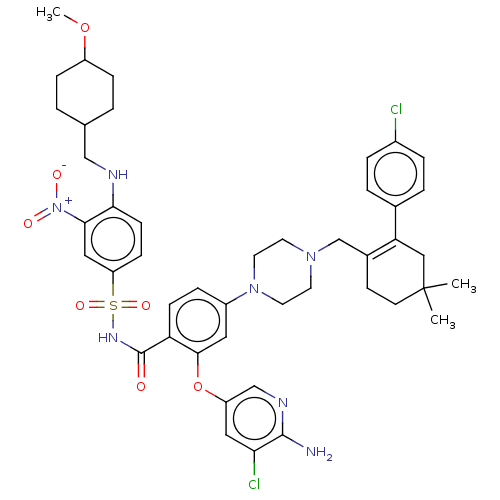
(US8952157, 345 | US9303025, 345)Show SMILES COC1CCC(CNc2ccc(cc2[N+]([O-])=O)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cnc(N)c(Cl)c2)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)CC1 |t:46,(-11.47,3.54,;-10.7,2.21,;-9.16,2.21,;-8.39,.88,;-6.85,.88,;-6.08,2.21,;-4.54,2.21,;-3.77,3.54,;-2.23,3.54,;-1.46,2.21,;.08,2.21,;.85,3.54,;.08,4.88,;-1.46,4.88,;-2.23,6.21,;-1.46,7.54,;-3.77,6.21,;2.39,3.54,;2.39,5.08,;3.93,3.54,;2.39,2,;3.72,1.23,;5.06,2,;3.72,-.31,;5.06,-1.08,;5.06,-2.62,;3.72,-3.39,;2.39,-2.62,;2.39,-1.08,;1.06,-.31,;-.28,-1.08,;-1.61,-.31,;-2.94,-1.08,;-2.94,-2.62,;-4.28,-3.39,;-1.61,-3.39,;-1.61,-4.93,;-.28,-2.62,;3.72,-4.93,;5.06,-5.7,;5.06,-7.24,;3.72,-8.01,;3.72,-9.55,;5.06,-10.32,;5.06,-11.86,;6.39,-12.63,;7.73,-11.86,;8.54,-13.16,;9.26,-11.91,;7.73,-10.32,;6.39,-9.55,;3.72,-12.63,;3.72,-14.17,;2.39,-14.94,;1.06,-14.17,;-.28,-14.94,;1.06,-12.63,;2.39,-11.86,;2.39,-7.24,;2.39,-5.7,;-6.85,3.54,;-8.39,3.54,)| Show InChI InChI=1S/C45H53Cl2N7O7S/c1-45(2)17-16-31(38(25-45)30-6-8-32(46)9-7-30)28-52-18-20-53(21-19-52)33-10-14-37(42(22-33)61-35-23-39(47)43(48)50-27-35)44(55)51-62(58,59)36-13-15-40(41(24-36)54(56)57)49-26-29-4-11-34(60-3)12-5-29/h6-10,13-15,22-24,27,29,34,49H,4-5,11-12,16-21,25-26,28H2,1-3H3,(H2,48,50)(H,51,55) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US8952157 (2015)
BindingDB Entry DOI: 10.7270/Q2QN65G9 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM144855
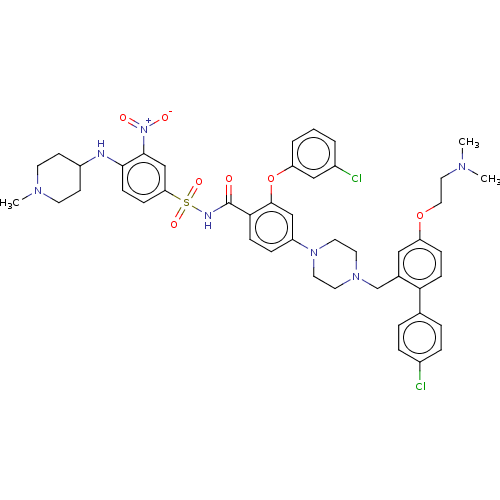
(US8952157, 37 | US9303025, 37)Show SMILES CN(C)CCOc1ccc(c(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NC4CCN(C)CC4)c(c3)[N+]([O-])=O)c(Oc3cccc(Cl)c3)c2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C46H51Cl2N7O7S/c1-51(2)25-26-61-38-12-15-41(32-7-9-34(47)10-8-32)33(27-38)31-53-21-23-54(24-22-53)37-11-14-42(45(29-37)62-39-6-4-5-35(48)28-39)46(56)50-63(59,60)40-13-16-43(44(30-40)55(57)58)49-36-17-19-52(3)20-18-36/h4-16,27-30,36,49H,17-26,31H2,1-3H3,(H,50,56) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US8952157 (2015)
BindingDB Entry DOI: 10.7270/Q2QN65G9 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM451099
