 Found 2415 hits with Last Name = 'dong' and Initial = 'j'
Found 2415 hits with Last Name = 'dong' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50305263
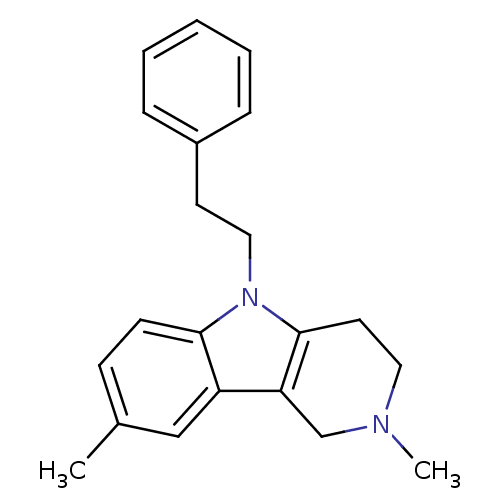
(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85052
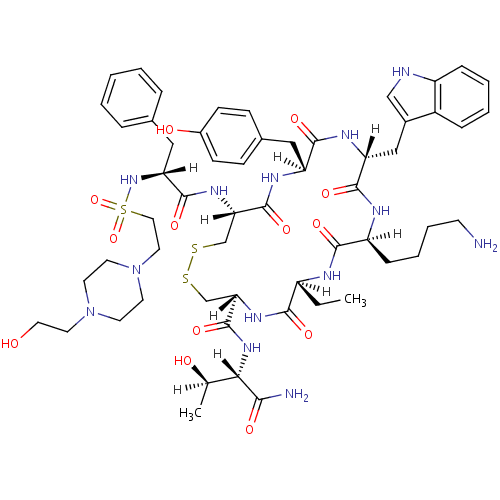
(BIM 23197 | BIM-23197)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@@H](Cc1ccccc1)N[S](=O)(=O)CCN1CCN(CCO)CC1 Show InChI InChI=1S/C57H81N13O13S3/c1-3-41-51(75)65-48(57(81)67-49(35(2)72)50(59)74)34-85-84-33-47(66-55(79)46(30-36-11-5-4-6-12-36)68-86(82,83)28-26-70-23-21-69(22-24-70)25-27-71)56(80)63-44(29-37-16-18-39(73)19-17-37)53(77)64-45(31-38-32-60-42-14-8-7-13-40(38)42)54(78)62-43(52(76)61-41)15-9-10-20-58/h4-8,11-14,16-19,32,35,41,43-49,60,68,71-73H,3,9-10,15,20-31,33-34,58H2,1-2H3,(H2,59,74)(H,61,76)(H,62,78)(H,63,80)(H,64,77)(H,65,75)(H,66,79)(H,67,81)/t35-,41+,43+,44+,45-,46-,47+,48+,49+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206734
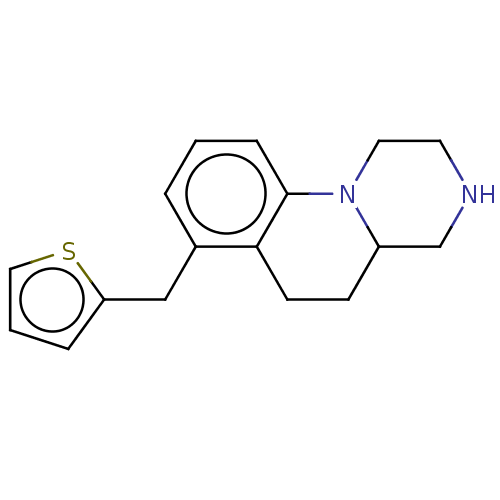
(CHEMBL3959570)Show InChI InChI=1S/C17H20N2S/c1-3-13(11-15-4-2-10-20-15)16-7-6-14-12-18-8-9-19(14)17(16)5-1/h1-5,10,14,18H,6-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85051
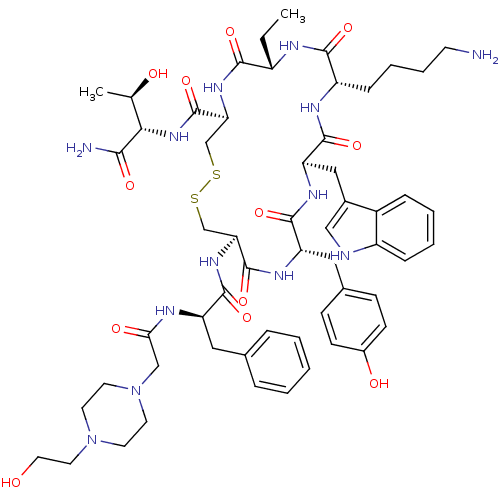
(BIM-23190)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CN1CCN(CCO)CC1 Show InChI InChI=1S/C57H79N13O12S2/c1-3-40-51(76)66-47(57(82)68-49(34(2)72)50(59)75)33-84-83-32-46(67-53(78)43(27-35-11-5-4-6-12-35)61-48(74)31-70-23-21-69(22-24-70)25-26-71)56(81)64-44(28-36-16-18-38(73)19-17-36)54(79)65-45(29-37-30-60-41-14-8-7-13-39(37)41)55(80)63-42(52(77)62-40)15-9-10-20-58/h4-8,11-14,16-19,30,34,40,42-47,49,60,71-73H,3,9-10,15,20-29,31-33,58H2,1-2H3,(H2,59,75)(H,61,74)(H,62,77)(H,63,80)(H,64,81)(H,65,79)(H,66,76)(H,67,78)(H,68,82)/t34-,40+,42+,43-,44+,45-,46+,47+,49+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50063839
