

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50167703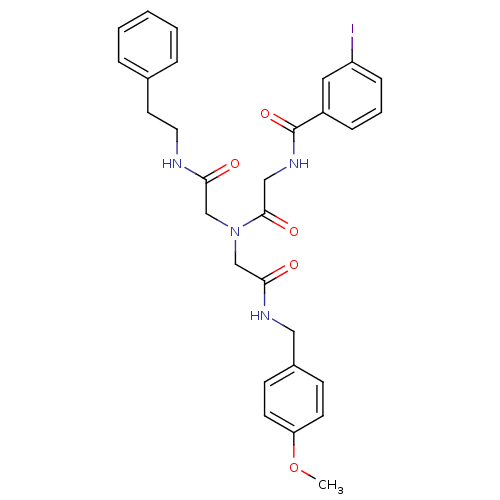 (3-Iodo-N-{[[(4-methoxy-benzylcarbamoyl)-methyl]-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition constant against AICAR formyltransferase | Bioorg Med Chem Lett 15: 2840-4 (2005) Article DOI: 10.1016/j.bmcl.2005.03.094 BindingDB Entry DOI: 10.7270/Q22V2FNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50167702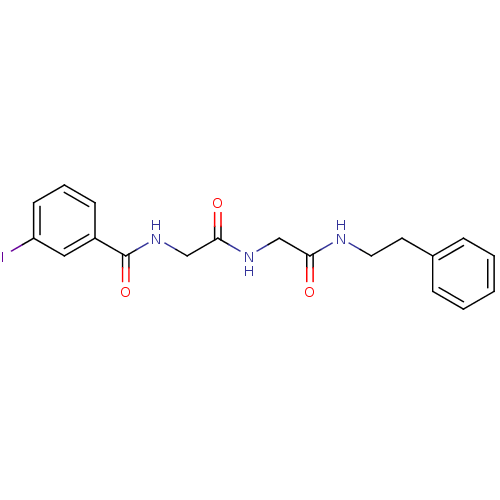 (3-Iodo-N-{[(phenethylcarbamoyl-methyl)-carbamoyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition constant against AICAR formyltransferase | Bioorg Med Chem Lett 15: 2840-4 (2005) Article DOI: 10.1016/j.bmcl.2005.03.094 BindingDB Entry DOI: 10.7270/Q22V2FNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317628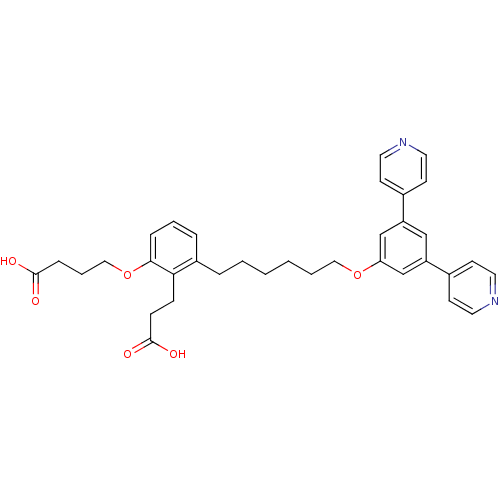 (4-{2-(2-Carboxy-ethyl)-3-[6-(3,5-di-pyridin-4-yl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317631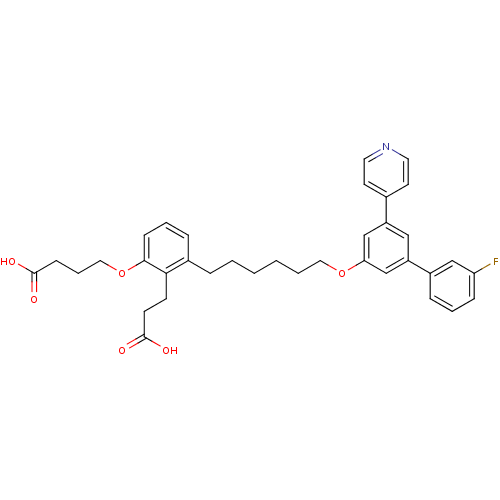 (4-{2-(2-Carboxy-ethyl)-3-[6-(3'-fluoro-5-pyridin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317632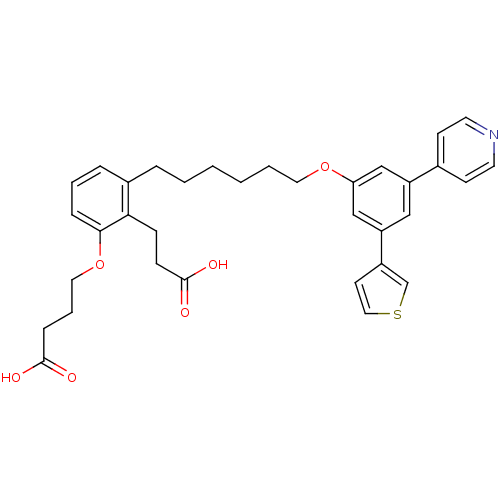 (4-{2-(2-Carboxy-ethyl)-3-[6-(3-pyridin-4-yl-5-thio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317625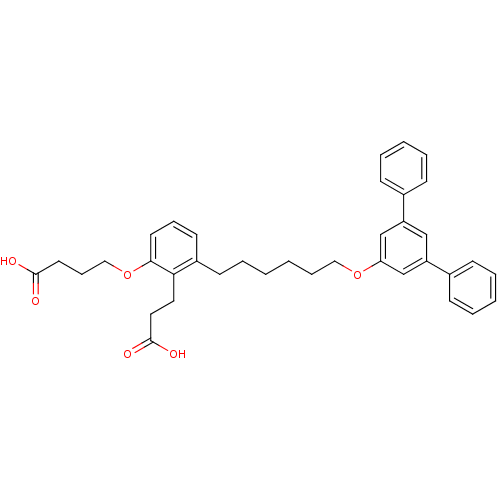 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317633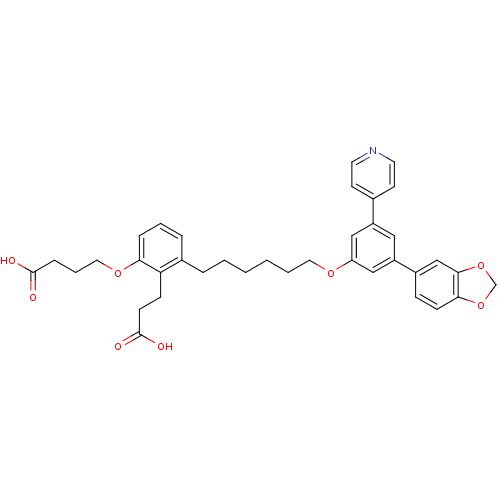 (4-{3-[6-(3-Benzo[1,3]dioxol-5-yl-5-pyridin-4-yl-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317634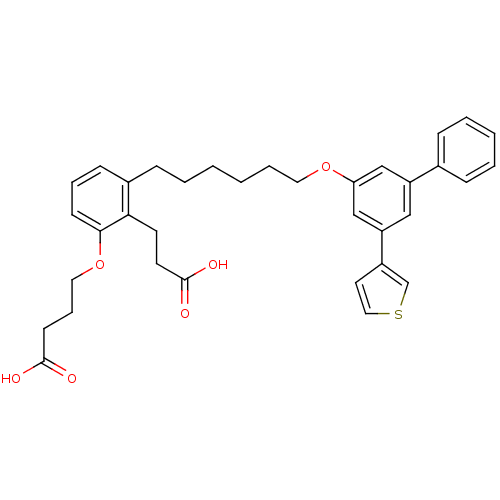 (4-{2-(2-Carboxyethyl)-3-[6-(5-thiophen-3-ylbipheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317635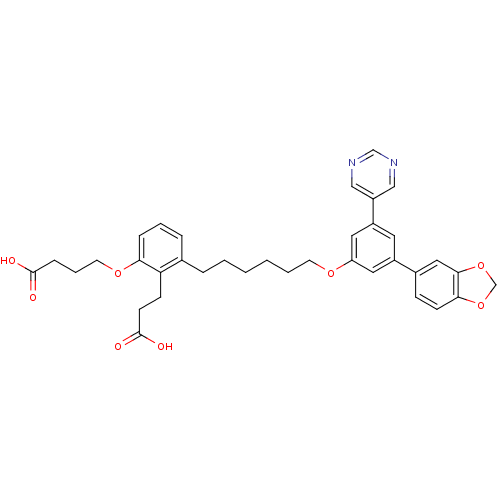 (4-{3-[6-(3-Benzo[1,3]dioxol-5-yl-5-pyrimidin-5-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317636 (4-{2-(2-Carboxy-ethyl)-3-[6-(3-pyrimidin-5-yl-5-th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317626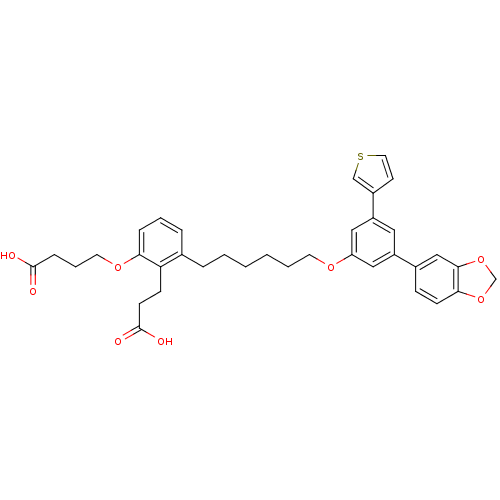 (4-{3-[6-(3-5-Benzo[1,3]dioxolyl-5-thiophen-3-ylphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263526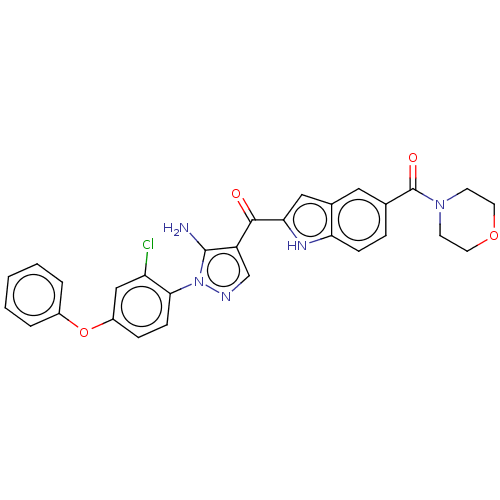 (US9556150, i-14 | [5-amino-1-(2-chloro-4- phenoxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317624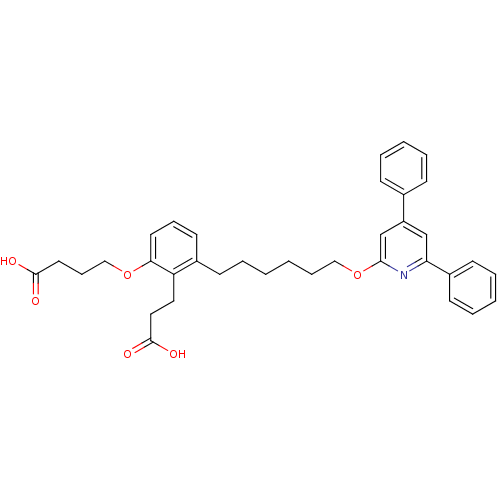 (4-(2-(2-carboxyethyl)-3-(6-(4,6-diphenylpyridin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM352195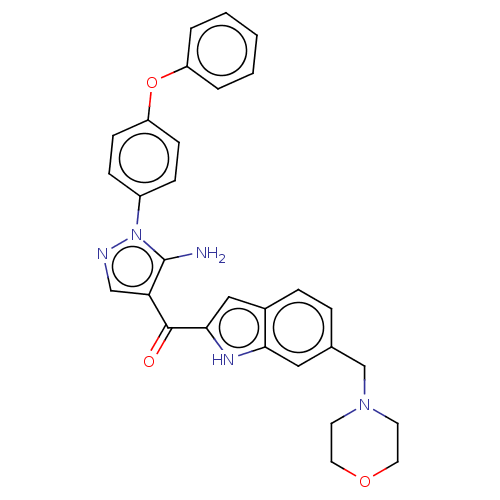 (US9802920, Compound I-19 | [5-Amino-1-(4-phenoxy- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc.; Chugai Pharamceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US9802920 (2017) BindingDB Entry DOI: 10.7270/Q29G5PXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317637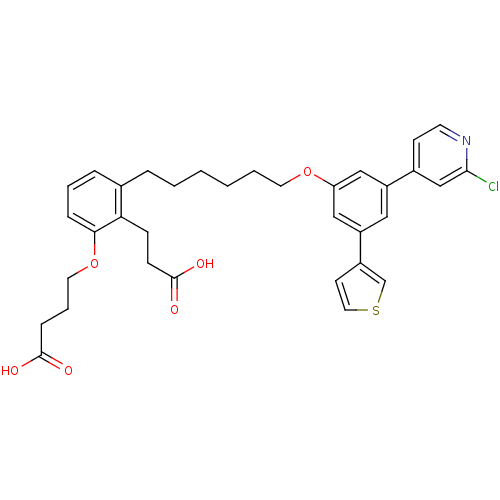 (4-(2-(2-Carboxy-ethyl)-3-{6-[3-(2-chloro-pyridin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317638 (4-{2-(2-Carboxy-ethyl)-3-[6-(3,5-di-thiophen-3-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317639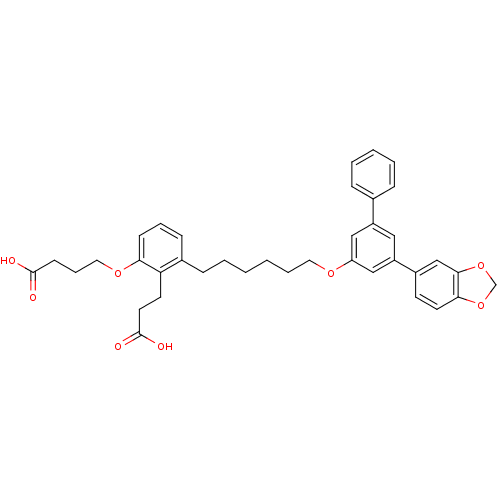 (4-[3-[6-(5-Benzo[1,3]dioxol-5-yl-biphenyl-3-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317630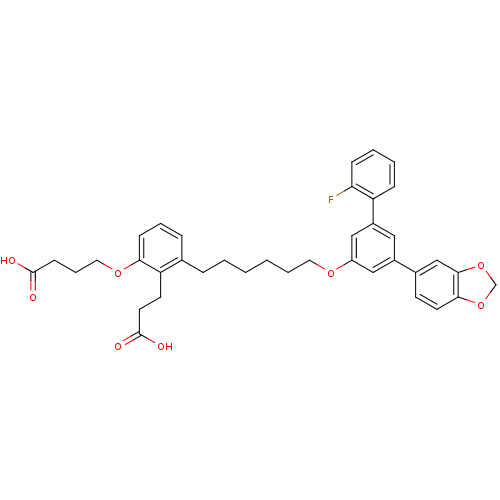 (4-(3-(6-(5-(benzo[d][1,3]dioxol-5-yl)-2'-fluorobip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263527 (US9556150, i-15 | {5-amino-1-[2-chloro-4-(2- fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM352196 (3-{4-[5-Amino-4-(6- morpholin-4-ylmethyl-1H- indol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc.; Chugai Pharamceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US9802920 (2017) BindingDB Entry DOI: 10.7270/Q29G5PXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263563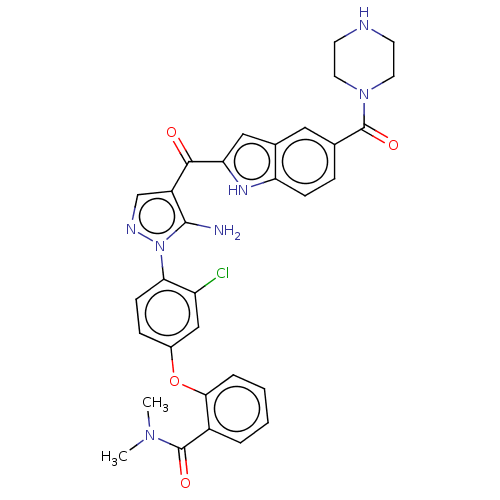 (2-(4-{5-amino-4-[5-(piperazine- 1-carbonyl)-1h-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317623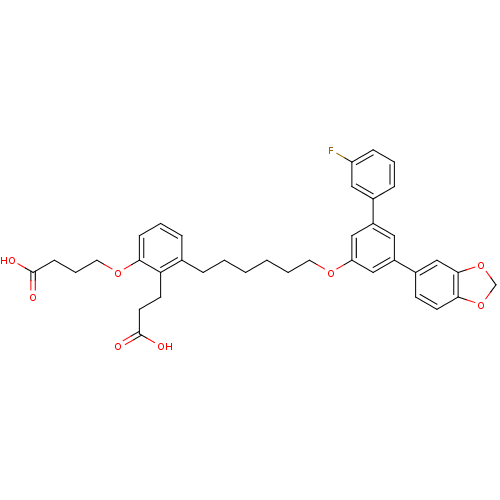 (4-(3-(6-(5-(benzo[d][1,3]dioxol-5-yl)-3'-fluorobip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263555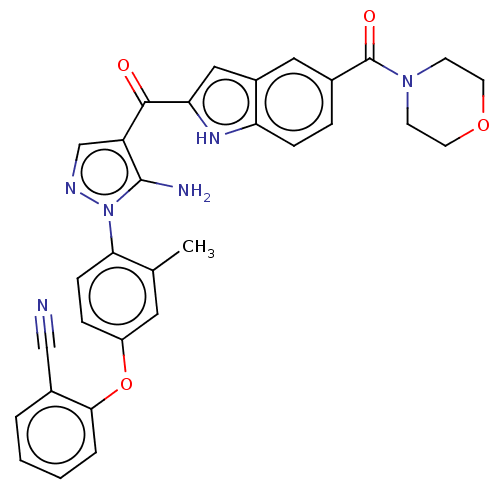 (2-(4-{5-amino-4-[5- (morpholine-4-carbonyl)-1h- in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263534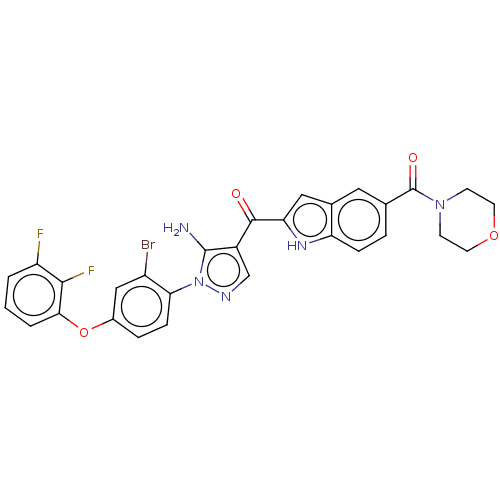 (US9556150, i-22 | {5-amino-1-[2-bromo-4-(2,3- difl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263561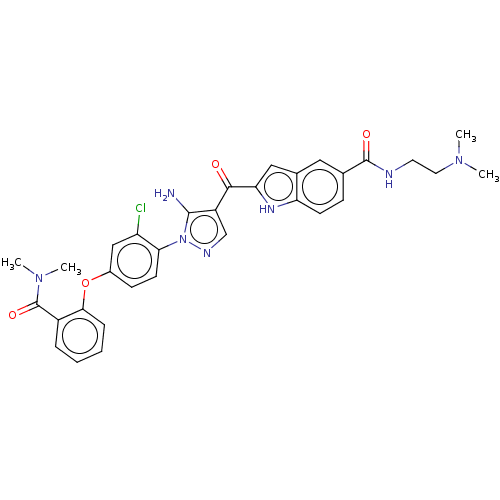 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263557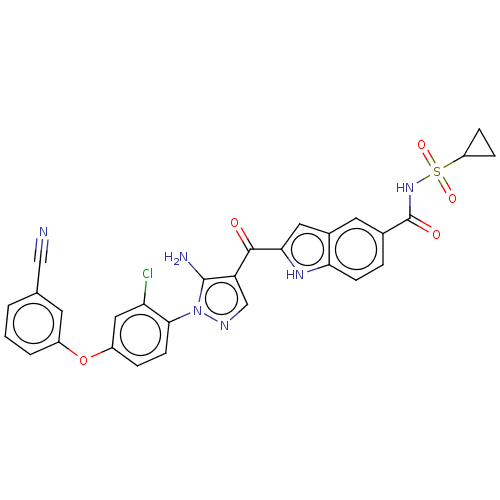 (US9556150, i-45 | cyclopropanesulfonic acid (2-{5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263565 (2-(4-{5-amino-4-[5-(3- dimethylamino-pyrrolidine-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263547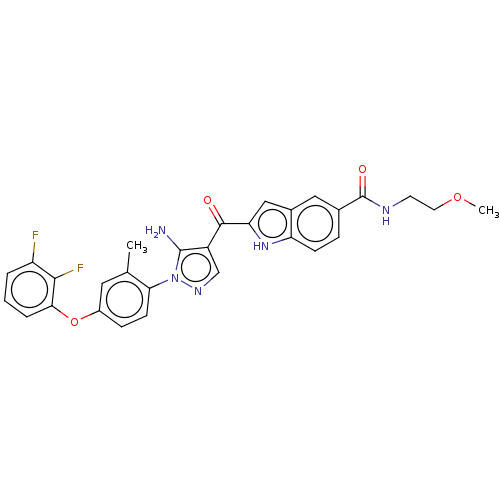 (2-{5-amino-1-[4-(2,3-difluoro- phenoxy)-2-methyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263570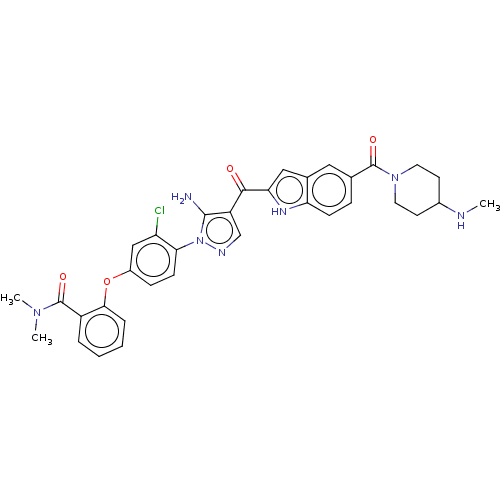 (2-(4-{5-amino-4-[5-(4- methylamino-piperidine-1- c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263517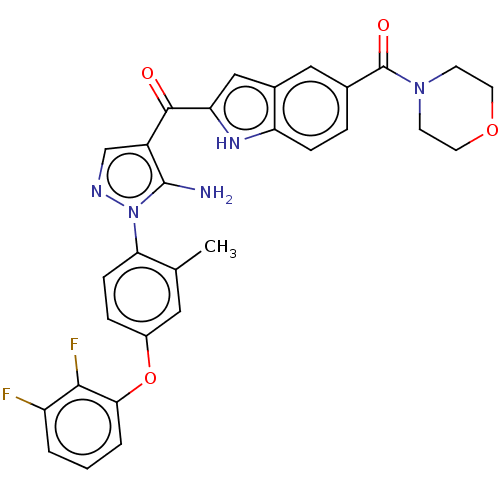 (US9556150, i-5 | {5-amino-1-[4-(2,3-difluoro- phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263562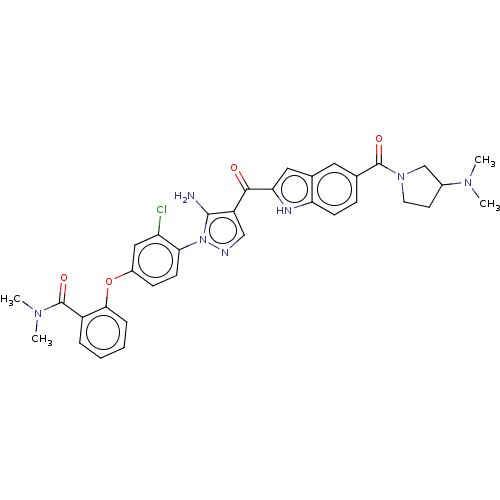 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263519 (US9556150, i-7 | {2-[5-amino-1-(2-methyl-4- phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263566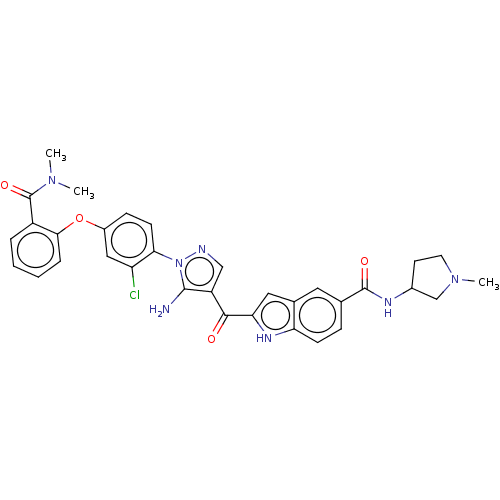 