

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542790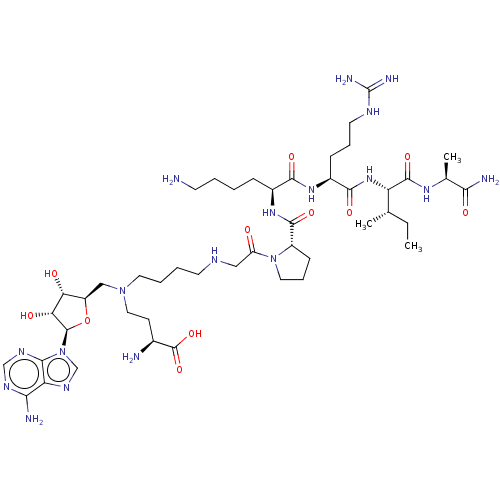 (CHEMBL4647584) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 120 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence ... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM294039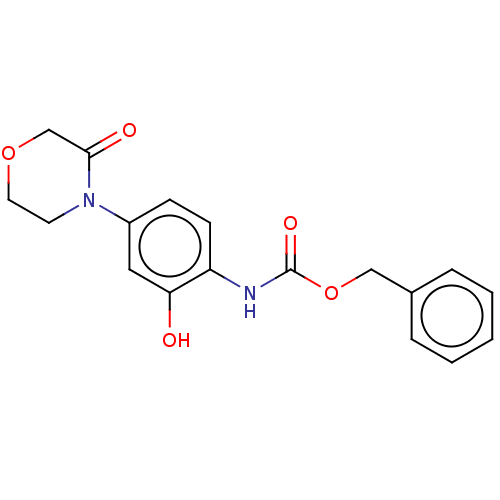 (Process 1 | US10106557, Compound 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The activity of the compound to be tested against prothrombinase was determined by the production of thrombin. In summary, 12.5 μL human factor ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542790 (CHEMBL4647584) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 30 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542790 (CHEMBL4647584) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 90 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542790 (CHEMBL4647584) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 60 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542789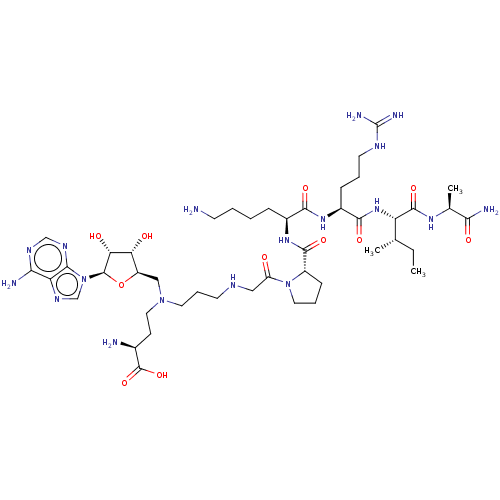 (CHEMBL4647726) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 60 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM294042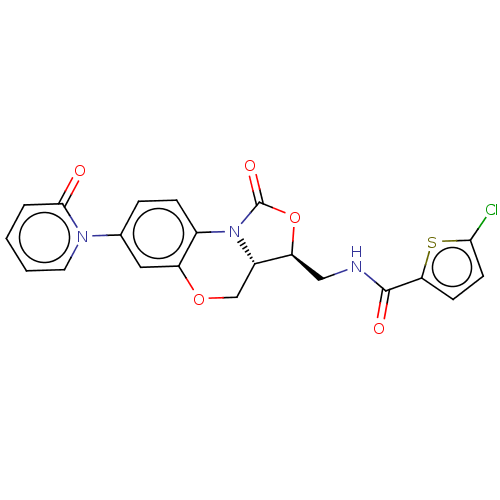 (US10106557, Compound 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The activity of the compound to be tested against prothrombinase was determined by the production of thrombin. In summary, 12.5 μL human factor ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM294039 (Process 1 | US10106557, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in human was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542789 (CHEMBL4647726) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 120 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence ... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM294042 (US10106557, Compound 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in human was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50024354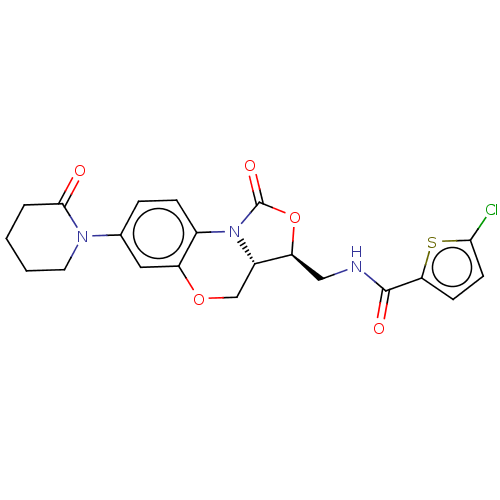 (CHEMBL3330444 | US10106557, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in human was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542789 (CHEMBL4647726) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 30 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50329367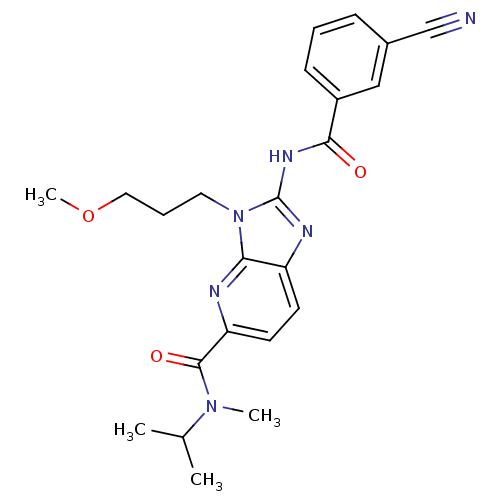 (2-(3-cyanobenzamido)-N-isopropyl-3-(3-methoxypropy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human recombinant A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542789 (CHEMBL4647726) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 90 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Rattus norvegicus (rat)) | BDBM294039 (Process 1 | US10106557, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.87 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in rats was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50 ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50329364 (2-(3-cyanobenzamido)-N,N-diethyl-1-(3-methoxypropy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human recombinant A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Rattus norvegicus (rat)) | BDBM294042 (US10106557, Compound 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.17 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in rats was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50 ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM294041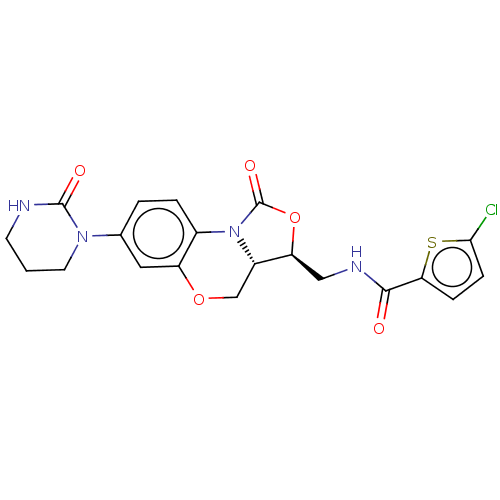 (US10106557, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.13 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in human was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50024354 (CHEMBL3330444 | US10106557, Compound 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.22 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The activity of the compound to be tested against prothrombinase was determined by the production of thrombin. In summary, 12.5 μL human factor ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50329367 (2-(3-cyanobenzamido)-N-isopropyl-3-(3-methoxypropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor by cAMP functional assay | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM294041 (US10106557, Compound 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.41 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The activity of the