 Found 455 hits with Last Name = 'donald' and Initial = 'cs'
Found 455 hits with Last Name = 'donald' and Initial = 'cs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743
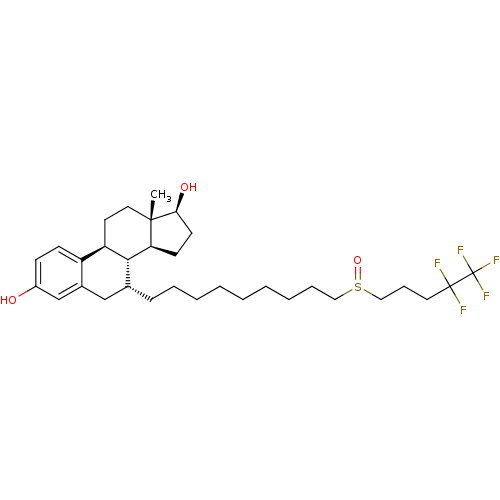
((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
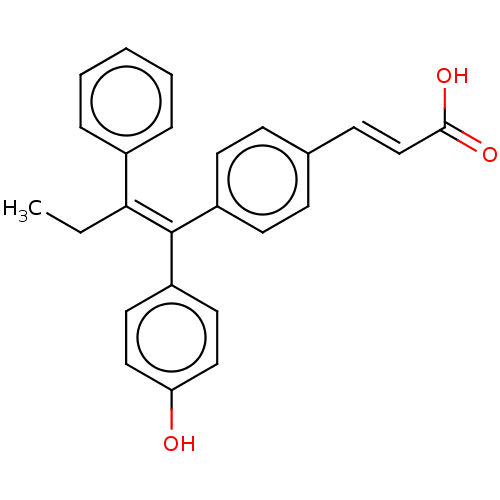
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells in presence of 0.25 uM tamoxifen |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
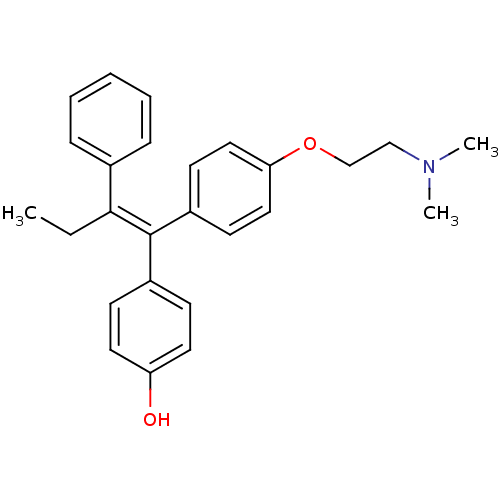
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055
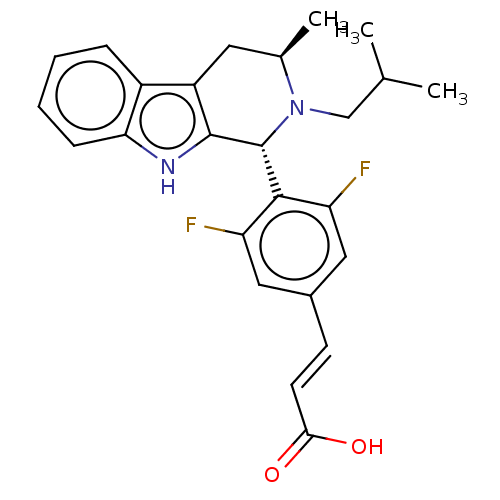
(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385398
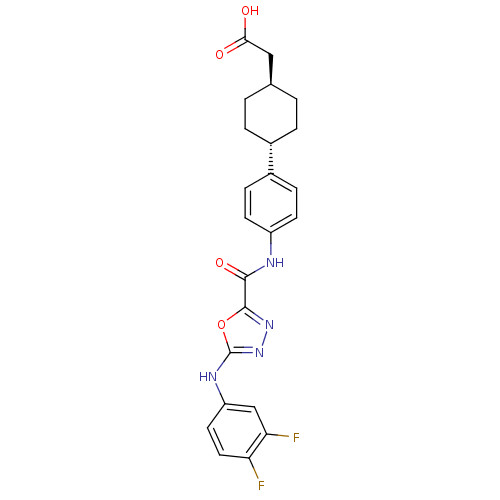
(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1-mediated triacylglycerol synthesis in human HuTu80 cells |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Rattus norvegicus (rat)) | BDBM50385398

(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of rat DGAT1 |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385398

(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM20608

(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116590
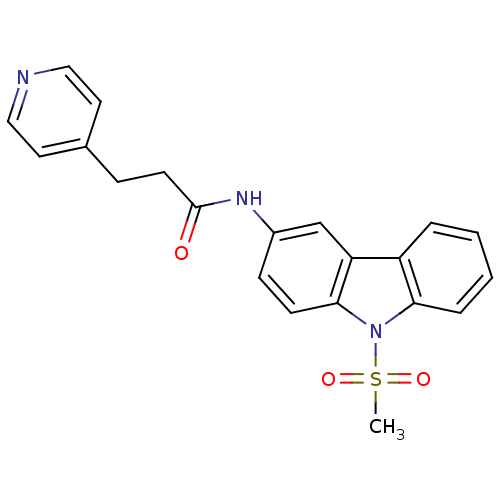
(CHEMBL325486 | N-(9-Methanesulfonyl-9H-carbazol-3-...)Show SMILES CS(=O)(=O)n1c2ccccc2c2cc(NC(=O)CCc3ccncc3)ccc12 Show InChI InChI=1S/C21H19N3O3S/c1-28(26,27)24-19-5-3-2-4-17(19)18-14-16(7-8-20(18)24)23-21(25)9-6-15-10-12-22-13-11-15/h2-5,7-8,10-14H,6,9H2,1H3,(H,23,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116610