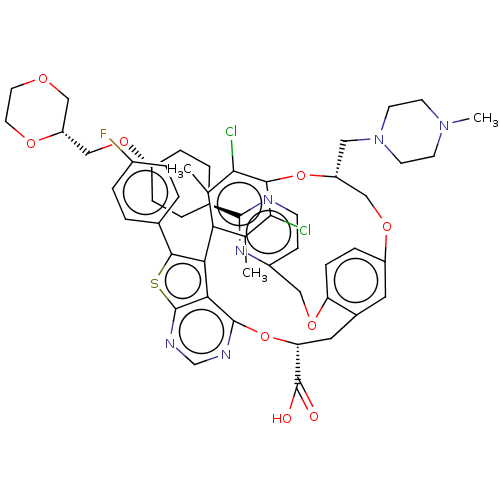
(US10676485, Example 9)Show SMILES CN1CCN(C[C@@H]2COc3ccc(OCc4ccnc(n4)[C@H]4CC[C@@H](CC4)OC[C@H]4COCCO4)c(C[C@@H](Oc4ncnc5sc(c(-c6c(C)c(Cl)c(O2)c(Cl)c6C)c45)-c2ccc(F)cc2)C(O)=O)c3)CC1 |r,wU:6.5,37.73,21.21,29.30,wD:24.28,(15.01,6.11,;13.52,5.71,;13.13,4.22,;11.64,3.82,;10.55,4.91,;9.06,4.51,;8.66,3.03,;7.18,2.63,;4.03,5.47,;3.26,4.13,;1.72,4.13,;.95,2.8,;1.72,1.47,;.95,.13,;-.59,.13,;-1.36,1.47,;-.59,2.8,;-1.36,4.13,;-2.9,4.13,;-3.67,2.8,;-2.9,1.47,;-5.21,2.8,;-5.98,1.47,;-7.52,1.47,;-8.29,2.8,;-7.52,4.13,;-5.98,4.13,;-9.83,2.8,;-10.6,1.47,;-12.14,1.47,;-12.91,.13,;-14.45,.13,;-15.22,1.47,;-14.45,2.8,;-12.91,2.8,;3.26,1.47,;4.03,.13,;4.03,-1.41,;5.36,-2.18,;5.36,-3.72,;4.03,-4.49,;4.03,-6.03,;5.36,-6.8,;6.69,-6.03,;8.16,-6.5,;9.06,-5.26,;8.16,-4.01,;8.56,-2.53,;7.47,-1.44,;5.98,-1.04,;7.87,.05,;6.78,1.14,;9.35,.45,;9.75,1.94,;10.44,-.64,;11.93,-.24,;10.04,-2.13,;11.13,-3.22,;6.69,-4.49,;10.6,-5.26,;11.37,-6.59,;12.91,-6.59,;13.68,-5.26,;15.22,-5.26,;12.91,-3.92,;11.37,-3.92,;2.69,-2.18,;1.36,-1.41,;2.69,-3.72,;4.03,2.8,;10.95,6.4,;12.44,6.8,)| Show InChI InChI=1S/C53H57Cl2FN6O9S/c1-30-43-31(2)47(55)48(46(30)54)70-39(24-62-18-16-61(3)17-19-62)27-68-38-12-13-41(69-25-36-14-15-57-50(60-36)33-6-10-37(11-7-33)67-28-40-26-65-20-21-66-40)34(22-38)23-42(53(63)64)71-51-45-44(43)49(72-52(45)59-29-58-51)32-4-8-35(56)9-5-32/h4-5,8-9,12-15,22,29,33,37,39-40,42H,6-7,10-11,16-21,23-28H2,1-3H3,(H,63,64)/t33-,37-,39-,40-,42-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The ability of the exemplary MCL-1 inhibitors of Examples 1 through 151 to bind MCL-1 was demonstrated using the Time Resolved-Fluorescence Resonance... |
US Patent US10676485 (2020)
BindingDB Entry DOI: 10.7270/Q2PZ5CWK |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM145154

