

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513345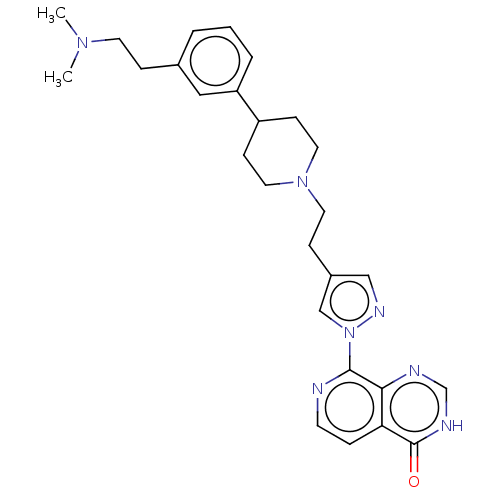 (CHEMBL4438830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM5B (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513361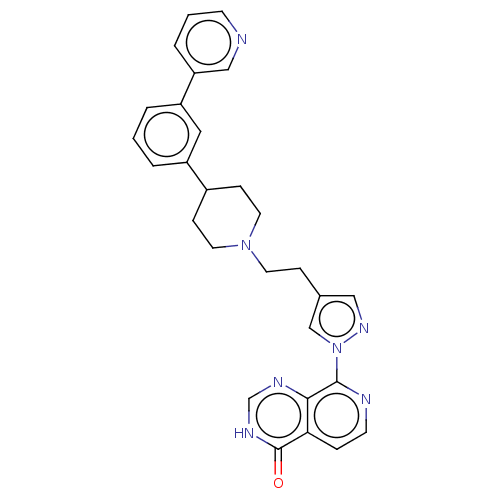 (CHEMBL4567766) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM5B (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513360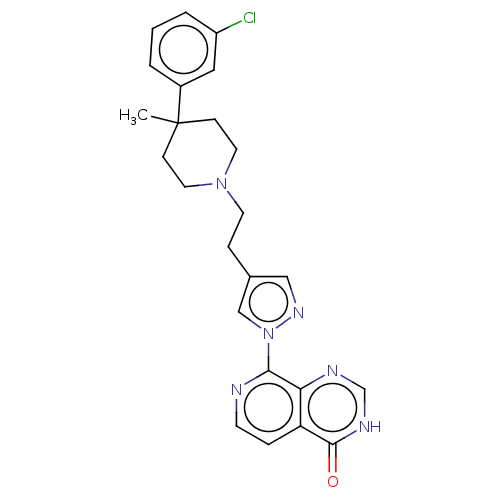 (CHEMBL4573390) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM5B (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50513345 (CHEMBL4438830) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM4A (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50151923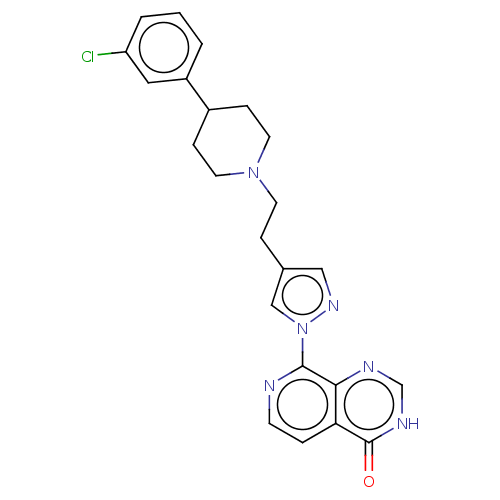 (CHEMBL3774537) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM5B (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50151923 (CHEMBL3774537) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM4A (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513344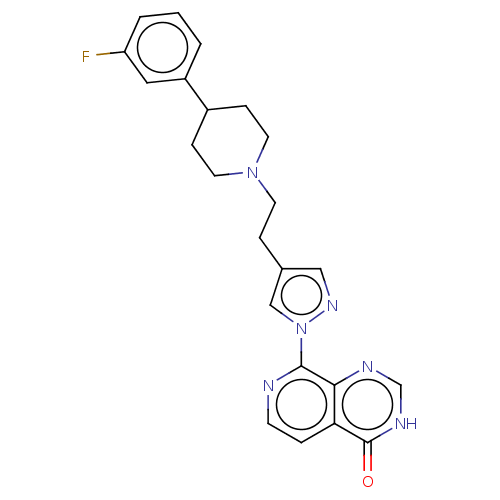 (CHEMBL4447515) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM5B (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50513361 (CHEMBL4567766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM4A (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50513343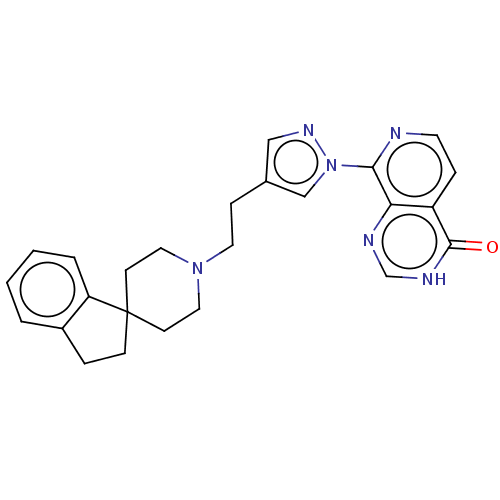 (CHEMBL4449500) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM4A (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50513344 (CHEMBL4447515) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM4A (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50513348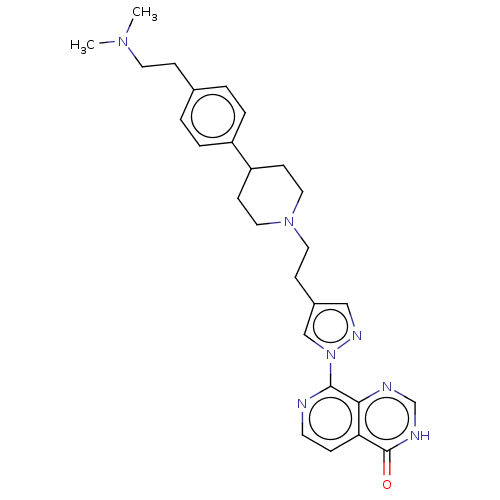 (CHEMBL4585876) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM4A (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513348 (CHEMBL4585876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM5B (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50513360 (CHEMBL4573390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM4A (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513343 (CHEMBL4449500) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM5B (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513346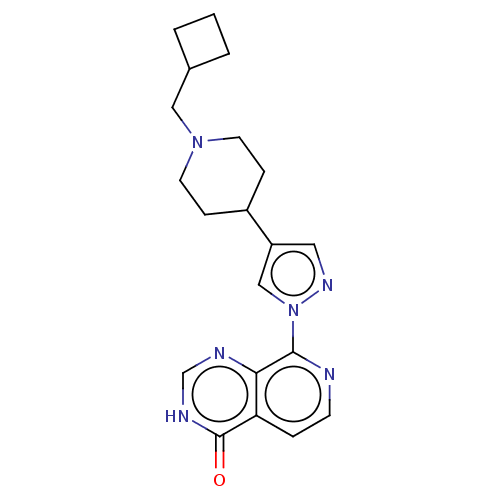 (CHEMBL4525269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM5B (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50513346 (CHEMBL4525269) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to KDM4A (unknown origin) | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24828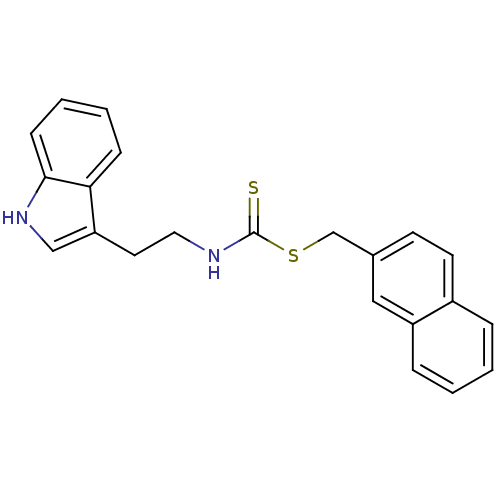 (Brassinin derivative, 16 | N-[2-(1H-indol-3-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.16E+4 | -29.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24825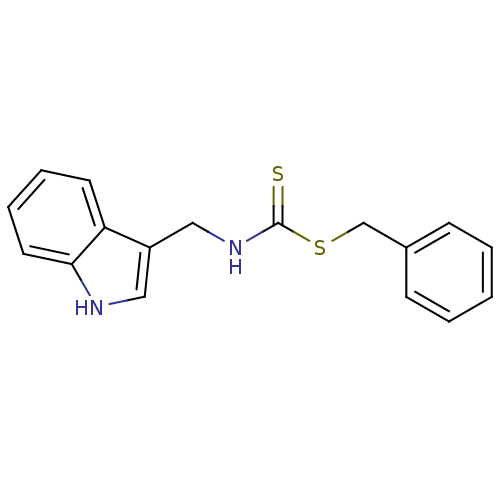 ((benzylsulfanyl)-N-(1H-indol-3-ylmethyl)carbothioa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.32E+4 | -29.