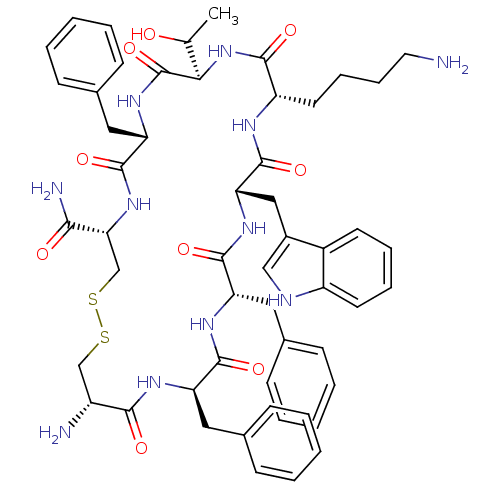
(BIM 23268 | CHEMBL263606 | H-cyclo[DCys-Phe-Phe-DT...)Show SMILES CC(O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O Show InChI InChI=1S/C54H67N11O9S2/c1-32(66)46-54(74)63-43(27-35-19-9-4-10-20-35)52(72)64-45(47(57)67)31-76-75-30-38(56)48(68)60-41(25-33-15-5-2-6-16-33)50(70)61-42(26-34-17-7-3-8-18-34)51(71)62-44(28-36-29-58-39-22-12-11-21-37(36)39)53(73)59-40(49(69)65-46)23-13-14-24-55/h2-12,15-22,29,32,38,40-46,58,66H,13-14,23-28,30-31,55-56H2,1H3,(H2,57,67)(H,59,73)(H,60,68)(H,61,70)(H,62,71)(H,63,74)(H,64,72)(H,65,69)/t32?,38-,40+,41-,42+,43+,44+,45-,46-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206719
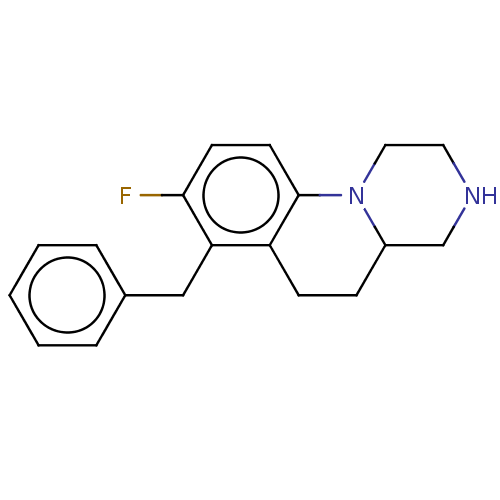
(CHEMBL3956394)Show InChI InChI=1S/C19H21FN2/c20-18-8-9-19-16(7-6-15-13-21-10-11-22(15)19)17(18)12-14-4-2-1-3-5-14/h1-5,8-9,15,21H,6-7,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206725
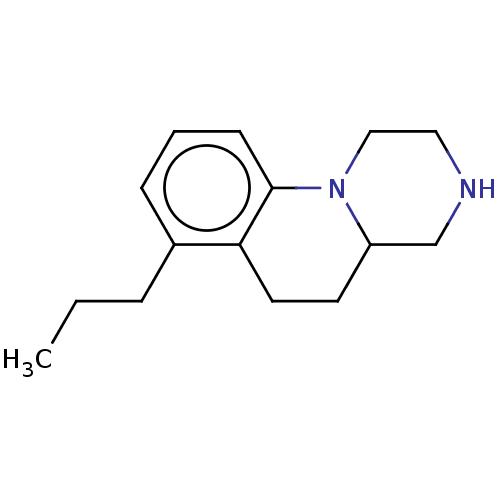
(CHEMBL3947276)Show InChI InChI=1S/C15H22N2/c1-2-4-12-5-3-6-15-14(12)8-7-13-11-16-9-10-17(13)15/h3,5-6,13,16H,2,4,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM82465
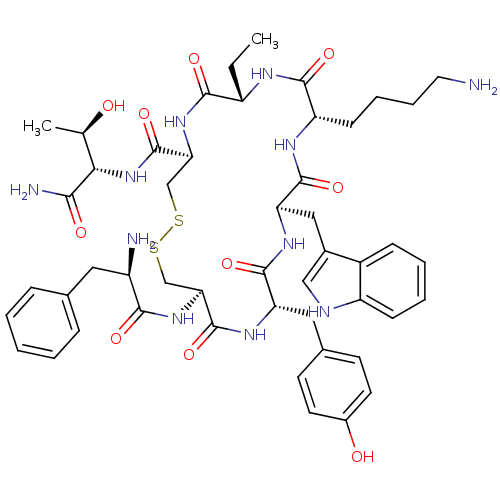
(BIM 23023 | BIM-23023 | D-Phe-L-Cys(1)-L-Tyr-D-Trp...)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C49H65N11O10S2/c1-3-34-44(65)59-40(49(70)60-41(27(2)61)42(52)63)26-72-71-25-39(58-43(64)33(51)21-28-11-5-4-6-12-28)48(69)56-37(22-29-16-18-31(62)19-17-29)46(67)57-38(23-30-24-53-35-14-8-7-13-32(30)35)47(68)55-36(45(66)54-34)15-9-10-20-50/h4-8,11-14,16-19,24,27,33-34,36-41,53,61-62H,3,9-10,15,20-23,25-26,50-51H2,1-2H3,(H2,52,63)(H,54,66)(H,55,68)(H,56,69)(H,57,67)(H,58,64)(H,59,65)(H,60,70)/t27-,33-,34+,36+,37+,38-,39+,40+,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50059090