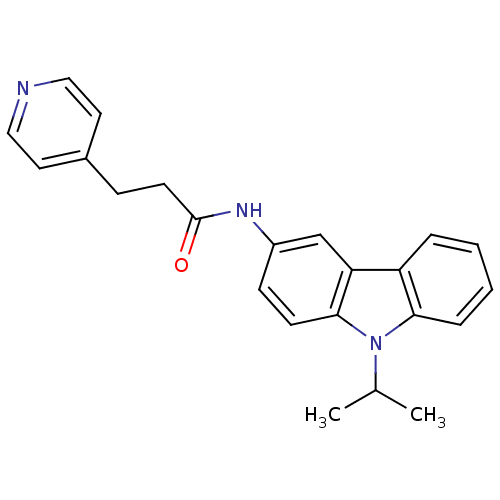
(CHEMBL119743 | N-(9-Isopropyl-9H-carbazol-3-yl)-3-...)Show InChI InChI=1S/C23H23N3O/c1-16(2)26-21-6-4-3-5-19(21)20-15-18(8-9-22(20)26)25-23(27)10-7-17-11-13-24-14-12-17/h3-6,8-9,11-16H,7,10H2,1-2H3,(H,25,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50385398

(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400147
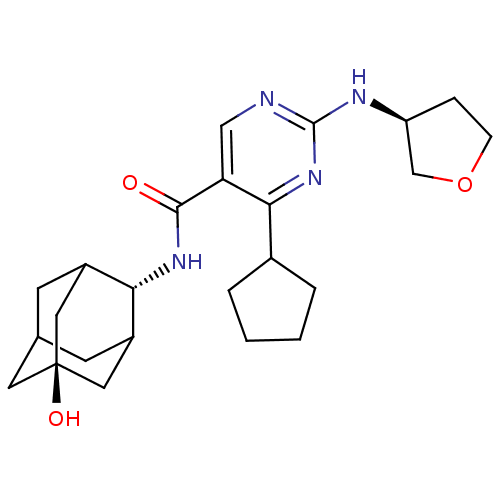
(CHEMBL2179014)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(N[C@H]4CCOC4)nc1C1CCCC1)C(C3)C2 |r,wU:7.8,16.16,wD:1.0,TLB:0:1:7:29.3.4,8:7:6.30.1:29.3.4,8:7:4:6.1.2,THB:0:1:7.28.29:4,2:1:7:29.3.4,2:3:7:6.30.1,30:28:4:6.1.2,30:1:7.28.29:4,(33.95,-38.8,;32.42,-38.79,;31.38,-37.67,;29.96,-38.21,;29.31,-39.49,;30.34,-40.56,;31.64,-40.01,;30.35,-42.31,;29.02,-43.08,;27.68,-42.32,;27.68,-40.78,;26.35,-43.09,;25.02,-42.33,;23.69,-43.1,;23.69,-44.64,;22.35,-45.41,;21.02,-44.64,;19.61,-45.27,;18.58,-44.13,;19.35,-42.79,;20.86,-43.11,;25.02,-45.41,;26.36,-44.64,;27.69,-45.41,;27.86,-46.94,;29.37,-47.26,;30.13,-45.92,;29.1,-44.78,;31.15,-40.98,;29.98,-39.66,;32.56,-40.42,)| Show InChI InChI=1S/C24H34N4O3/c29-22(27-20-16-7-14-8-17(20)11-24(30,9-14)10-16)19-12-25-23(26-18-5-6-31-13-18)28-21(19)15-3-1-2-4-15/h12,14-18,20,30H,1-11,13H2,(H,27,29)(H,25,26,28)/t14?,16?,17?,18-,20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HPLC assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116600
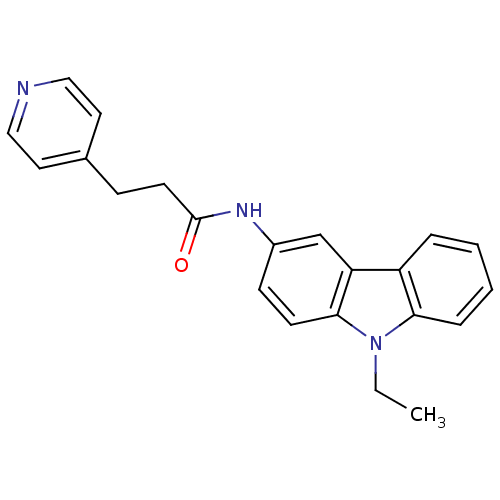