(US8952157, 345 | US9303025, 345)Show SMILES COC1CCC(CNc2ccc(cc2[N+]([O-])=O)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cnc(N)c(Cl)c2)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)CC1 |t:46,(-11.47,3.54,;-10.7,2.21,;-9.16,2.21,;-8.39,.88,;-6.85,.88,;-6.08,2.21,;-4.54,2.21,;-3.77,3.54,;-2.23,3.54,;-1.46,2.21,;.08,2.21,;.85,3.54,;.08,4.88,;-1.46,4.88,;-2.23,6.21,;-1.46,7.54,;-3.77,6.21,;2.39,3.54,;2.39,5.08,;3.93,3.54,;2.39,2,;3.72,1.23,;5.06,2,;3.72,-.31,;5.06,-1.08,;5.06,-2.62,;3.72,-3.39,;2.39,-2.62,;2.39,-1.08,;1.06,-.31,;-.28,-1.08,;-1.61,-.31,;-2.94,-1.08,;-2.94,-2.62,;-4.28,-3.39,;-1.61,-3.39,;-1.61,-4.93,;-.28,-2.62,;3.72,-4.93,;5.06,-5.7,;5.06,-7.24,;3.72,-8.01,;3.72,-9.55,;5.06,-10.32,;5.06,-11.86,;6.39,-12.63,;7.73,-11.86,;8.54,-13.16,;9.26,-11.91,;7.73,-10.32,;6.39,-9.55,;3.72,-12.63,;3.72,-14.17,;2.39,-14.94,;1.06,-14.17,;-.28,-14.94,;1.06,-12.63,;2.39,-11.86,;2.39,-7.24,;2.39,-5.7,;-6.85,3.54,;-8.39,3.54,)| Show InChI InChI=1S/C45H53Cl2N7O7S/c1-45(2)17-16-31(38(25-45)30-6-8-32(46)9-7-30)28-52-18-20-53(21-19-52)33-10-14-37(42(22-33)61-35-23-39(47)43(48)50-27-35)44(55)51-62(58,59)36-13-15-40(41(24-36)54(56)57)49-26-29-4-11-34(60-3)12-5-29/h6-10,13-15,22-24,27,29,34,49H,4-5,11-12,16-21,25-26,28H2,1-3H3,(H2,48,50)(H,51,55) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... |
US Patent US9303025 (2016)
BindingDB Entry DOI: 10.7270/Q2XD10JP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM144855

(US8952157, 37 | US9303025, 37)Show SMILES CN(C)CCOc1ccc(c(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NC4CCN(C)CC4)c(c3)[N+]([O-])=O)c(Oc3cccc(Cl)c3)c2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C46H51Cl2N7O7S/c1-51(2)25-26-61-38-12-15-41(32-7-9-34(47)10-8-32)33(27-38)31-53-21-23-54(24-22-53)37-11-14-42(45(29-37)62-39-6-4-5-35(48)28-39)46(56)50-63(59,60)40-13-16-43(44(30-40)55(57)58)49-36-17-19-52(3)20-18-36/h4-16,27-30,36,49H,17-26,31H2,1-3H3,(H,50,56) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... |
US Patent US9303025 (2016)
BindingDB Entry DOI: 10.7270/Q2XD10JP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178639
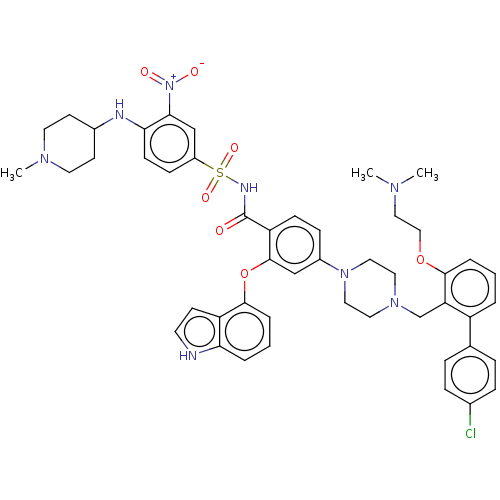