(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206724
(CHEMBL3912721)Show InChI InChI=1S/C17H24N2/c1-3-13(4-1)11-14-5-2-6-17-16(14)8-7-15-12-18-9-10-19(15)17/h2,5-6,13,15,18H,1,3-4,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206714
(CHEMBL3919795)Show InChI InChI=1S/C19H22N2/c1-2-5-15(6-3-1)13-16-7-4-8-19-18(16)10-9-17-14-20-11-12-21(17)19/h1-8,17,20H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206719

(CHEMBL3956394)Show InChI InChI=1S/C19H21FN2/c20-18-8-9-19-16(7-6-15-13-21-10-11-22(15)19)17(18)12-14-4-2-1-3-5-14/h1-5,8-9,15,21H,6-7,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2A receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM82470
(3-(2-Naphtyl)-D-Ala-L-Cys(1)-L-Tyr-D-Trp-L-Lys-L-V...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H](N)Cc1ccc2ccccc2c1 Show InChI InChI=1S/C54H69N11O10S2/c1-29(2)45-54(75)63-44(53(74)65-46(30(3)66)47(57)68)28-77-76-27-43(62-48(69)38(56)23-32-15-18-33-10-4-5-11-34(33)22-32)52(73)60-41(24-31-16-19-36(67)20-17-31)50(71)61-42(25-35-26-58-39-13-7-6-12-37(35)39)51(72)59-40(49(70)64-45)14-8-9-21-55/h4-7,10-13,15-20,22,26,29-30,38,40-46,58,66-67H,8-9,14,21,23-25,27-28,55-56H2,1-3H3,(H2,57,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,75)(H,64,70)(H,65,74)/t30-,38-,40+,41+,42-,43+,44+,45+,46+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50206716
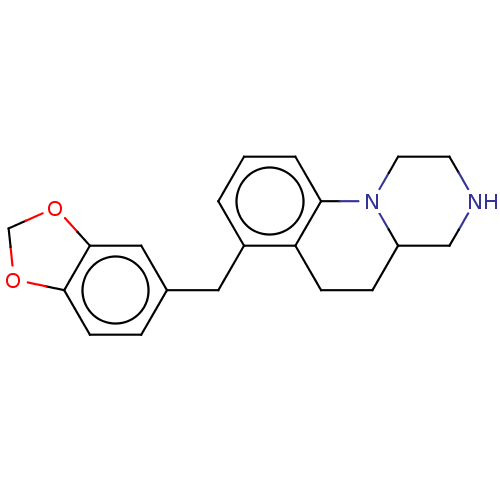
(CHEMBL3935339)Show InChI InChI=1S/C20H22N2O2/c1-2-15(10-14-4-7-19-20(11-14)24-13-23-19)17-6-5-16-12-21-8-9-22(16)18(17)3-1/h1-4,7,11,16,21H,5-6,8-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50305263

(2,8-Dimethyl-5-phenethyl-2,3,4,5-tetrahydro-1H-pyr...)Show InChI InChI=1S/C21H24N2/c1-16-8-9-20-18(14-16)19-15-22(2)12-11-21(19)23(20)13-10-17-6-4-3-5-7-17/h3-9,14H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM82253