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM352190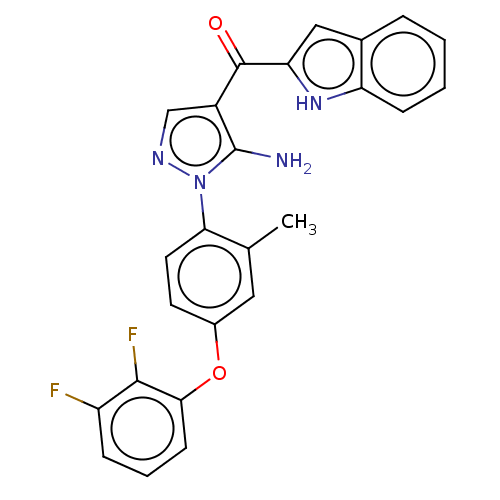 (US9802920, Compound I-14 | {5-Amino-1-[4-(2,3- dif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc.; Chugai Pharamceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US9802920 (2017) BindingDB Entry DOI: 10.7270/Q29G5PXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263568 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263560 (2-{5-amino-1-[2-chloro-4-(3- cyano-2-fluoro-phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317641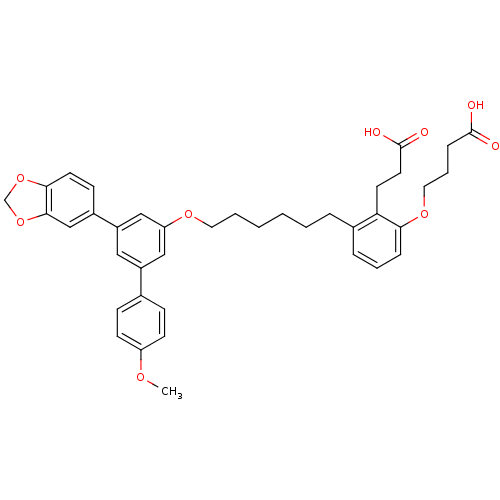 (4-(3-(6-(5-(benzo[d][1,3]dioxol-5-yl)-4'-methoxybi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263569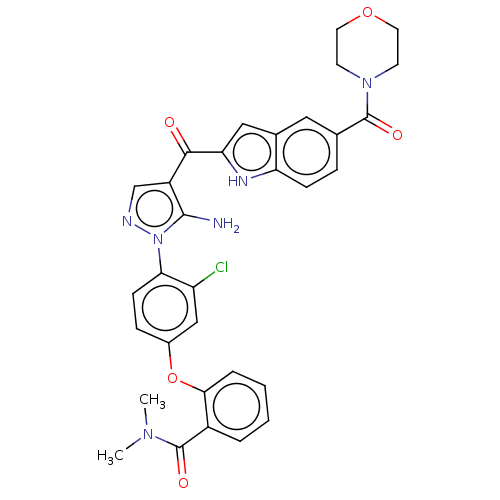 (2-(4-{5-amino-4-[5- (morpholine-4-carbonyl)-1h- in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263529 ((2-{5-amino-1-[4-(3-bromo- phenoxy)-2-methyl-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263528 ((2-{5-amino-1-[4-(3-chloro- phenoxy)-2-methyl-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263539 (US9556150, i-27 | {5-amino-1-[4-(4-chloro- benzylo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263558 (2-(4-{5-amino-4-[5-(4-methyl- piperazine-1-carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM317621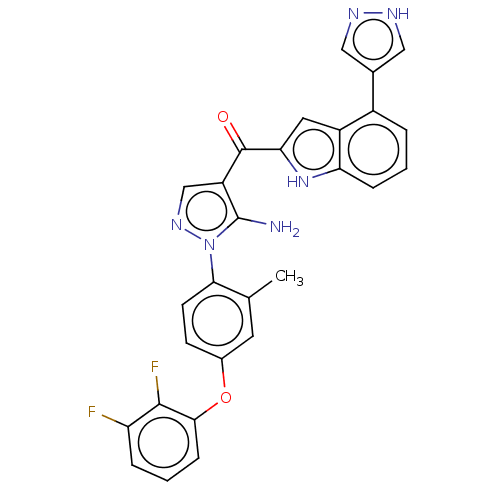 (US10640491, Compound 10 | US11104668, Compound 10 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49TX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM317621 (US10640491, Compound 10 | US11104668, Compound 10 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a |
HOFFMANN-LA ROCHE INC.; CHUGAI PHARMACEUTICAL CO. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of Btk, biotinylated SH2 peptide substrate (Src... | US Patent US9624201 (2017) BindingDB Entry DOI: 10.7270/Q2TQ63NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM317621 (US10640491, Compound 10 | US11104668, Compound 10 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US10640491 (2020) BindingDB Entry DOI: 