(US9125913, 205)Show SMILES CN(C)CCOc1cccc(c1CN1CCN(CC1)c1ccc(C(=O)NS(=O)(=O)c2ccc(NC3CCN(C)CC3)c(c2)[N+]([O-])=O)c(Oc2cccc3[nH]ccc23)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C48H53ClN8O7S/c1-53(2)28-29-63-45-8-4-6-38(33-10-12-34(49)13-11-33)41(45)32-55-24-26-56(27-25-55)36-14-16-40(47(30-36)64-46-9-5-7-42-39(46)18-21-50-42)48(58)52-65(61,62)37-15-17-43(44(31-37)57(59)60)51-35-19-22-54(3)23-20-35/h4-18,21,30-31,35,50-51H,19-20,22-29,32H2,1-3H3,(H,52,58) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM451104
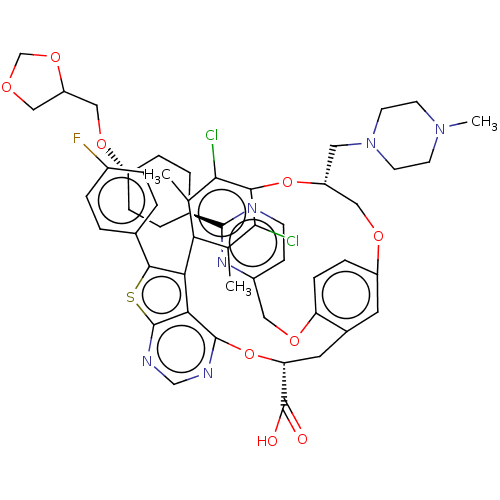
(US10676485, Example 14)Show SMILES CN1CCN(C[C@@H]2COc3ccc(OCc4ccnc(n4)[C@H]4CC[C@@H](CC4)OCC4COCO4)c(C[C@@H](Oc4ncnc5sc(c(-c6c(C)c(Cl)c(O2)c(Cl)c6C)c45)-c2ccc(F)cc2)C(O)=O)c3)CC1 |r,wU:36.72,6.5,21.21,wD:24.28,(14.66,6.11,;13.17,5.71,;12.77,4.22,;11.28,3.82,;10.19,4.91,;8.71,4.51,;8.31,3.03,;6.82,2.63,;3.67,5.47,;2.9,4.13,;1.36,4.13,;.59,2.8,;1.36,1.47,;.59,.13,;-.95,.13,;-1.72,1.47,;-.95,2.8,;-1.72,4.13,;-3.26,4.13,;-4.03,2.8,;-3.26,1.47,;-5.57,2.8,;-6.34,1.47,;-7.88,1.47,;-8.65,2.8,;-7.88,4.13,;-6.34,4.13,;-10.19,2.8,;-10.96,1.47,;-12.5,1.47,;-13.4,.22,;-14.87,.7,;-14.87,2.24,;-13.4,2.71,;2.9,1.47,;3.67,.13,;3.67,-1.41,;5.01,-2.18,;5.01,-3.72,;3.67,-4.49,;3.67,-6.03,;5.01,-6.8,;6.34,-6.03,;7.8,-6.5,;8.71,-5.26,;7.8,-4.01,;8.2,-2.53,;7.11,-1.44,;5.63,-1.04,;7.51,.05,;6.42,1.14,;9,.45,;9.4,1.94,;10.09,-.64,;11.58,-.24,;9.69,-2.13,;10.78,-3.22,;6.34,-4.49,;10.25,-5.26,;11.02,-6.59,;12.56,-6.59,;13.33,-5.26,;14.87,-5.26,;12.56,-3.92,;11.02,-3.92,;2.34,-2.18,;1,-1.41,;2.34,-3.72,;3.67,2.8,;10.59,6.4,;12.08,6.8,)| Show InChI InChI=1S/C52H55Cl2FN6O9S/c1-29-42-30(2)46(54)47(45(29)53)69-38(22-61-18-16-60(3)17-19-61)25-66-37-12-13-40(67-23-35-14-15-56-49(59-35)32-6-10-36(11-7-32)65-26-39-24-64-28-68-39)33(20-37)21-41(52(62)63)70-50-44-43(42)48(71-51(44)58-27-57-50)31-4-8-34(55)9-5-31/h4-5,8-9,12-15,20,27,32,36,38-39,41H,6-7,10-11,16-19,21-26,28H2,1-3H3,(H,62,63)/t32-,36-,38-,39?,41-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The ability of the exemplary MCL-1 inhibitors of Examples 1 through 151 to bind MCL-1 was demonstrated using the Time Resolved-Fluorescence Resonance... |
US Patent US10676485 (2020)
BindingDB Entry DOI: 10.7270/Q2PZ5CWK |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178793
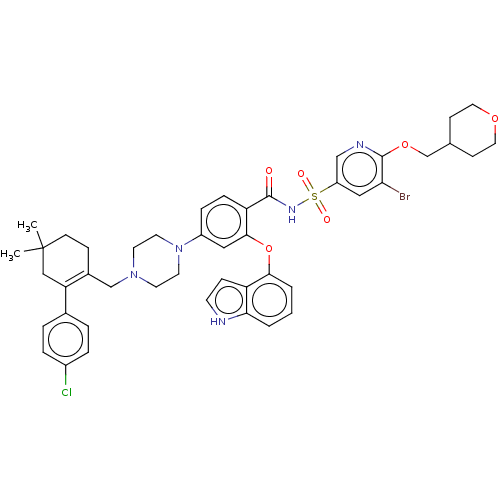