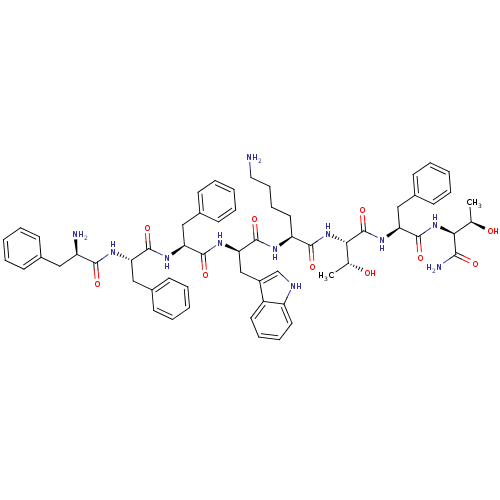
(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206716

(CHEMBL3935339)Show InChI InChI=1S/C20H22N2O2/c1-2-15(10-14-4-7-19-20(11-14)24-13-23-19)17-6-5-16-12-21-8-9-22(16)18(17)3-1/h1-4,7,11,16,21H,5-6,8-10,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2A receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206714

(CHEMBL3919795)Show InChI InChI=1S/C19H22N2/c1-2-5-15(6-3-1)13-16-7-4-8-19-18(16)10-9-17-14-20-11-12-21(17)19/h1-8,17,20H,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2A receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50231951
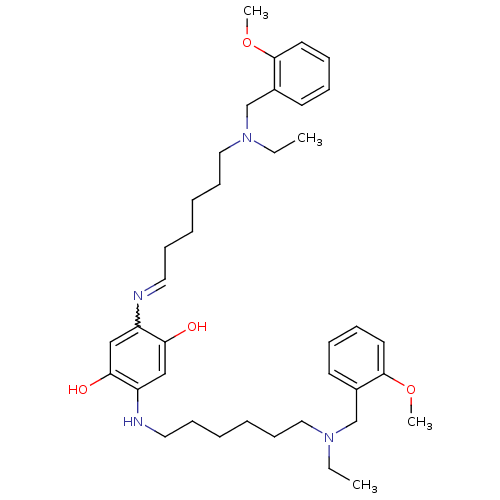
(2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...)Show SMILES CCN(CCCCCCNc1cc(O)c(cc1O)N=CCCCCCN(CC)Cc1ccccc1OC)Cc1ccccc1OC |w:18.18| Show InChI InChI=1S/C38H56N4O4/c1-5-41(29-31-19-11-13-21-37(31)45-3)25-17-9-7-15-23-39-33-27-36(44)34(28-35(33)43)40-24-16-8-10-18-26-42(6-2)30-32-20-12-14-22-38(32)46-4/h11-14,19-23,27-28,40,43-44H,5-10,15-18,24-26,29-30H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114464
BindingDB Entry DOI: 10.7270/Q2FB56XK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354607
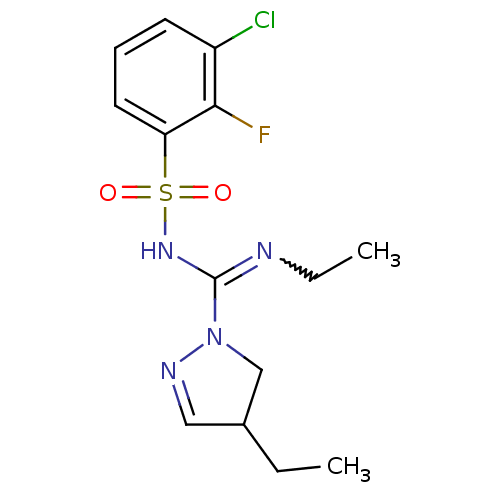
(CHEMBL1834337)Show SMILES CCN=C(NS(=O)(=O)c1cccc(Cl)c1F)N1CC(CC)C=N1 |w:2.1,c:22| Show InChI InChI=1S/C14H18ClFN4O2S/c1-3-10-8-18-20(9-10)14(17-4-2)19-23(21,22)12-7-5-6-11(15)13(12)16/h5-8,10H,3-4,9H2,1-2H3,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354585
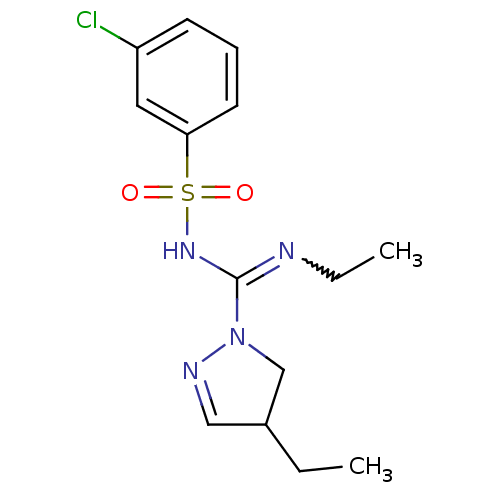
(CHEMBL1834226)Show SMILES CCN=C(NS(=O)(=O)c1cccc(Cl)c1)N1CC(CC)C=N1 |w:2.1,c:21| Show InChI InChI=1S/C14H19ClN4O2S/c1-3-11-9-17-19(10-11)14(16-4-2)18-22(20,21)13-7-5-6-12(15)8-13/h5-9,11H,3-4,10H2,1-2H3,(H,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354585