10.7270/Q2SJ1PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263541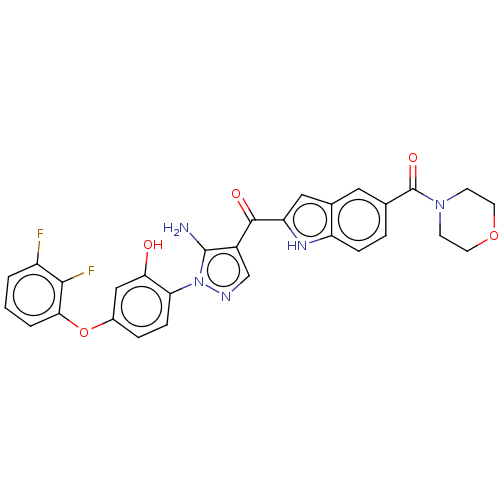 (US9556150, i-29 | {5-amino-1-[4-(2,3-difluoro- phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263514 (US9556150, i-2 | {5-amino-1-[4-(2-fluoro- phenoxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263548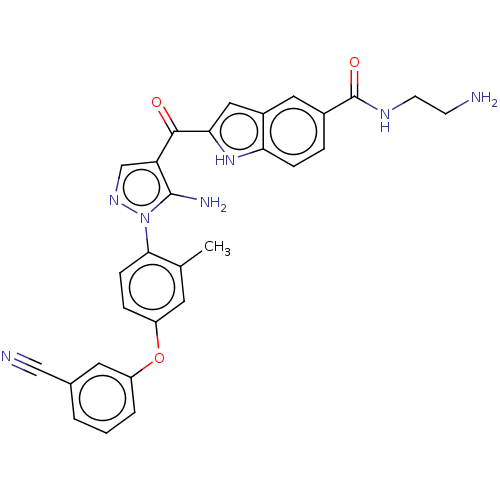 (2-{5-amino-1-[4-(3-cyano- phenoxy)-2-methyl-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM352194 (US9802920, Compound I-18 | {5-Amino-1-[4-(2,3-difl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc.; Chugai Pharamceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US9802920 (2017) BindingDB Entry DOI: 10.7270/Q29G5PXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50444358 (CHEMBL3094437) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of DYRK1A (unknown origin) | Bioorg Med Chem Lett 23: 6610-5 (2013) Article DOI: 10.1016/j.bmcl.2013.10.055 BindingDB Entry DOI: 10.7270/Q20G3MNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 291 total ) | Next | Last >> |