(US9125913, 364)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3cnc(OCC4CCOCC4)c(Br)c3)c(Oc3cccc4[nH]ccc34)c2)=C(C1)c1ccc(Cl)cc1 |c:55| Show InChI InChI=1S/C45H49BrClN5O6S/c1-45(2)16-12-32(38(26-45)31-6-8-33(47)9-7-31)28-51-18-20-52(21-19-51)34-10-11-37(42(24-34)58-41-5-3-4-40-36(41)13-17-48-40)43(53)50-59(54,55)35-25-39(46)44(49-27-35)57-29-30-14-22-56-23-15-30/h3-11,13,17,24-25,27,30,48H,12,14-16,18-23,26,28-29H2,1-2H3,(H,50,53) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178854
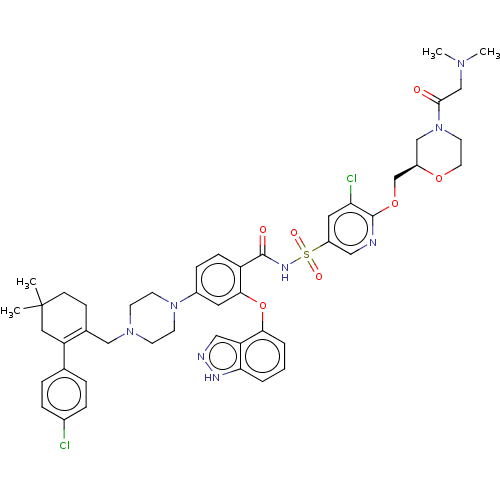
(US9125913, 426)Show SMILES CN(C)CC(=O)N1CCO[C@@H](COc2ncc(cc2Cl)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cccc3[nH]ncc23)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)C1 |t:51| Show InChI InChI=1S/C47H54Cl2N8O7S/c1-47(2)15-14-32(38(24-47)31-8-10-33(48)11-9-31)27-55-16-18-56(19-17-55)34-12-13-37(43(22-34)64-42-7-5-6-41-39(42)26-51-52-41)45(59)53-65(60,61)36-23-40(49)46(50-25-36)63-30-35-28-57(20-21-62-35)44(58)29-54(3)4/h5-13,22-23,25-26,35H,14-21,24,27-30H2,1-4H3,(H,51,52)(H,53,59)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178891
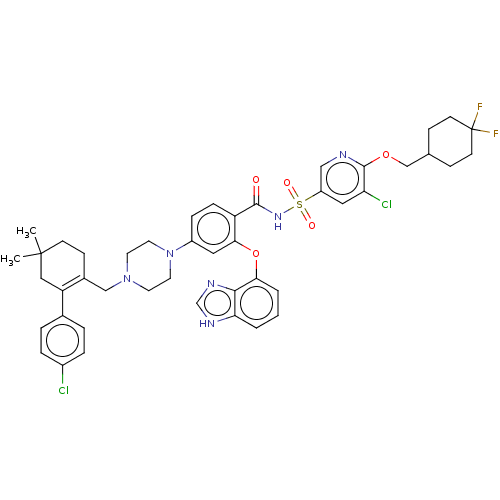
(US9125913, 463)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3cnc(OCC4CCC(F)(F)CC4)c(Cl)c3)c(Oc3cccc4[nH]cnc34)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H48Cl2F2N6O5S/c1-44(2)15-14-31(36(24-44)30-6-8-32(46)9-7-30)26-54-18-20-55(21-19-54)33-10-11-35(40(22-33)60-39-5-3-4-38-41(39)52-28-51-38)42(56)53-61(57,58)34-23-37(47)43(50-25-34)59-27-29-12-16-45(48,49)17-13-29/h3-11,22-23,25,28-29H,12-21,24,26-27H2,1-2H3,(H,51,52)(H,53,56) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178842
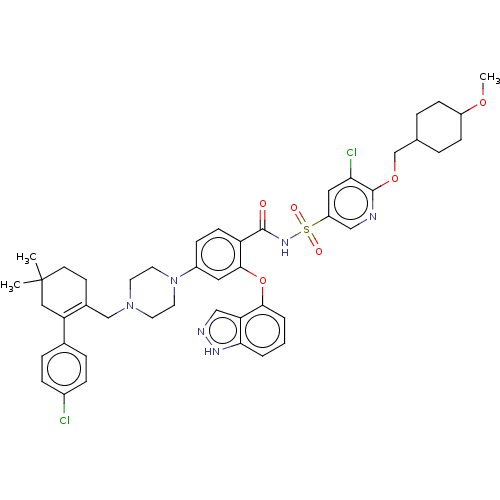
(US9125913, 414)Show SMILES COC1CCC(COc2ncc(cc2Cl)S(=O)(=O)NC(=O)c2ccc(cc2Oc2cccc3[nH]ncc23)N2CCN(CC3=C(CC(C)(C)CC3)c3ccc(Cl)cc3)CC2)CC1 |t:46,(17.62,-8.45,;16.29,-9.22,;14.96,-8.45,;14.96,-6.91,;13.62,-6.14,;12.29,-6.91,;10.96,-6.14,;9.62,-6.91,;8.29,-6.14,;6.95,-6.91,;5.62,-6.14,;5.62,-4.6,;6.95,-3.83,;8.29,-4.6,;9.62,-3.83,;4.29,-3.83,;5.06,-2.5,;3.52,-5.17,;2.95,-3.06,;1.62,-3.83,;1.62,-5.37,;.29,-3.06,;-1.05,-3.83,;-2.38,-3.06,;-2.38,-1.52,;-1.05,-.75,;.29,-1.52,;1.62,-.75,;1.62,.79,;2.95,1.56,;2.95,3.1,;1.62,3.87,;.29,3.1,;-1.18,3.57,;-2.08,2.33,;-1.18,1.08,;.29,1.56,;-3.71,-.75,;-5.05,-1.52,;-6.38,-.75,;-6.38,.79,;-7.72,1.56,;-7.72,3.1,;-9.05,3.87,;-9.05,5.41,;-7.72,6.18,;-8.44,7.54,;-6.99,7.54,;-6.38,5.41,;-6.38,3.87,;-10.38,3.1,;-10.38,1.56,;-11.72,.79,;-13.05,1.56,;-14.38,.79,;-13.05,3.1,;-11.72,3.87,;-5.05,1.56,;-3.71,.79,;12.29,-8.45,;13.62,-9.22,)| Show InChI InChI=1S/C46H52Cl2N6O6S/c1-46(2)18-17-32(38(25-46)31-9-11-33(47)12-10-31)28-53-19-21-54(22-20-53)34-13-16-37(43(23-34)60-42-6-4-5-41-39(42)27-50-51-41)44(55)52-61(56,57)36-24-40(48)45(49-26-36)59-29-30-7-14-35(58-3)15-8-30/h4-6,9-13,16,23-24,26-27,30,35H,7-8,14-15,17-22,25,28-29H2,1-3H3,(H,50,51)(H,52,55) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178576

(US9125913, 135)Show SMILES CN(C)CCOc1ccc(c(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCCCN4CCCC4)c(c3)[N+]([O-])=O)c(Oc3ccc4[nH]ccc4c3)c2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C49H55ClN8O7S/c1-54(2)28-29-64-40-11-15-43(35-6-8-38(50)9-7-35)37(31-40)34-56-24-26-57(27-25-56)39-10-14-44(48(32-39)65-41-12-16-45-36(30-41)18-20-52-45)49(59)53-66(62,63)42-13-17-46(47(33-42)58(60)61)51-19-5-23-55-21-3-4-22-55/h6-18,20,30-33,51-52H,3-5,19,21-29,34H2,1-2H3,(H,53,59) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM451117
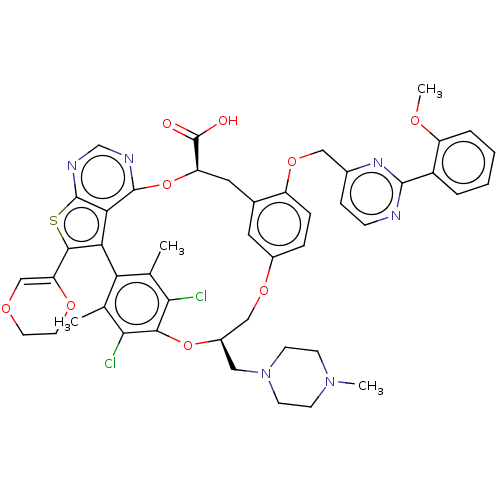
(US10676485, Example 27)Show SMILES COc1ccccc1-c1nccc(COc2ccc3OC[C@@H](CN4CCN(C)CC4)Oc4c(Cl)c(C)c(-c5c(sc6ncnc(O[C@H](Cc2c3)C(O)=O)c56)C2=COCCO2)c(C)c4Cl)n1 |r,t:60| Show InChI InChI=1S/C47H46Cl2N6O9S/c1-26-37-27(2)41(49)42(40(26)48)63-31(21-55-15-13-54(3)14-16-55)23-61-30-9-10-33(62-22-29-11-12-50-44(53-29)32-7-5-6-8-34(32)58-4)28(19-30)20-35(47(56)57)64-45-39-38(37)43(36-24-59-17-18-60-36)65-46(39)52-25-51-45/h5-12,19,24-25,31,35H,13-18,20-23H2,1-4H3,(H,56,57)/t31-,35-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The ability of the exemplary MCL-1 inhibitors of Examples 1 through 151 to bind MCL-1 was demonstrated using the Time Resolved-Fluorescence Resonance... |
US Patent US10676485 (2020)