(CHEMBL325475 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyri...)Show InChI InChI=1S/C22H21N3O/c1-2-25-20-6-4-3-5-18(20)19-15-17(8-9-21(19)25)24-22(26)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,2,7,10H2,1H3,(H,24,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116602
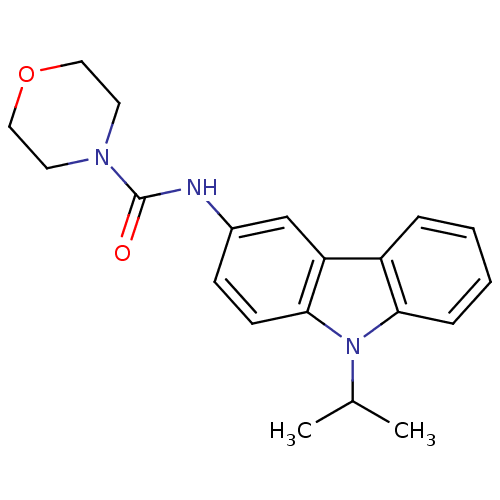
(CHEMBL419951 | Morpholine-4-carboxylic acid (9-iso...)Show InChI InChI=1S/C20H23N3O2/c1-14(2)23-18-6-4-3-5-16(18)17-13-15(7-8-19(17)23)21-20(24)22-9-11-25-12-10-22/h3-8,13-14H,9-12H2,1-2H3,(H,21,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116619
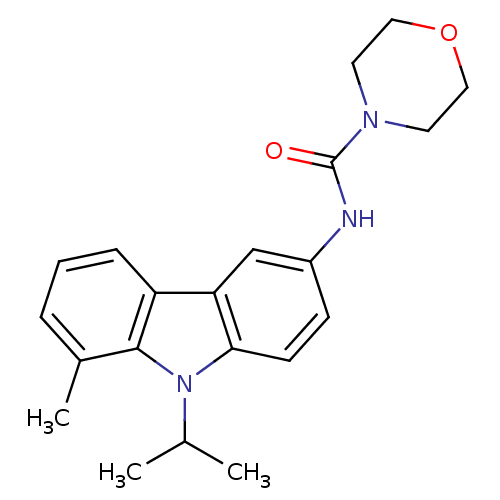
(CHEMBL117563 | Morpholine-4-carboxylic acid (9-iso...)Show SMILES CC(C)n1c2ccc(NC(=O)N3CCOCC3)cc2c2cccc(C)c12 Show InChI InChI=1S/C21H25N3O2/c1-14(2)24-19-8-7-16(22-21(25)23-9-11-26-12-10-23)13-18(19)17-6-4-5-15(3)20(17)24/h4-8,13-14H,9-12H2,1-3H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM454567

(US10717746, Example 48)Show SMILES CC(=O)N[C@@H]1CC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CC(C)(C)Cc23)c(Cl)cn1 |r| Show InChI InChI=1S/C21H26ClN5O2/c1-12(28)25-14-5-4-13(6-14)20(29)26-19-7-15(17(22)10-23-19)16-9-24-27-11-21(2,3)8-18(16)27/h7,9-10,13-14H,4-6,8,11H2,1-3H3,(H,25,28)(H,23,26,29)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM454570
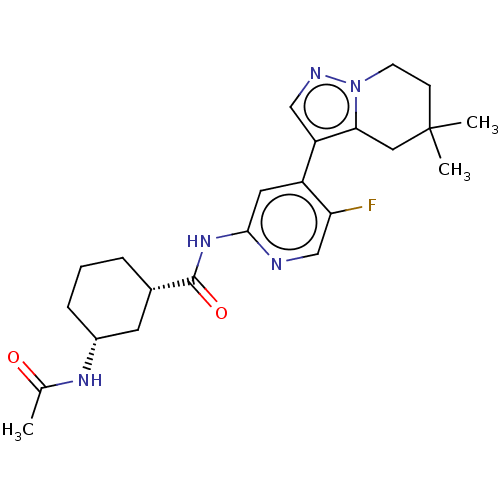
(US10717746, Example 51)Show SMILES CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CCC(C)(C)Cc23)c(F)cn1 |r| Show InChI InChI=1S/C23H30FN5O2/c1-14(30)27-16-6-4-5-15(9-16)22(31)28-21-10-17(19(24)13-25-21)18-12-26-29-8-7-23(2,3)11-20(18)29/h10,12-13,15-16H,4-9,11H2,1-3H3,(H,27,30)(H,25,28,31)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116592
