

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030575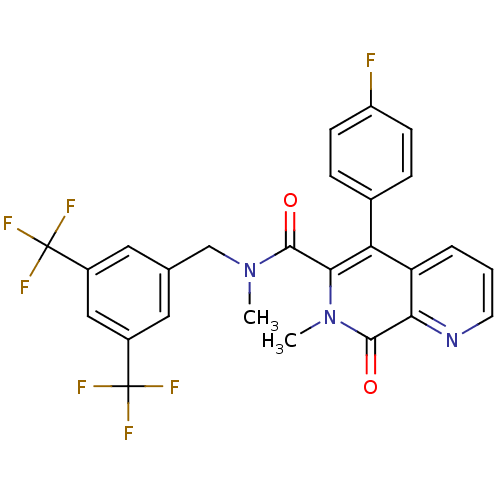 (5-(4-Fluoro-phenyl)-7-methyl-8-oxo-7,8-dihydro-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity at Tachykinin receptor 1 by measuring its ability to inhibit [125I]BH-SP binding in human IM-9 cells (Lymphoblast cells). | J Med Chem 41: 4232-9 (1998) Article DOI: 10.1021/jm980042m BindingDB Entry DOI: 10.7270/Q280538T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030573 (7-Methyl-8-oxo-5-phenyl-7,8-dihydro-[1,7]naphthyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50081415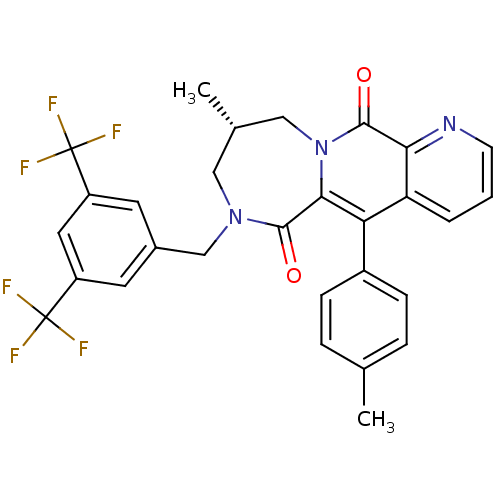 ((S)-7-(3,5-Bis-trifluoromethyl-benzyl)-9-methyl-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50081413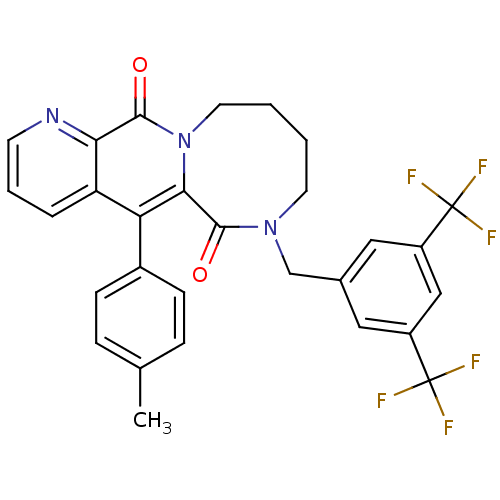 ((R)-7-(3,5-Bis-trifluoromethyl-benzyl)-5-p-tolyl-8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030576 (4-(4-Fluoro-phenyl)-2-methyl-1-oxo-1,2-dihydro-iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067516 (7-METHYL-8-OXO-5-P-TOLYL-7,8-DIHYDRO-[1,7]NAPHTHYR...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity at Tachykinin receptor 1 by measuring its ability to inhibit [125I]BH-SP binding in human IM-9 cells (Lymphoblast cells). | J Med Chem 41: 4232-9 (1998) Article DOI: 10.1021/jm980042m BindingDB Entry DOI: 10.7270/Q280538T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067516 (7-METHYL-8-OXO-5-P-TOLYL-7,8-DIHYDRO-[1,7]NAPHTHYR...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity at Tachykinin receptor 1 by measuring its ability to inhibit [125I]BH-SP binding in human IM-9 cells (Lymphoblast cells). | J Med Chem 41: 4232-9 (1998) Article DOI: 10.1021/jm980042m BindingDB Entry DOI: 10.7270/Q280538T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50081414 (7-(3,5-Bis-trifluoromethyl-benzyl)-5-p-tolyl-7,8,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030584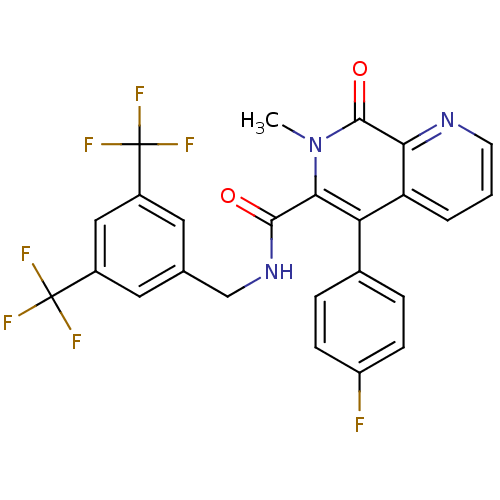 (5-(4-Fluoro-phenyl)-7-methyl-8-oxo-7,8-dihydro-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030581 (2-Methyl-1-oxo-4-phenyl-1,2-dihydro-isoquinoline-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030572 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity at Tachykinin receptor 1 by measuring its ability to inhibit [125I]BH-SP binding in human IM-9 cells (Lymphoblast cells). | J Med Chem 41: 4232-9 (1998) Article DOI: 10.1021/jm980042m BindingDB Entry DOI: 10.7270/Q280538T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50081412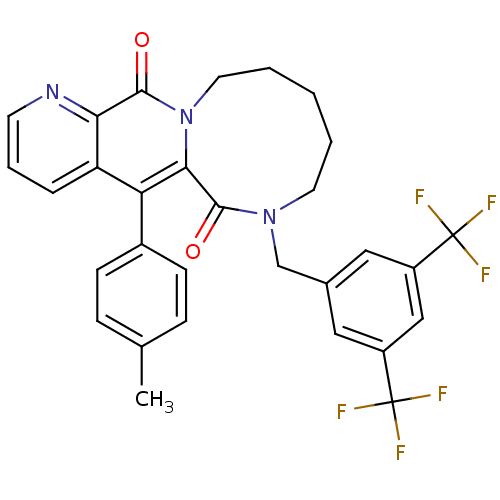 (7-(3,5-Bis-trifluoromethyl-benzyl)-5-p-tolyl-7,8,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50081417 (6-(3,5-Bis-trifluoromethyl-benzyl)-10-p-tolyl-7,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030611 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030607 (5-(4-Fluoro-phenyl)-7-methyl-8-oxo-7,8-dihydro-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030574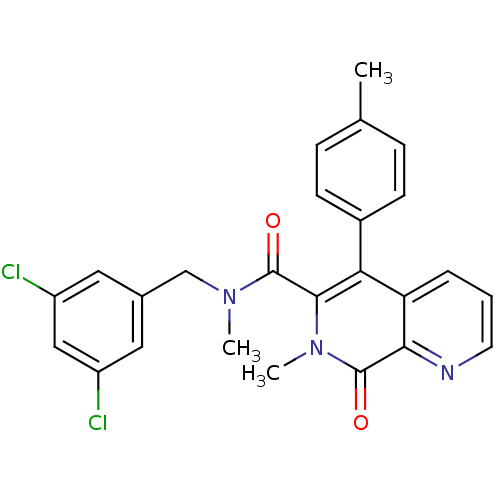 (7-Methyl-8-oxo-5-p-tolyl-7,8-dihydro-[1,7]naphthyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030591 (6-Chloro-2-methyl-1-oxo-4-phenyl-1,2-dihydro-isoqu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030569 (1-(2-Chloro-benzyl)-1-methyl-3-(2,6,7-trimethyl-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030589 (N-(3,5-bis(trifluoromethyl)benzyl)-N,7-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030582 (7-Methyl-8-oxo-5-p-tolyl-7,8-dihydro-[1,7]naphthyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030585 (5-(4-Fluoro-phenyl)-7-methyl-8-oxo-7,8-dihydro-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030594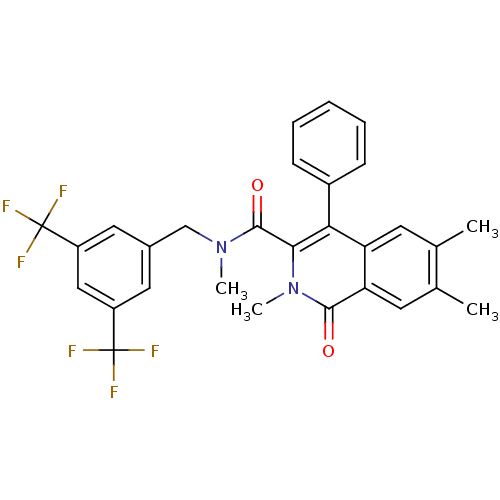 (2,6,7-Trimethyl-1-oxo-4-phenyl-1,2-dihydro-isoquin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50081419 ((R)-7-(3,5-Bis-trifluoromethyl-benzyl)-9-methyl-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50311792 (4'-butoxy-3'-((2-fluoro-4-(trifluoromethyl)benzami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at