(CHEMBL1834226)Show SMILES CCN=C(NS(=O)(=O)c1cccc(Cl)c1)N1CC(CC)C=N1 |w:2.1,c:21| Show InChI InChI=1S/C14H19ClN4O2S/c1-3-11-9-17-19(10-11)14(16-4-2)18-22(20,21)13-7-5-6-12(15)8-13/h5-9,11H,3-4,10H2,1-2H3,(H,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354608
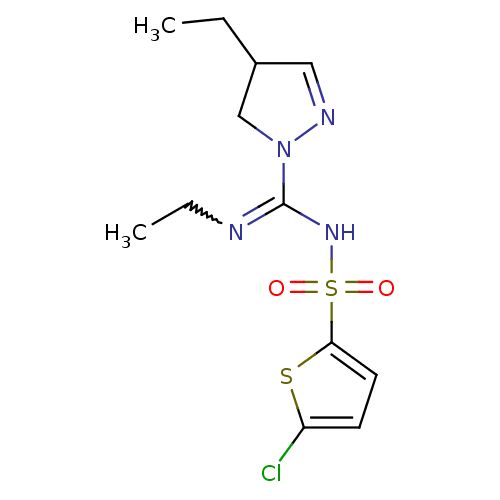
(CHEMBL1834342)Show SMILES CCN=C(NS(=O)(=O)c1ccc(Cl)s1)N1CC(CC)C=N1 |w:2.1,c:20| Show InChI InChI=1S/C12H17ClN4O2S2/c1-3-9-7-15-17(8-9)12(14-4-2)16-21(18,19)11-6-5-10(13)20-11/h5-7,9H,3-4,8H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM82465

(BIM 23023 | BIM-23023 | D-Phe-L-Cys(1)-L-Tyr-D-Trp...)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H](N)Cc1ccccc1 Show InChI InChI=1S/C49H65N11O10S2/c1-3-34-44(65)59-40(49(70)60-41(27(2)61)42(52)63)26-72-71-25-39(58-43(64)33(51)21-28-11-5-4-6-12-28)48(69)56-37(22-29-16-18-31(62)19-17-29)46(67)57-38(23-30-24-53-35-14-8-7-13-32(30)35)47(68)55-36(45(66)54-34)15-9-10-20-50/h4-8,11-14,16-19,24,27,33-34,36-41,53,61-62H,3,9-10,15,20-23,25-26,50-51H2,1-2H3,(H2,52,63)(H,54,66)(H,55,68)(H,56,69)(H,57,67)(H,58,64)(H,59,65)(H,60,70)/t27-,33-,34+,36+,37+,38-,39+,40+,41+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM82470

(3-(2-Naphtyl)-D-Ala-L-Cys(1)-L-Tyr-D-Trp-L-Lys-L-V...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H](N)Cc1ccc2ccccc2c1 Show InChI InChI=1S/C54H69N11O10S2/c1-29(2)45-54(75)63-44(53(74)65-46(30(3)66)47(57)68)28-77-76-27-43(62-48(69)38(56)23-32-15-18-33-10-4-5-11-34(33)22-32)52(73)60-41(24-31-16-19-36(67)20-17-31)50(71)61-42(25-35-26-58-39-13-7-6-12-37(35)39)51(72)59-40(49(70)64-45)14-8-9-21-55/h4-7,10-13,15-20,22,26,29-30,38,40-46,58,66-67H,8-9,14,21,23-25,27-28,55-56H2,1-3H3,(H2,57,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,75)(H,64,70)(H,65,74)/t30-,38-,40+,41+,42-,43+,44+,45+,46+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354609