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24827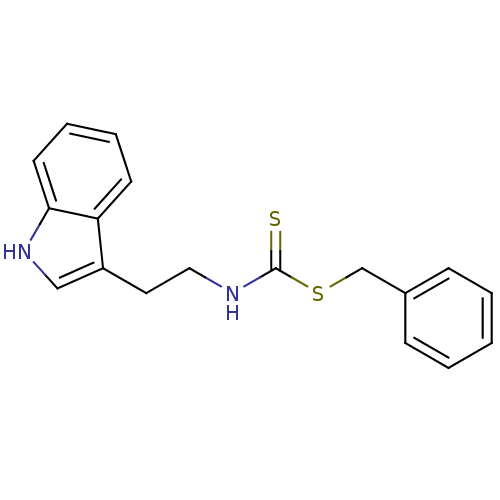 ((benzylsulfanyl)-N-[2-(1H-indol-3-yl)ethyl]carboth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.72E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24830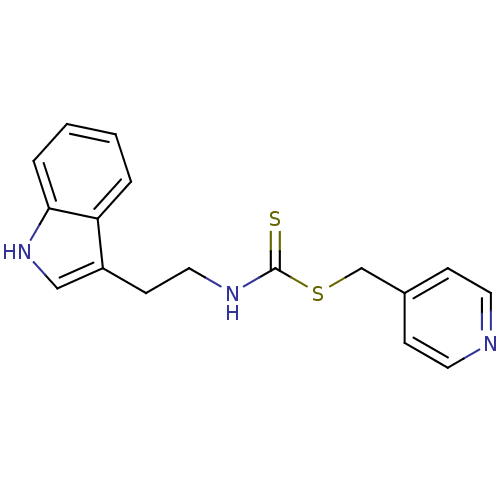 (Brassinin derivative, 18 | N-[2-(1H-indol-3-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.05E+4 | -27.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24829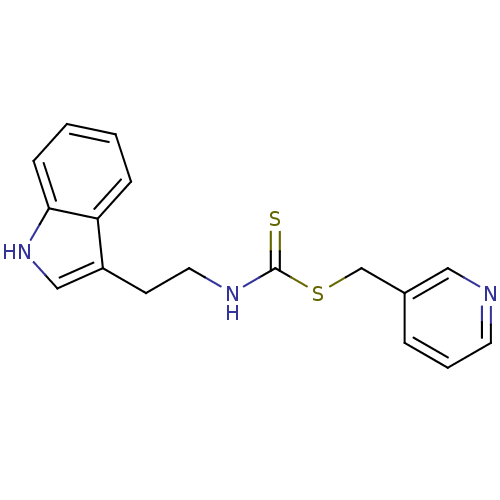 (Brassinin derivative, 17 | N-[2-(1H-indol-3-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.84E+4 | -27.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24816 (Brassinin derivative, 4 | N-[3-(1H-indol-3-yl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24824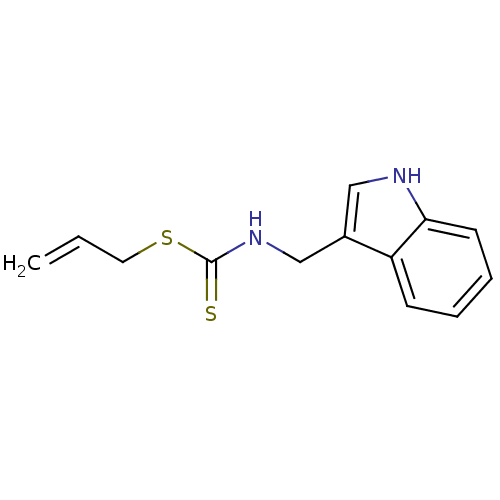 (Brassinin derivative, 12 | N-(1H-indol-3-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+4 | -26.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24815 (Brassinin derivative, 3 | N-[2-(1-benzothiophen-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.10E+4 | -26.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24817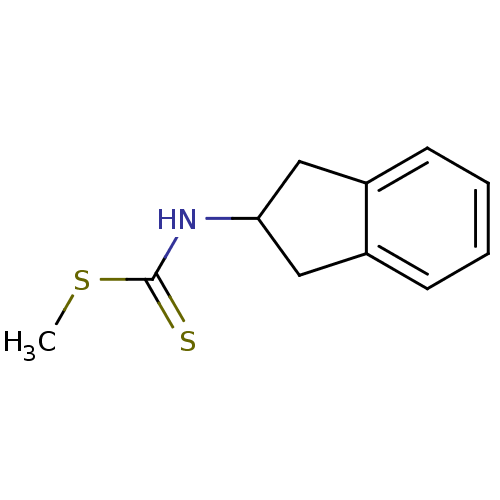 (Brassinin derivative, 5 | N-(2,3-dihydro-1H-inden-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.21E+4 | -26.