PPARgamma cotransfected with GAL4-PPAR fusion protein assessed as inhibition of TIPP703-induced response by luciferase reporte... | Bioorg Med Chem Lett 19: 6595-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.021 BindingDB Entry DOI: 10.7270/Q2736R23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50311792 (4'-butoxy-3'-((2-fluoro-4-(trifluoromethyl)benzami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at PPARdelta cotransfected with GAL4-PPAR fusion protein assessed as inhibition of GW501516-induced response by luciferase report... | Bioorg Med Chem Lett 19: 6595-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.021 BindingDB Entry DOI: 10.7270/Q2736R23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50311792 (4'-butoxy-3'-((2-fluoro-4-(trifluoromethyl)benzami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at PPARalpha cotransfected with GAL4-PPAR fusion protein assessed as inhibition of TIPP703-induced response by luciferase reporte... | Bioorg Med Chem Lett 19: 6595-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.021 BindingDB Entry DOI: 10.7270/Q2736R23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030604 (3-[(2-Methoxy-benzylamino)-methyl]-2,6,7-trimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030571 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030583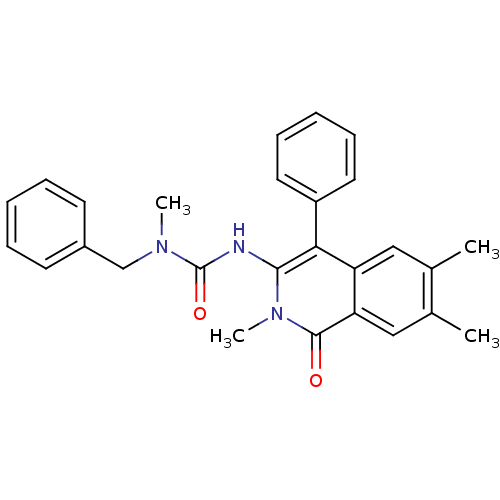 (1-Benzyl-1-methyl-3-(2,6,7-trimethyl-1-oxo-4-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030593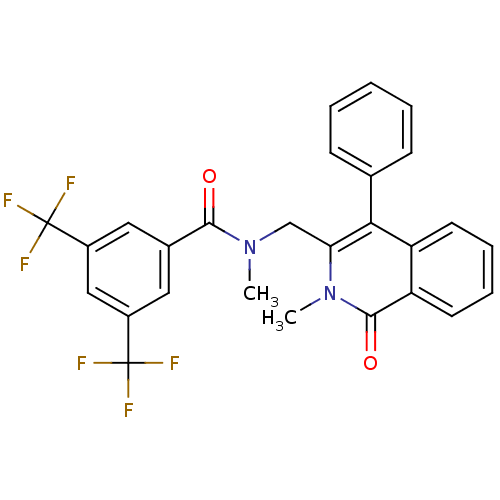 (CHEMBL106326 | N-Methyl-N-(2-methyl-1-oxo-4-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030579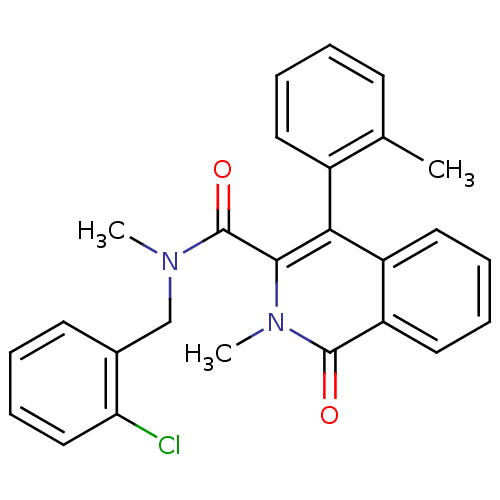 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50360307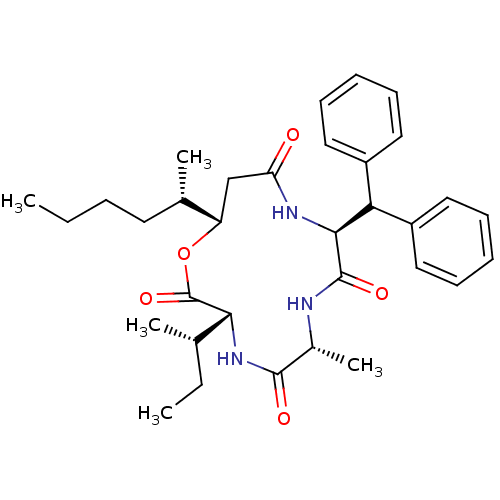 (CHEMBL449307) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibition of ACAT1- mediated cholesteryl ester synthesis in macrophages | Bioorg Med Chem Lett 22: 696-9 (2011) Article DOI: 10.1016/j.bmcl.2011.10.045 BindingDB Entry