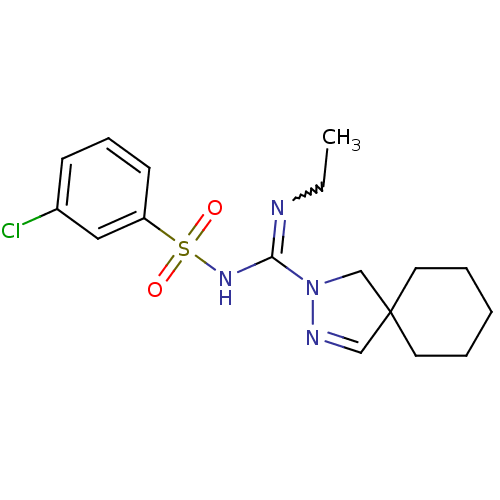
(CHEMBL1834348)Show SMILES CCN=C(NS(=O)(=O)c1cccc(Cl)c1)N1CC2(CCCCC2)C=N1 |w:2.1,c:25| Show InChI InChI=1S/C17H23ClN4O2S/c1-2-19-16(21-25(23,24)15-8-6-7-14(18)11-15)22-13-17(12-20-22)9-4-3-5-10-17/h6-8,11-12H,2-5,9-10,13H2,1H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM82253

(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354610
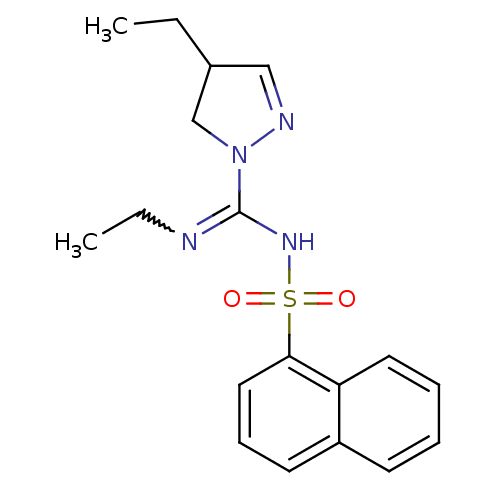
(CHEMBL1834338)Show SMILES CCN=C(NS(=O)(=O)c1cccc2ccccc12)N1CC(CC)C=N1 |w:2.1,c:25| Show InChI InChI=1S/C18H22N4O2S/c1-3-14-12-20-22(13-14)18(19-4-2)21-25(23,24)17-11-7-9-15-8-5-6-10-16(15)17/h5-12,14H,3-4,13H2,1-2H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206725

(CHEMBL3947276)Show InChI InChI=1S/C15H22N2/c1-2-4-12-5-3-6-15-14(12)8-7-13-11-16-9-10-17(13)15/h3,5-6,13,16H,2,4,7-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2A receptor (unknown origin) expressed in HEK293 cells assessed as [3H]inositol phosphate accumulation after 2 hrs by scintil... |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50206718
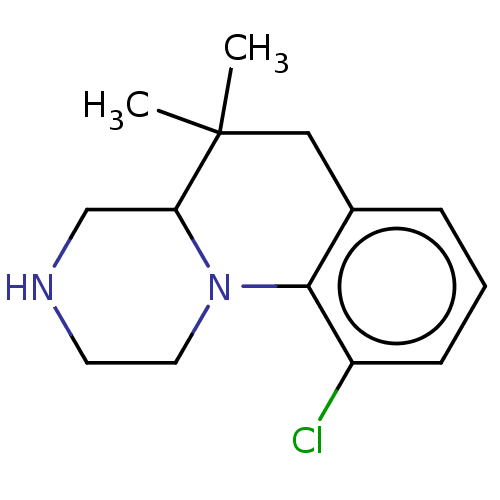
(CHEMBL3895972)Show InChI InChI=1S/C14H19ClN2/c1-14(2)8-10-4-3-5-11(15)13(10)17-7-6-16-9-12(14)17/h3-5,12,16H,6-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2B receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354611
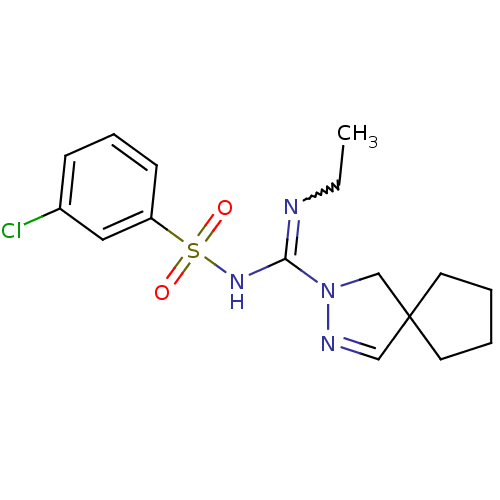
(CHEMBL1834347)Show SMILES CCN=C(NS(=O)(=O)c1cccc(Cl)c1)N1CC2(CCCC2)C=N1 |w:2.1,c:24| Show InChI InChI=1S/C16H21ClN4O2S/c1-2-18-15(21-12-16(11-19-21)8-3-4-9-16)20-24(22,23)14-7-5-6-13(17)10-14/h5-7,10-11H,2-4,8-9,12H2,1H3,(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50059090

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cedars-Sinai Research Institute