compound to be tested against prothrombinase was determined by the production of thrombin. In summary, 12.5 μL human factor ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Rattus norvegicus (rat)) | BDBM50024354 (CHEMBL3330444 | US10106557, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.45 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in rats was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50 ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542788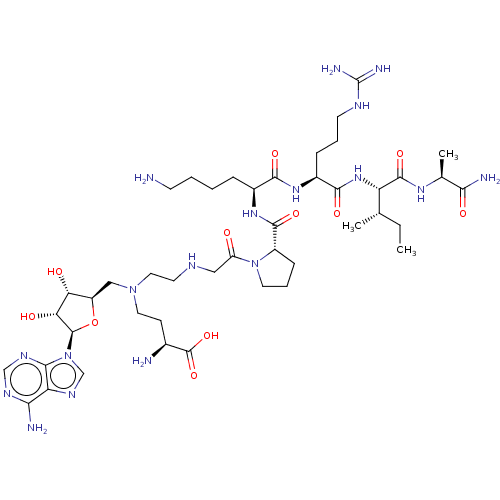 (CHEMBL4645959) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 90 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50300121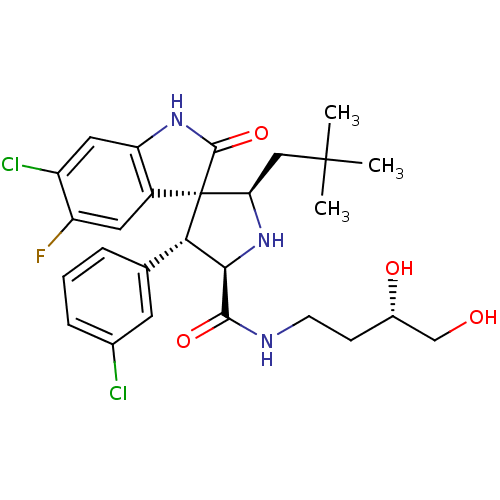 ((2'R,3S,4'R,5'R)-6-chloro-4'-(3-chlorophenyl)-N-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to MDM2 | Eur J Med Chem 56: 10-16 (2012) Article DOI: 10.1016/j.ejmech.2012.08.003 BindingDB Entry DOI: 10.7270/Q2K938SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542788 (CHEMBL4645959) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 120 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence ... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50329348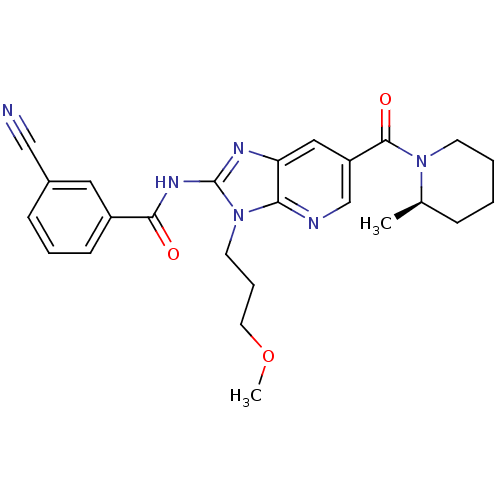 ((R)-3-cyano-N-(3-(3-methoxypropyl)-6-(2-methylpipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor by cAMP functional assay | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542788 (CHEMBL4645959) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 30 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50329348 ((R)-3-cyano-N-(3-(3-methoxypropyl)-6-(2-methylpipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human recombinant A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50249989 (CHEMBL1387422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542788 (CHEMBL4645959) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 60 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DCN1-like protein 1 (Homo sapiens) | BDBM50584167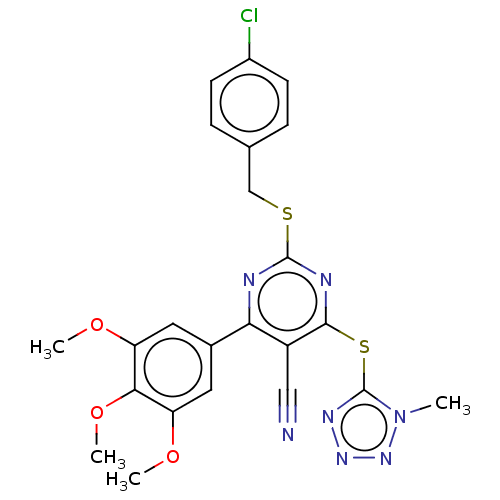 (CHEMBL5085822) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to DCN1 (unknown origin) assessed as inhibitory constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01207 BindingDB