BindingDB Entry DOI: 10.7270/Q2PZ5CWK |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM209097
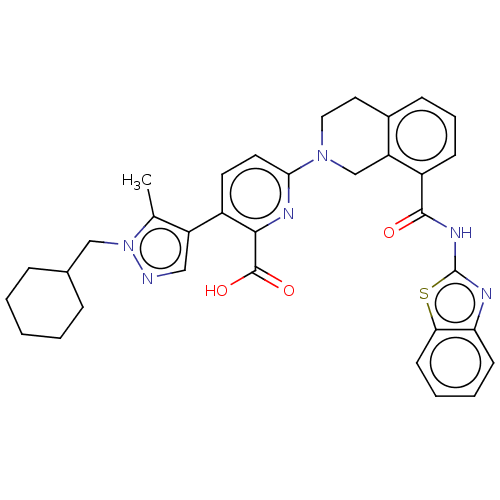
(US9266877, 43)Show SMILES Cc1c(cnn1CC1CCCCC1)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C34H34N6O3S/c1-21-26(18-35-40(21)19-22-8-3-2-4-9-22)24-14-15-30(37-31(24)33(42)43)39-17-16-23-10-7-11-25(27(23)20-39)32(41)38-34-36-28-12-5-6-13-29(28)44-34/h5-7,10-15,18,22H,2-4,8-9,16-17,19-20H2,1H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM451131
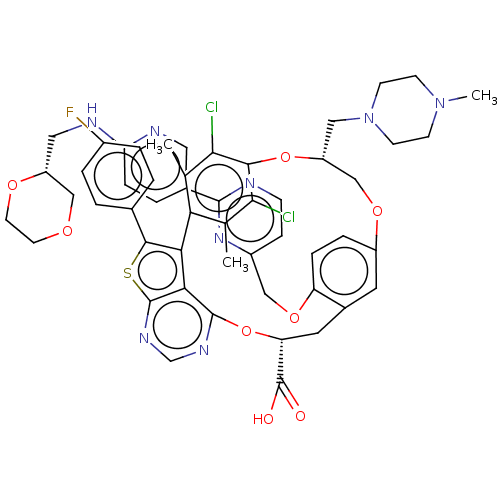
(US10676485, Example 41)Show SMILES CN1CCN(C[C@@H]2COc3ccc(OCc4ccnc(n4)-c4ccc(NC[C@@H]5COCCO5)nc4)c(C[C@@H](Oc4ncnc5sc(c(-c6c(C)c(Cl)c(O2)c(Cl)c6C)c45)-c2ccc(F)cc2)C(O)=O)c3)CC1 |r| Show InChI InChI=1S/C52H51Cl2FN8O8S/c1-29-42-30(2)46(54)47(45(29)53)70-38(24-63-16-14-62(3)15-17-63)27-68-36-9-10-39(69-25-35-12-13-56-49(61-35)32-6-11-41(57-22-32)58-23-37-26-66-18-19-67-37)33(20-36)21-40(52(64)65)71-50-44-43(42)48(72-51(44)60-28-59-50)31-4-7-34(55)8-5-31/h4-13,20,22,28,37-38,40H,14-19,21,23-27H2,1-3H3,(H,57,58)(H,64,65)/t37-,38-,40-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The ability of the exemplary MCL-1 inhibitors of Examples 1 through 151 to bind MCL-1 was demonstrated using the Time Resolved-Fluorescence Resonance... |
US Patent US10676485 (2020)
BindingDB Entry DOI: 10.7270/Q2PZ5CWK |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM145152
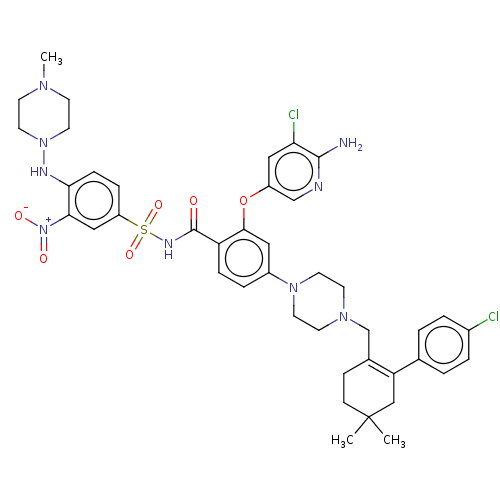
(US8952157, 343 | US9303025, 343)Show SMILES CN1CCN(CC1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1Oc1cnc(N)c(Cl)c1)N1CCN(CC2=C(CC(C)(C)CC2)c2ccc(Cl)cc2)CC1 |t:47| Show InChI InChI=1S/C42H49Cl2N9O6S/c1-42(2)13-12-29(35(25-42)28-4-6-30(43)7-5-28)27-50-16-18-51(19-17-50)31-8-10-34(39(22-31)59-32-23-36(44)40(45)46-26-32)41(54)48-60(57,58)33-9-11-37(38(24-33)53(55)56)47-52-20-14-49(3)15-21-52/h4-11,22-24,26,47H,12-21,25,27H2,1-3H3,(H2,45,46)(H,48,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00900 | -63.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... |
US Patent US9303025 (2016)
BindingDB Entry DOI: 10.7270/Q2XD10JP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178579