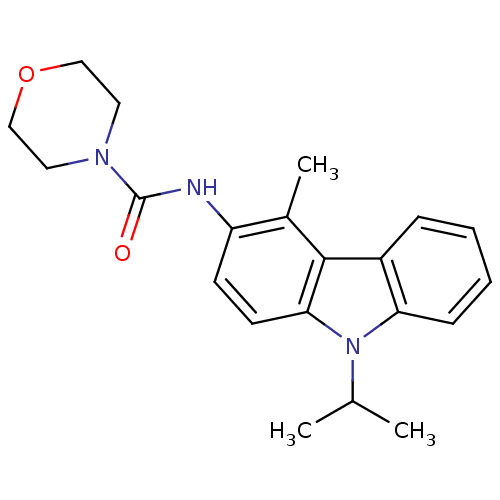
(CHEMBL325226 | Morpholine-4-carboxylic acid (9-iso...)Show SMILES CC(C)n1c2ccccc2c2c(C)c(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C21H25N3O2/c1-14(2)24-18-7-5-4-6-16(18)20-15(3)17(8-9-19(20)24)22-21(25)23-10-12-26-13-11-23/h4-9,14H,10-13H2,1-3H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116605
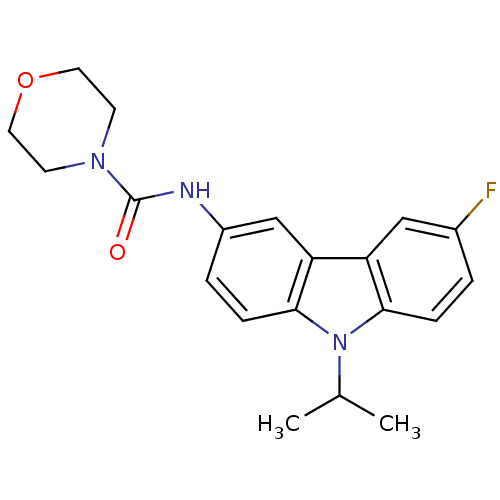
(CHEMBL432628 | Morpholine-4-carboxylic acid (6-flu...)Show SMILES CC(C)n1c2ccc(F)cc2c2cc(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C20H22FN3O2/c1-13(2)24-18-5-3-14(21)11-16(18)17-12-15(4-6-19(17)24)22-20(25)23-7-9-26-10-8-23/h3-6,11-13H,7-10H2,1-2H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116617
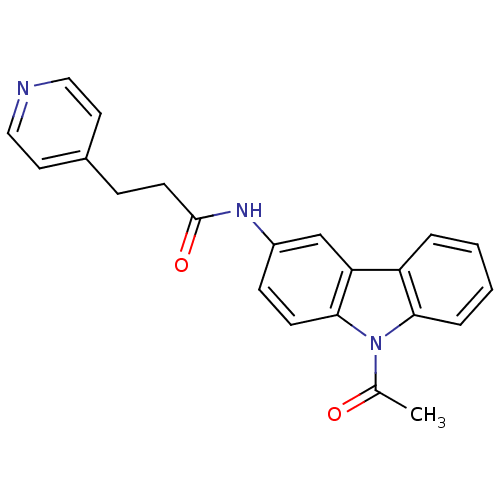
(CHEMBL116210 | N-(9-Acetyl-9H-carbazol-3-yl)-3-pyr...)Show SMILES CC(=O)n1c2ccccc2c2cc(NC(=O)CCc3ccncc3)ccc12 Show InChI InChI=1S/C22H19N3O2/c1-15(26)25-20-5-3-2-4-18(20)19-14-17(7-8-21(19)25)24-22(27)9-6-16-10-12-23-13-11-16/h2-5,7-8,10-14H,6,9H2,1H3,(H,24,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50041611
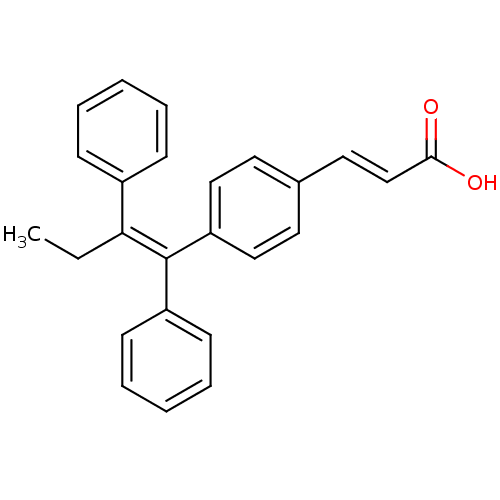
((2E)-3-{4-[(1E)-1,2-DIPHENYLBUT-1-ENYL]PHENYL}ACRY...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O2/c1-2-23(20-9-5-3-6-10-20)25(21-11-7-4-8-12-21)22-16-13-19(14-17-22)15-18-24(26)27/h3-18H,2H2,1H3,(H,26,27)/b18-15+,25-23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50528817