Entry DOI: 10.7270/Q2JD51NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583530 (CHEMBL5092661) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583533 (CHEMBL5084153) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50329342 (3-cyano-N-(3-(3-methoxypropyl)-6-(piperidine-1-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human recombinant A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM294043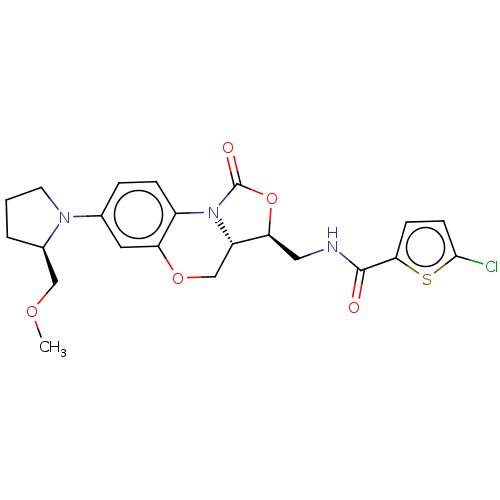 (US10106557, Compound 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.71 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in human was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50329349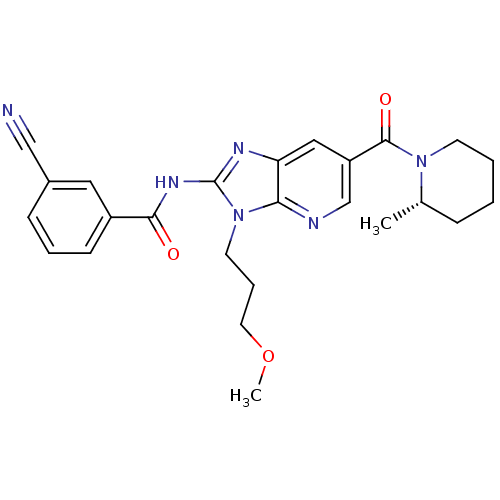 ((S)-3-cyano-N-(3-(3-methoxypropyl)-6-(2-methylpipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human recombinant A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50329366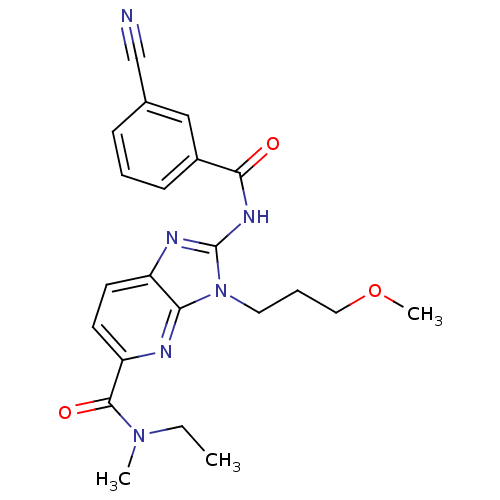 (2-(3-cyanobenzamido)-N-ethyl-3-(3-methoxypropyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human recombinant A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50329347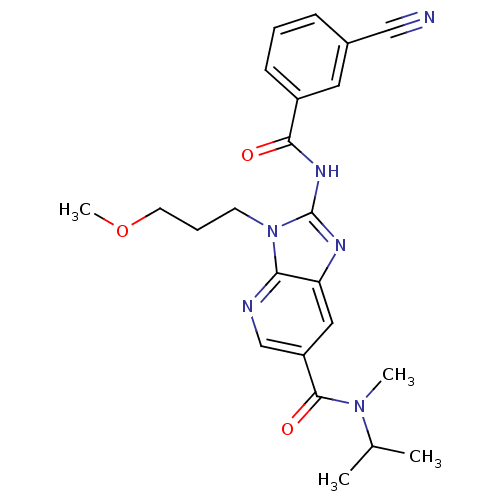 (2-(3-cyanobenzamido)-N-isopropyl-3-(3-methoxypropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor by cAMP functional assay | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50329347 (2-(3-cyanobenzamido)-N-isopropyl-3-(3-methoxypropy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human recombinant A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DCN1-like protein 1 (Homo sapiens) | BDBM50525313 (CHEMBL4592844) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to DCN1 (unknown origin) assessed as inhibitory constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01207 BindingDB Entry DOI: 10.7270/Q2JD51NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50329349 ((S)-3-cyano-N-(3-(3-methoxypropyl)-6-(2-methylpipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor by cAMP functional assay | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583534 (CHEMBL5089219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-terminal Xaa-Pro-Lys N-methyltransferase 1 (Homo sapiens) | BDBM50542790 (CHEMBL4647584) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... | J Med Chem 63: 8419-8431 (2020) Article DOI: 10.1021/acs.jmedchem.0c00770 BindingDB Entry DOI: 10.7270/Q2B85CQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301329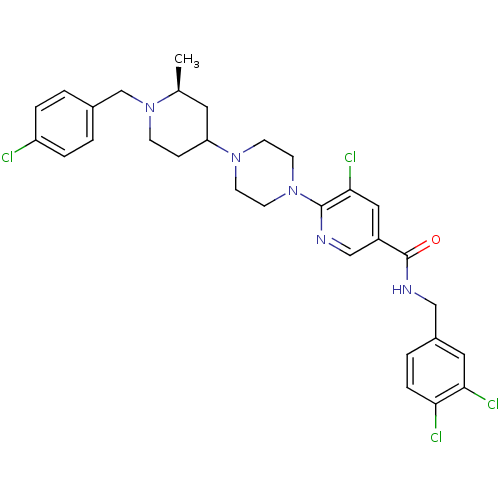 (5-chloro-6-(4-((2S)-1-(4-chlorobenzyl)-2-methylpip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50329346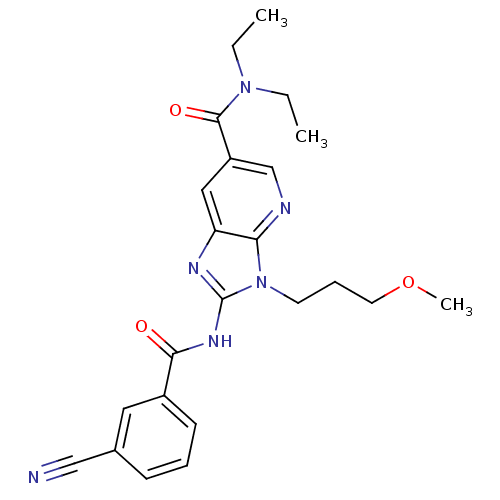 (2-(3-cyanobenzamido)-N,N-diethyl-3-(3-methoxypropy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human recombinant A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50329364 (2-(3-cyanobenzamido)-N,N-diethyl-1-(3-methoxypropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor by cAMP functional assay | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM294043 (US10106557, Compound 30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The activity of the compound to be tested against prothrombinase was determined by the production of thrombin. In summary, 12.5 μL human factor ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Rattus norvegicus (rat)) | BDBM294041 (US10106557, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in rats was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50 ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50329365 (3-cyano-N-(1-(3-methoxypropyl)-6-(piperidine-1-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]SCH58261 from human recombinant A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6845-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.064 BindingDB Entry DOI: 10.7270/Q2NS0V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Rattus norvegicus (rat)) | BDBM294043 (US10106557, Compound 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21.5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
NORTH CHINA PHARMACEUTICAL NEW DRUG R&D CO., LTD. US Patent | Assay Description The inhibitory activity on coagulation factor Xa activity in rats was measured by using Tris-HCl buffer (50 mM, pH 8.3, 150 mM NaCl). A buffer of 50 ... | US Patent US10106557 (2018) BindingDB Entry DOI: 10.7270/Q2X63Q0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1761 total ) | Next | Last >> |