DOI: 10.7270/Q2ZC839D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50081420 ((7R,9R)-7-(3,5-Bis-trifluoromethyl-benzyl)-9-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tachykinin receptor 1 antagonistic activity as ability to inhibit [125I]-BH-SP binding in human IM-9 cells (lymphoblast cells) | J Med Chem 42: 3982-93 (1999) BindingDB Entry DOI: 10.7270/Q2SF2VC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome assembly chaperone 3 (Homo sapiens (Human)) | BDBM50520988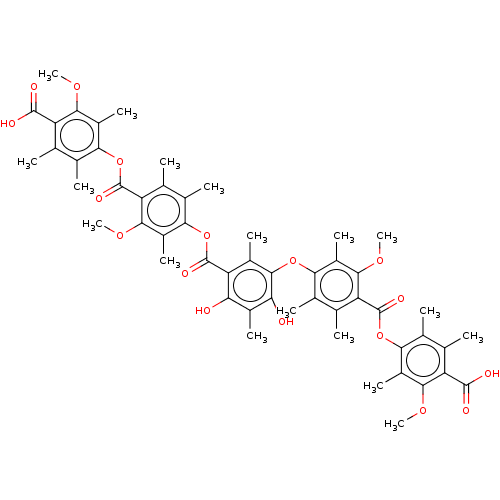 (CHEMBL4435319) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University Curated by ChEMBL | Assay Description Inhibition of PAC3 homodimer (unknown origin) protein-protein interaction | Bioorg Med Chem 26: 6023-6034 (2018) Article DOI: 10.1016/j.bmc.2018.11.001 BindingDB Entry DOI: 10.7270/Q24X5C69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030587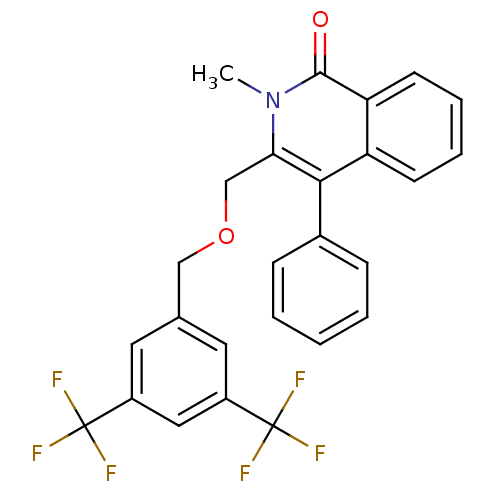 (3-(3,5-Bis-trifluoromethyl-benzyloxymethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030598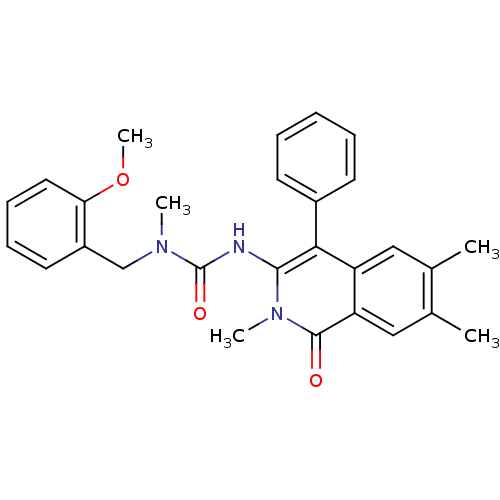 (1-(2-Methoxy-benzyl)-1-methyl-3-(2,6,7-trimethyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030608 (1-(3,5-Dimethyl-benzyl)-1-methyl-3-(2,6,7-trimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030588 (2-Methyl-1-oxo-4-phenyl-1,2-dihydro-isoquinoline-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030597 (5-(4-Fluoro-phenyl)-7-methyl-8-oxo-7,8-dihydro-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030606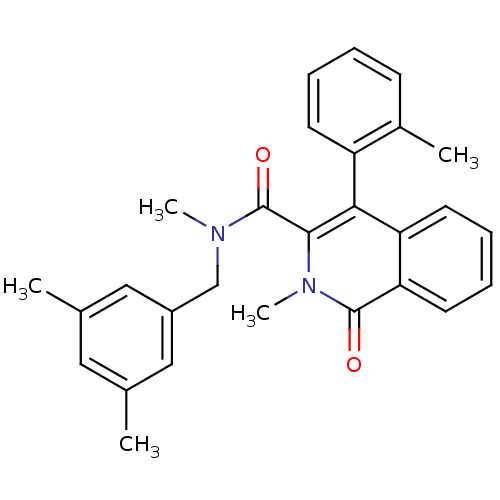 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030601 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030578 (2-Methyl-1-oxo-4-o-tolyl-1,2-dihydro-isoquinoline-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]-BH-Substance P binding to tachykinin receptor 1 in human IM-9 cells | J Med Chem 38: 3106-20 (1995) BindingDB Entry DOI: 10.7270/Q2ZS2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 163 total ) | Next | Last >> |