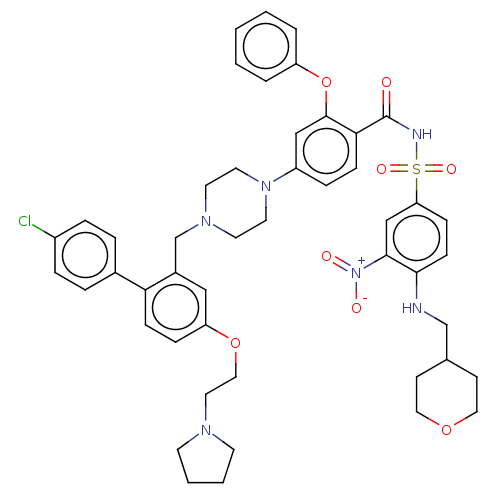
(US9125913, 138)Show SMILES [O-][N+](=O)c1cc(ccc1NCC1CCOCC1)S(=O)(=O)NC(=O)c1ccc(cc1Oc1ccccc1)N1CCN(Cc2cc(OCCN3CCCC3)ccc2-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C48H53ClN6O8S/c49-38-10-8-36(9-11-38)43-16-13-41(62-29-26-52-20-4-5-21-52)30-37(43)34-53-22-24-54(25-23-53)39-12-15-44(47(31-39)63-40-6-2-1-3-7-40)48(56)51-64(59,60)42-14-17-45(46(32-42)55(57)58)50-33-35-18-27-61-28-19-35/h1-3,6-17,30-32,35,50H,4-5,18-29,33-34H2,(H,51,56) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Mus musculus (Mouse)) | BDBM178574
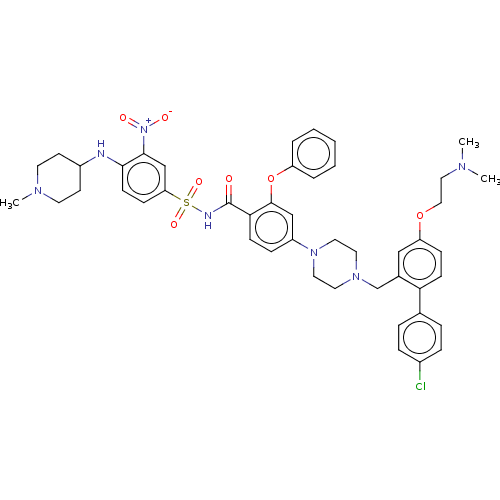
(US9125913, 133)Show SMILES CN(C)CCOc1ccc(c(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NC4CCN(C)CC4)c(c3)[N+]([O-])=O)c(Oc3ccccc3)c2)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C46H52ClN7O7S/c1-50(2)27-28-60-39-14-17-41(33-9-11-35(47)12-10-33)34(29-39)32-52-23-25-53(26-24-52)37-13-16-42(45(30-37)61-38-7-5-4-6-8-38)46(55)49-62(58,59)40-15-18-43(44(31-40)54(56)57)48-36-19-21-51(3)22-20-36/h4-18,29-31,36,48H,19-28,32H2,1-3H3,(H,49,55) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... |
US Patent US9125913 (2015)
BindingDB Entry DOI: 10.7270/Q29Z93PP |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM145152

(US8952157, 343 | US9303025, 343)Show SMILES CN1CCN(CC1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1Oc1cnc(N)c(Cl)c1)N1CCN(CC2=C(CC(C)(C)CC2)c2ccc(Cl)cc2)CC1 |t:47| Show InChI InChI=1S/C42H49Cl2N9O6S/c1-42(2)13-12-29(35(25-42)28-4-6-30(43)7-5-28)27-50-16-18-51(19-17-50)31-8-10-34(39(22-31)59-32-23-36(44)40(45)46-26-32)41(54)48-60(57,58)33-9-11-37(38(24-33)53(55)56)47-52-20-14-49(3)15-21-52/h4-11,22-24,26,47H,12-21,25,27H2,1-3H3,(H2,45,46)(H,48,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00900 | -63.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research
US Patent
| Assay Description
Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... |
US Patent US8952157 (2015)
BindingDB Entry DOI: 10.7270/Q2QN65G9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