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24819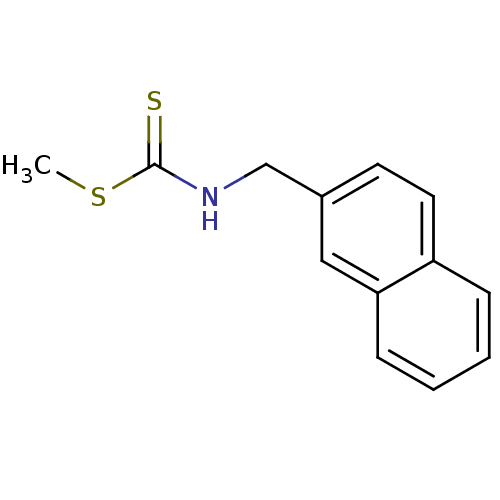 ((methylsulfanyl)-N-(naphthalen-2-ylmethyl)carbothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.76E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24821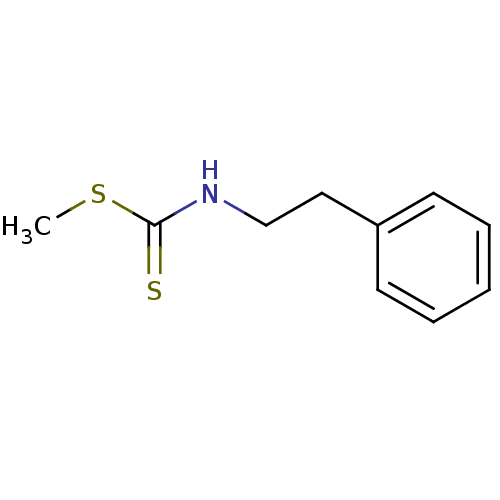 ((methylsulfanyl)-N-(2-phenylethyl)carbothioamide |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 6.24E+4 | -25.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24820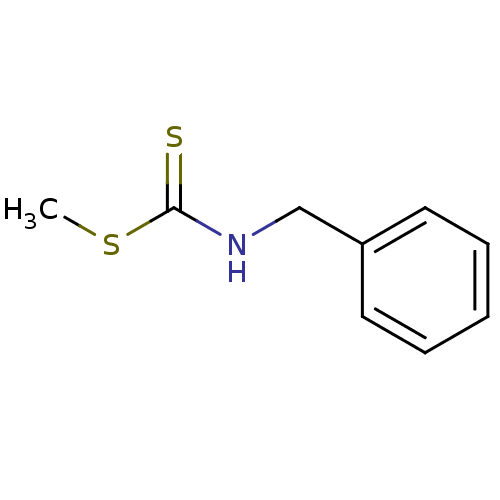 (Brassinin derivative, 8 | N-benzyl(methylsulfanyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7.24E+4 | -24.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24814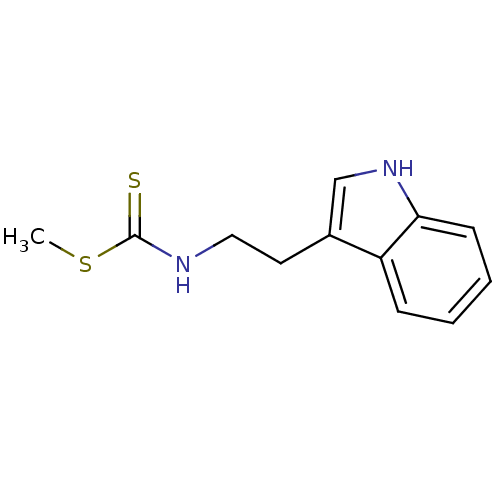 (Brassinin derivative, 2 | N-[2-(1H-indol-3-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.25E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24813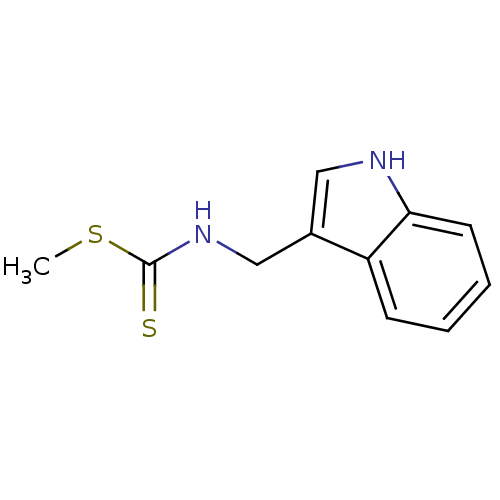 (Brassinin, 1 | N-(1H-indol-3-ylmethyl)(methylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.77E+4 | -23.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24822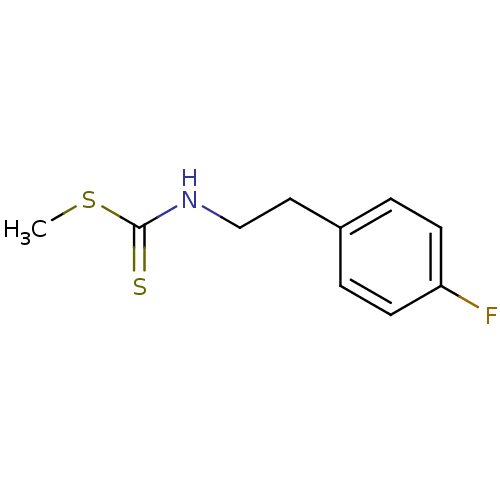 (Brassinin derivative, 10 | N-[2-(4-fluorophenyl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49E+5 | -22.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24818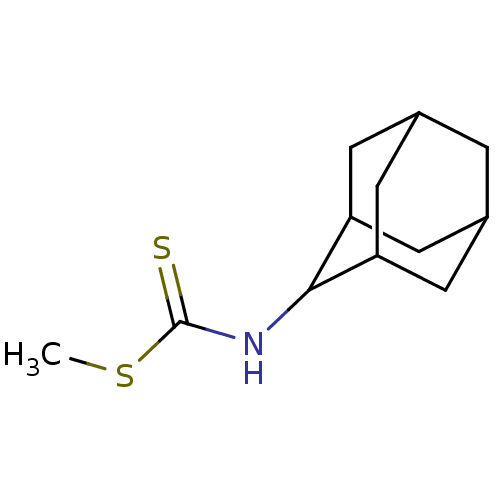 (Brassinin derivative, 6 | N-(adamantan-2-yl)(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+5 | -22.