(CHEMBL4462530 | US10717746, Example 14 | US2023041...)Show SMILES CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CC(C)(C)Cc23)c(Cl)cn1 |r| Show InChI InChI=1S/C22H28ClN5O2/c1-13(29)26-15-6-4-5-14(7-15)21(30)27-20-8-16(18(23)11-24-20)17-10-25-28-12-22(2,3)9-19(17)28/h8,10-11,14-15H,4-7,9,12H2,1-3H3,(H,26,29)(H,24,27,30)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM454542

(US10717746, Example 25 | US10717746, Example 84)Show SMILES CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CC(C)(C)Cc23)c(F)cn1 |r| Show InChI InChI=1S/C22H28FN5O2/c1-13(29)26-15-6-4-5-14(7-15)21(30)27-20-8-16(18(23)11-24-20)17-10-25-28-12-22(2,3)9-19(17)28/h8,10-11,14-15H,4-7,9,12H2,1-3H3,(H,26,29)(H,24,27,30)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385439
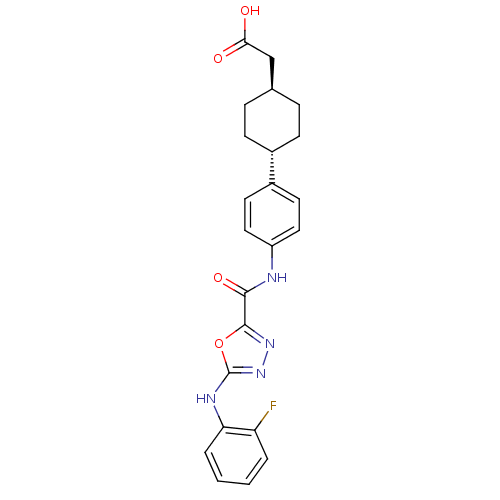
(CHEMBL2036745)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccccc3F)o2)cc1 |r,wU:7.10,wD:4.3,(45.29,-27.64,;44.52,-28.98,;42.98,-28.97,;45.28,-30.31,;46.82,-30.32,;47.6,-31.66,;49.13,-31.66,;49.9,-30.34,;49.14,-29,;47.6,-28.99,;51.43,-30.34,;52.21,-29.01,;53.75,-29.02,;54.51,-30.36,;56.05,-30.37,;56.83,-29.04,;56.07,-27.71,;58.38,-29.05,;58.85,-27.59,;60.38,-27.59,;60.86,-29.05,;62.19,-29.81,;63.52,-29.04,;63.51,-27.5,;64.83,-26.72,;66.18,-27.49,;66.19,-29.03,;64.86,-29.81,;64.86,-31.35,;59.62,-29.96,;53.74,-31.69,;52.2,-31.69,)| Show InChI InChI=1S/C23H23FN4O4/c24-18-3-1-2-4-19(18)26-23-28-27-22(32-23)21(31)25-17-11-9-16(10-12-17)15-7-5-14(6-8-15)13-20(29)30/h1-4,9-12,14-15H,5-8,13H2,(H,25,31)(H,26,28)(H,29,30)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385429