Curated by PDSP Ki Database
| |
J Clin Invest 99: 789-98 (1997)
Article DOI: 10.1172/JCI119225
BindingDB Entry DOI: 10.7270/Q2H70DCK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354612
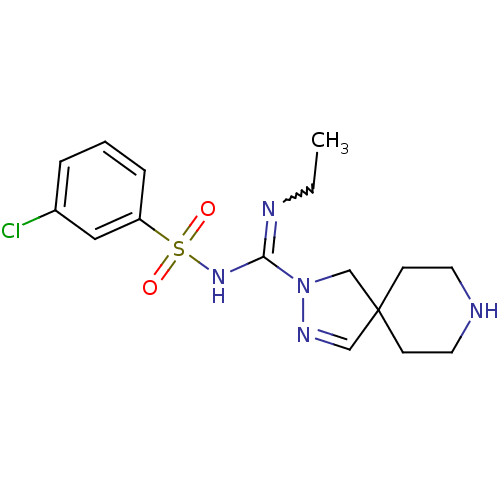
(CHEMBL1834350)Show SMILES CCN=C(NS(=O)(=O)c1cccc(Cl)c1)N1CC2(CCNCC2)C=N1 |w:2.1,c:25| Show InChI InChI=1S/C16H22ClN5O2S/c1-2-19-15(21-25(23,24)14-5-3-4-13(17)10-14)22-12-16(11-20-22)6-8-18-9-7-16/h3-5,10-11,18H,2,6-9,12H2,1H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354585

(CHEMBL1834226)Show SMILES CCN=C(NS(=O)(=O)c1cccc(Cl)c1)N1CC(CC)C=N1 |w:2.1,c:21| Show InChI InChI=1S/C14H19ClN4O2S/c1-3-11-9-17-19(10-11)14(16-4-2)18-22(20,21)13-7-5-6-12(15)8-13/h5-9,11H,3-4,10H2,1-2H3,(H,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001915
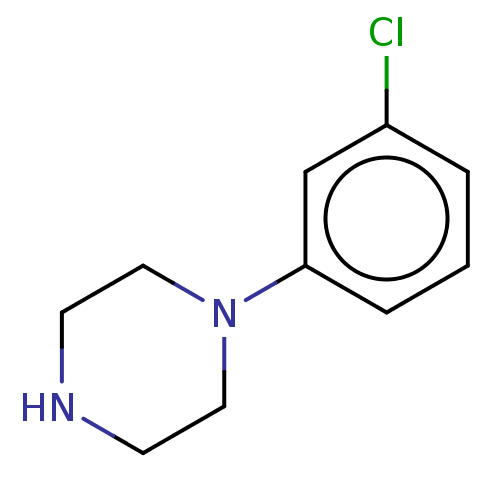
(1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...)Show InChI InChI=1S/C10H13ClN2/c11-9-2-1-3-10(8-9)13-6-4-12-5-7-13/h1-3,8,12H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2C receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50206724

(CHEMBL3912721)Show InChI InChI=1S/C17H24N2/c1-3-13(4-1)11-14-5-2-6-17-16(14)8-7-15-12-18-9-10-19(15)17/h2,5-6,13,15,18H,1,3-4,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from recombinant human 5-HT2A receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting |
Bioorg Med Chem Lett 26: 5877-5882 (2016)
Article DOI: 10.1016/j.bmcl.2016.11.016
BindingDB Entry DOI: 10.7270/Q29025R3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354614
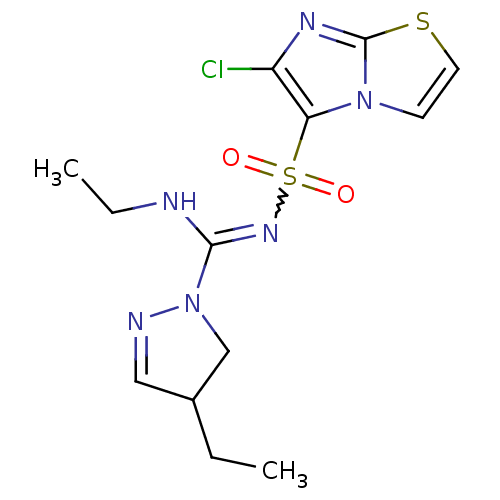
(CHEMBL1834341)Show SMILES CCNC(=NS(=O)(=O)c1c(Cl)nc2sccn12)N1CC(CC)C=N1 |w:4.4,c:24| Show InChI InChI=1S/C13H17ClN6O2S2/c1-3-9-7-16-20(8-9)12(15-4-2)18-24(21,22)11-10(14)17-13-19(11)5-6-23-13/h5-7,9H,3-4,8H2,1-2H3,(H,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354613