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24834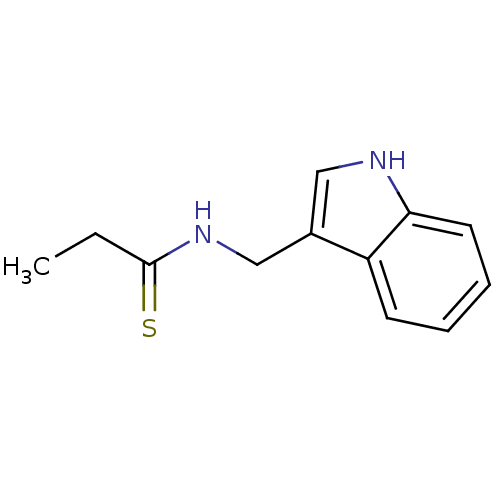 (N-(1H-indol-3-ylmethyl)propanethioamide | thioamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.02E+5 | -21.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24836 (4-(1H-indol-3-ylmethyl)-2-methyl-1,3-thiazole | th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.29E+5 | -20.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24832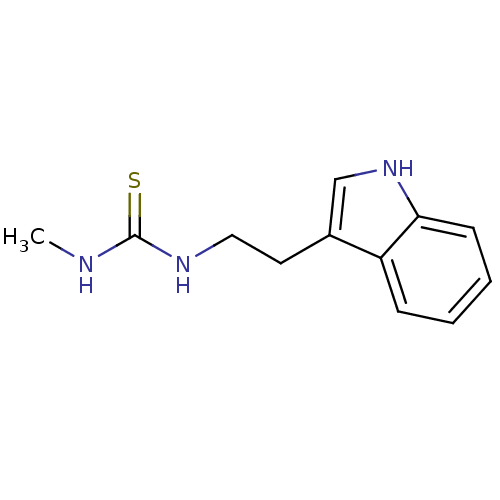 (1-[2-(1H-indol-3-yl)ethyl]-3-methylthiourea | thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.42E+5 | -20.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24826 ((hexylsulfanyl)-N-(1H-indol-3-ylmethyl)carbothioam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.64E+5 | -20.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24823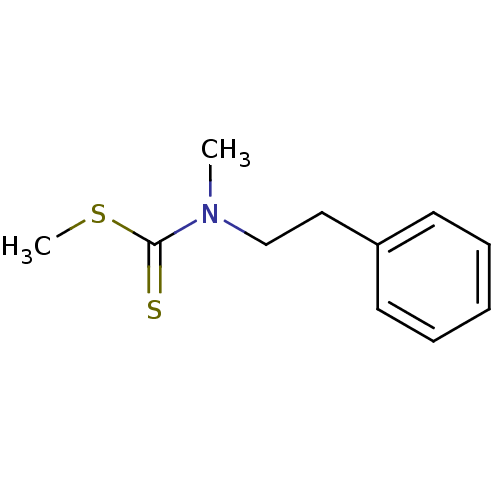 (Brassinin derivative, 11 | N-methyl(methylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.27E+6 | -17.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24835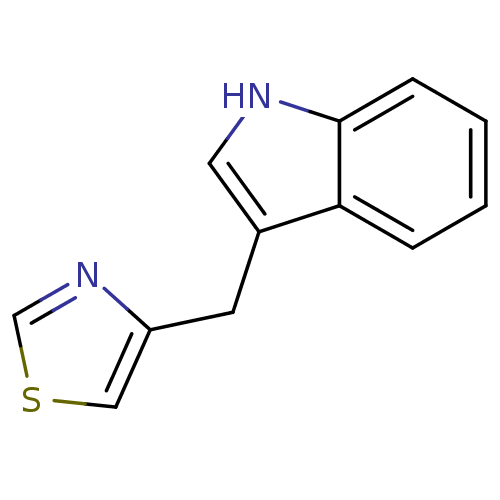 (4-(1H-indol-3-ylmethyl)-1,3-thiazole | thiazole, 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.29E+6 | -17.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50513345 (CHEMBL4438830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human KDM4B assessed as decrease in demethylation of substrate using peptide (ARTKQTARK(Me3)STGGKAPRKQLA-GGKbiotin) as subs... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50513343 (CHEMBL4449500) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human KDM4B assessed as decrease in demethylation of substrate using peptide (ARTKQTARK(Me3)STGGKAPRKQLA-GGKbiotin) as subs... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513343 (CHEMBL4449500) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human KDM5B assessed as decrease in demethylation of substrate using peptide ARTK(me3)QTARKSTGGKAPRKQLA-GGK-biotin as subst... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513345 (CHEMBL4438830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) assessed as decrease in demethylation of substrate using peptide (H3(1-21)K4-Me3-GGKBiotin) as substrate and 2OG... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50513345 (CHEMBL4438830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human KDM4B assessed as decrease in demethylation of substrate using peptide (ARTKQTARK(Me3)STGGKAPRKQLA-GGKbiotin) as subs... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513343 (CHEMBL4449500) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human KDM5B assessed as decrease in demethylation of substrate using peptide ARTK(me3)QTARKSTGGKAPRKQLA-GGK-biotin as subst... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153099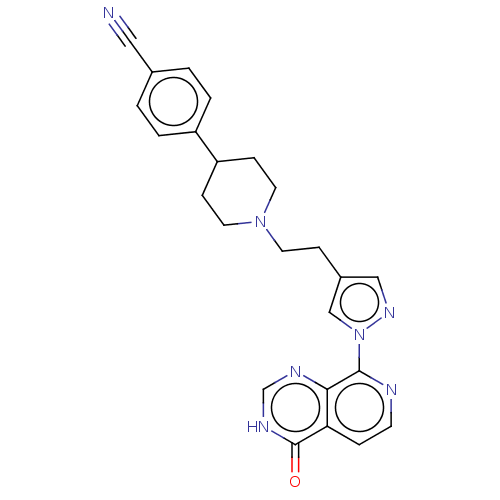 (CHEMBL3775814) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50513345 (CHEMBL4438830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged recombinant human KDM4B (1 to 500 residues) expressed in baculovirus infected Sf9 insect cells assessed as decrea... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50513343 (CHEMBL4449500) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human KDM4B assessed as decrease in demethylation of substrate using peptide (ARTKQTARK(Me3)STGGKAPRKQLA-GGKbiotin) as subs... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50513345 (CHEMBL4438830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human KDM4B assessed as decrease in demethylation of substrate using peptide (ARTKQTARK(Me3)STGGKAPRKQLA-GGKbiotin) as subs... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50513343 (CHEMBL4449500) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human KDM5B assessed as decrease in demethylation of substrate using peptide ARTK(me3)QTARKSTGGKAPRKQLA-GGK-biotin as subst... | Eur J Med Chem 177: 316-337 (2019) Article DOI: 10.1016/j.ejmech.2019.05.041 BindingDB Entry DOI: 10.7270/Q25B05TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153101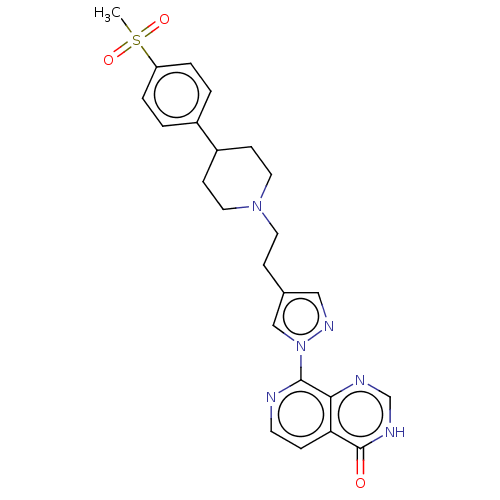 (CHEMBL3774665) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 551 total ) | Next | Last >> |