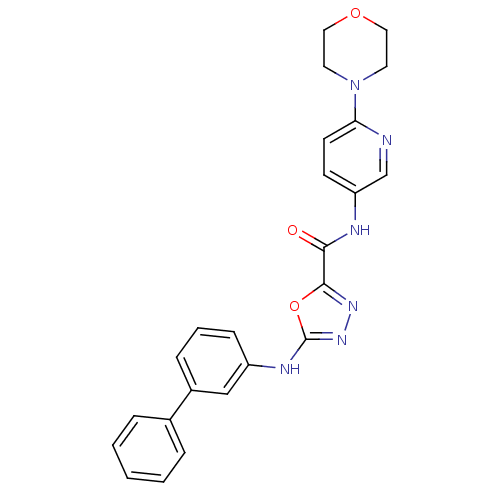
(CHEMBL2036734)Show SMILES O=C(Nc1ccc(nc1)N1CCOCC1)c1nnc(Nc2cccc(c2)-c2ccccc2)o1 Show InChI InChI=1S/C24H22N6O3/c31-22(26-20-9-10-21(25-16-20)30-11-13-32-14-12-30)23-28-29-24(33-23)27-19-8-4-7-18(15-19)17-5-2-1-3-6-17/h1-10,15-16H,11-14H2,(H,26,31)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116592

(CHEMBL325226 | Morpholine-4-carboxylic acid (9-iso...)Show SMILES CC(C)n1c2ccccc2c2c(C)c(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C21H25N3O2/c1-14(2)24-18-7-5-4-6-16(18)20-15(3)17(8-9-19(20)24)22-21(25)23-10-12-26-13-11-23/h4-9,14H,10-13H2,1-3H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Compound was evaluated for functional antagonism of Neuropeptide Y receptor Y5 activity in cellular Ca flux |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385424
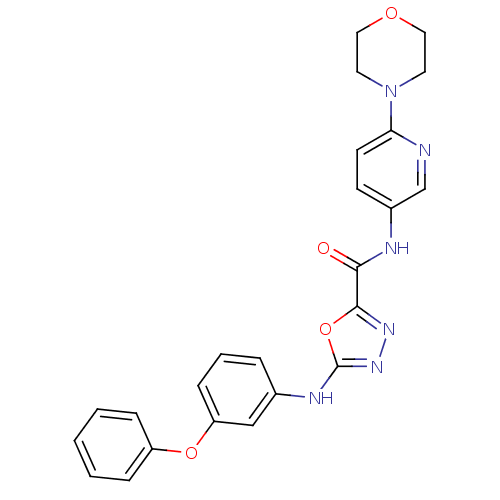
(CHEMBL2036596)Show SMILES O=C(Nc1ccc(nc1)N1CCOCC1)c1nnc(Nc2cccc(Oc3ccccc3)c2)o1 Show InChI InChI=1S/C24H22N6O4/c31-22(26-18-9-10-21(25-16-18)30-11-13-32-14-12-30)23-28-29-24(34-23)27-17-5-4-8-20(15-17)33-19-6-2-1-3-7-19/h1-10,15-16H,11-14H2,(H,26,31)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385412
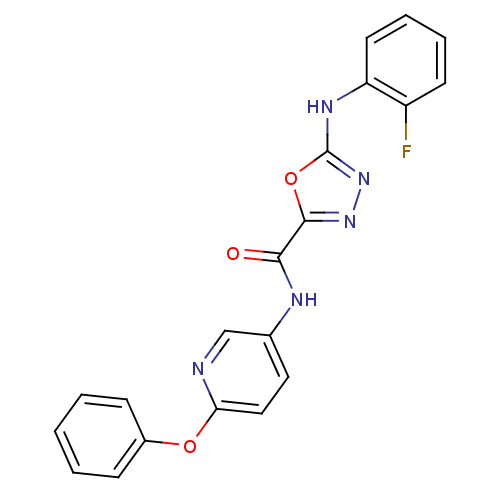
(CHEMBL2036583)Show SMILES Fc1ccccc1Nc1nnc(o1)C(=O)Nc1ccc(Oc2ccccc2)nc1 Show InChI InChI=1S/C20H14FN5O3/c21-15-8-4-5-9-16(15)24-20-26-25-19(29-20)18(27)23-13-10-11-17(22-12-13)28-14-6-2-1-3-7-14/h1-12H,(H,23,27)(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50116600