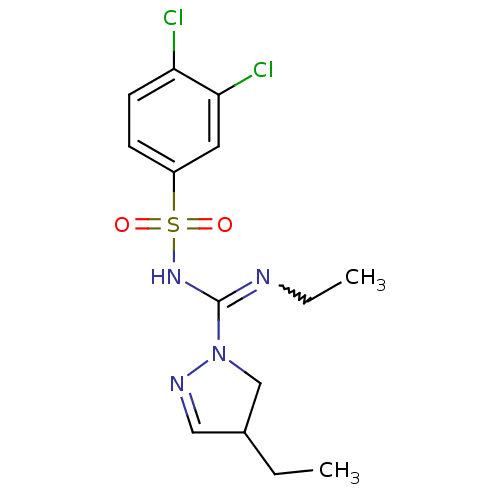
(CHEMBL1834333)Show SMILES CCN=C(NS(=O)(=O)c1ccc(Cl)c(Cl)c1)N1CC(CC)C=N1 |w:2.1,c:22| Show InChI InChI=1S/C14H18Cl2N4O2S/c1-3-10-8-18-20(9-10)14(17-4-2)19-23(21,22)11-5-6-12(15)13(16)7-11/h5-8,10H,3-4,9H2,1-2H3,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354615
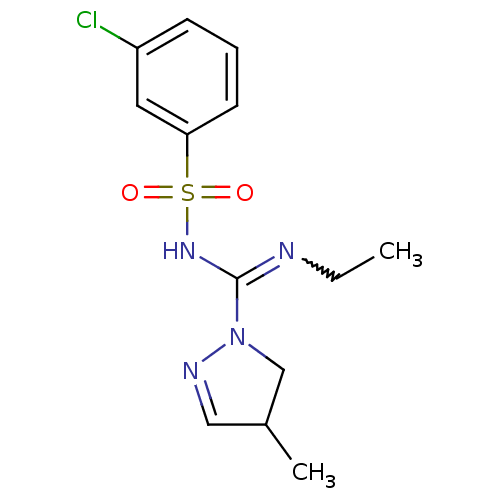
(CHEMBL1834230)Show SMILES CCN=C(NS(=O)(=O)c1cccc(Cl)c1)N1CC(C)C=N1 |w:2.1,c:20| Show InChI InChI=1S/C13H17ClN4O2S/c1-3-15-13(18-9-10(2)8-16-18)17-21(19,20)12-6-4-5-11(14)7-12/h4-8,10H,3,9H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354616
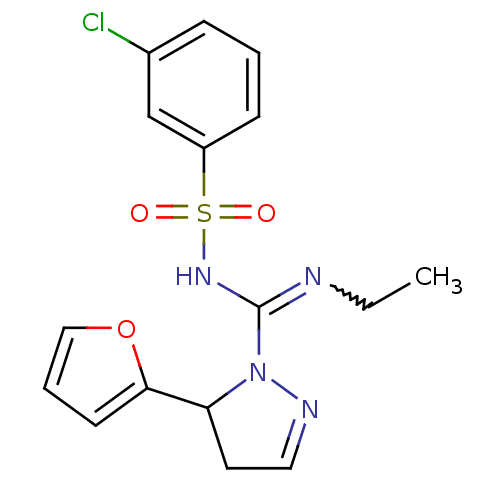
(CHEMBL1834238)Show SMILES CCN=C(NS(=O)(=O)c1cccc(Cl)c1)N1N=CCC1c1ccco1 |w:2.1,c:17| Show InChI InChI=1S/C16H17ClN4O3S/c1-2-18-16(20-25(22,23)13-6-3-5-12(17)11-13)21-14(8-9-19-21)15-7-4-10-24-15/h3-7,9-11,14H,2,8H2,1H3,(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50584167
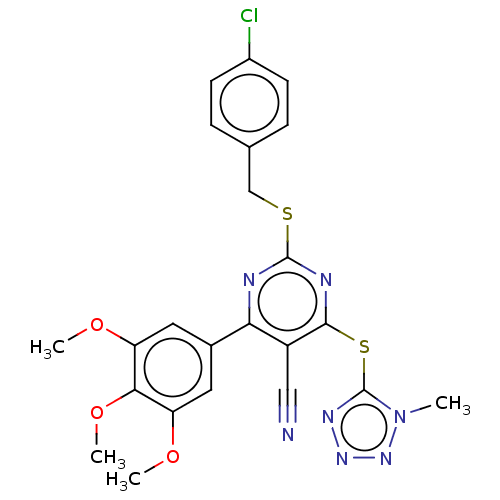
(CHEMBL5085822)Show SMILES COc1cc(cc(OC)c1OC)-c1nc(SCc2ccc(Cl)cc2)nc(Sc2nnnn2C)c1C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to DCN1 (unknown origin) assessed as inhibitory constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01207
BindingDB Entry DOI: 10.7270/Q2JD51NJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50354617
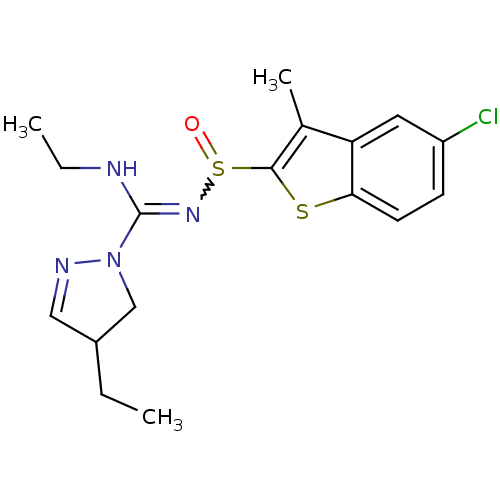
(CHEMBL1834340)Show SMILES CCNC(=NS(=O)c1sc2ccc(Cl)cc2c1C)N1CC(CC)C=N1 |w:4.4,c:25| Show InChI InChI=1S/C17H21ClN4OS2/c1-4-12-9-20-22(10-12)17(19-5-2)21-25(23)16-11(3)14-8-13(18)6-7-15(14)24-16/h6-9,12H,4-5,10H2,1-3H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting |
J Med Chem 54: 7030-54 (2011)
Article DOI: 10.1021/jm200466r
BindingDB Entry DOI: 10.7270/Q2J103JB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data