(CHEMBL325475 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyri...)Show InChI InChI=1S/C22H21N3O/c1-2-25-20-6-4-3-5-18(20)19-15-17(8-9-21(19)25)24-22(26)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,2,7,10H2,1H3,(H,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity against rat Neuropeptide Y receptor Y5 |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116597
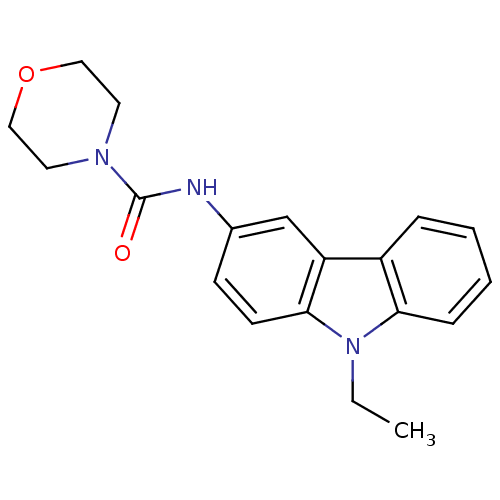
(CHEMBL119247 | Morpholine-4-carboxylic acid (9-eth...)Show InChI InChI=1S/C19H21N3O2/c1-2-22-17-6-4-3-5-15(17)16-13-14(7-8-18(16)22)20-19(23)21-9-11-24-12-10-21/h3-8,13H,2,9-12H2,1H3,(H,20,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
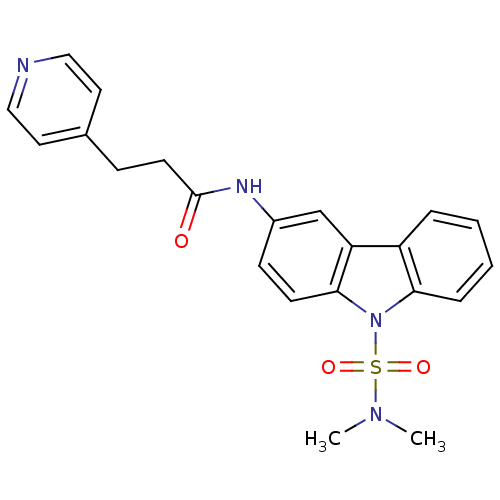
(Homo sapiens (Human)) | BDBM50116608
(CHEMBL117922 | N-(9-Dimethylsulfamoyl-9H-carbazol-...)Show SMILES CN(C)S(=O)(=O)n1c2ccccc2c2cc(NC(=O)CCc3ccncc3)ccc12 Show InChI InChI=1S/C22H22N4O3S/c1-25(2)30(28,29)26-20-6-4-3-5-18(20)19-15-17(8-9-21(19)26)24-22(27)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,7,10H2,1-2H3,(H,24,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM454563

(US10717746, Example 44)Show SMILES CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(ccn1)-c1cnn2CCC(C)(C)Cc12 |r| Show InChI InChI=1S/C23H31N5O2/c1-15(29)26-18-6-4-5-17(11-18)22(30)27-21-12-16(7-9-24-21)19-14-25-28-10-8-23(2,3)13-20(19)28/h7,9,12,14,17-18H,4-6,8,10-11,13H2,1-3H3,(H,26,29)(H,24,27,30)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400149
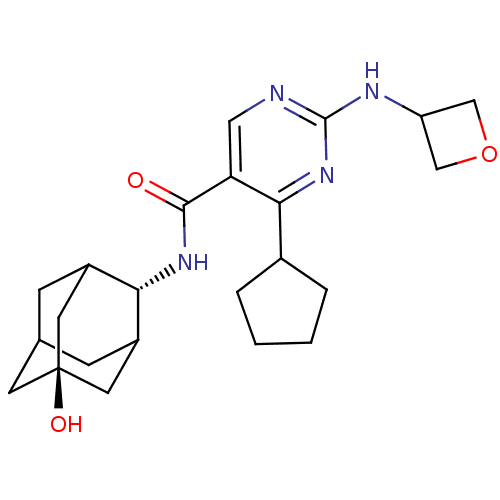
(CHEMBL2179456)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(NC4COC4)nc1C1CCCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:28.3.4,8:7:6.29.1:28.3.4,8:7:4:6.1.2,THB:0:1:7.27.28:4,2:1:7:28.3.4,2:3:7:6.29.1,29:27:4:6.1.2,29:1:7.27.28:4,(75.52,-27,;73.99,-26.98,;72.94,-25.86,;71.53,-26.41,;70.88,-27.68,;71.91,-28.76,;73.21,-28.2,;71.92,-30.5,;70.59,-31.27,;69.25,-30.51,;69.24,-28.97,;67.92,-31.28,;66.58,-30.52,;65.26,-31.29,;65.25,-32.84,;63.92,-33.6,;62.59,-32.83,;62.19,-31.35,;60.71,-31.74,;61.1,-33.23,;66.59,-33.61,;67.93,-32.83,;69.26,-33.6,;69.42,-35.13,;70.93,-35.45,;71.7,-34.11,;70.67,-32.97,;72.72,-29.17,;71.55,-27.85,;74.13,-28.61,)| Show InChI InChI=1S/C23H32N4O3/c28-21(26-19-15-5-13-6-16(19)9-23(29,7-13)8-15)18-10-24-22(25-17-11-30-12-17)27-20(18)14-3-1-2-4-14/h10,13-17,19,29H,1-9,11-12H2,(H,26,28)(H,24,25,27)/t13?,15?,16?,19-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM454570

(US10717746, Example 51)Show SMILES CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CCC(C)(C)Cc23)c(F)cn1 |r| Show InChI InChI=1S/C23H30FN5O2/c1-14(30)27-16-6-4-5-15(9-16)22(31)28-21-10-17(19(24)13-25-21)18-12-26-29-8-7-23(2,3)11-20(18)29/h10,12-13,15-16H,4-9,11H2,1-3H3,(H,27,30)(H,25,28,31)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9 in human MCF7 cells assessed as reduction in RNA Polymerase 2 CTD phosphorylation at Ser2 residues measured after 6 hrs by immunof... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data