

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123462 (US8741923, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.230 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123463 (US8741923, 36) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.490 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM86210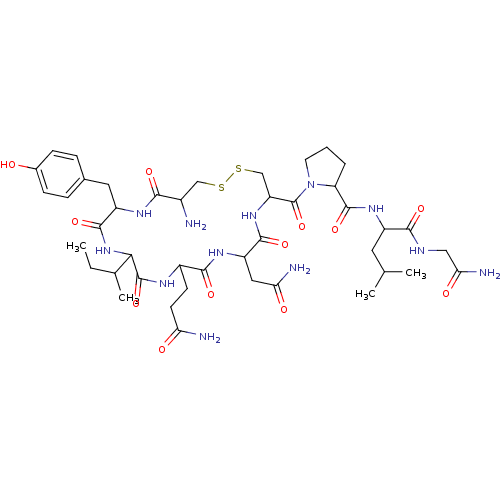 (CAS_50-56-6 | NSC_439302 | Oxytocin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123466 (US8741923, 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326722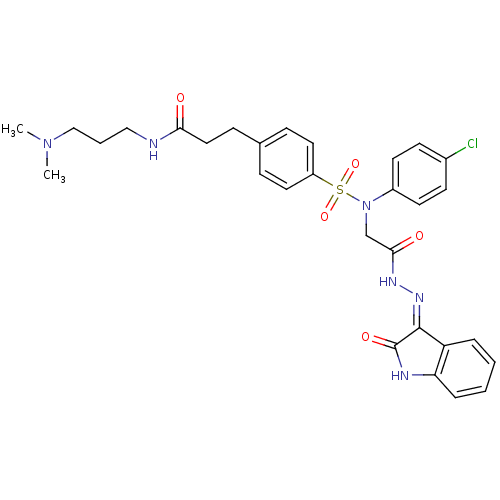 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326722 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123457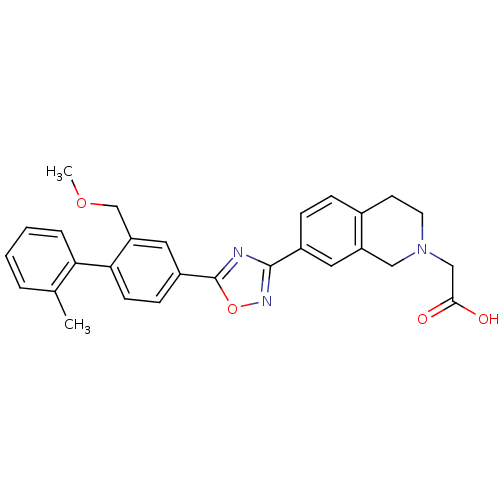 (US8741923, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM86210 (CAS_50-56-6 | NSC_439302 | Oxytocin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123464 (US8741923, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.75 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123455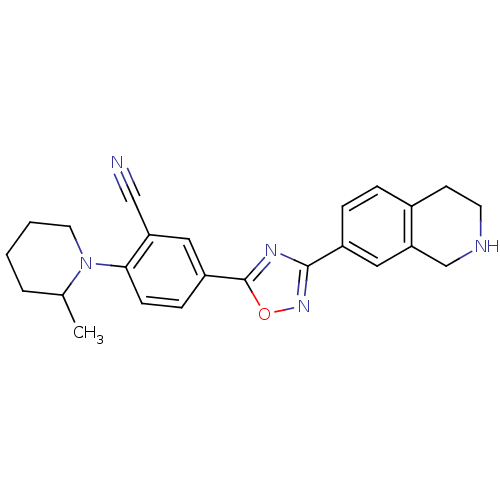 (US8741923, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.800 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123465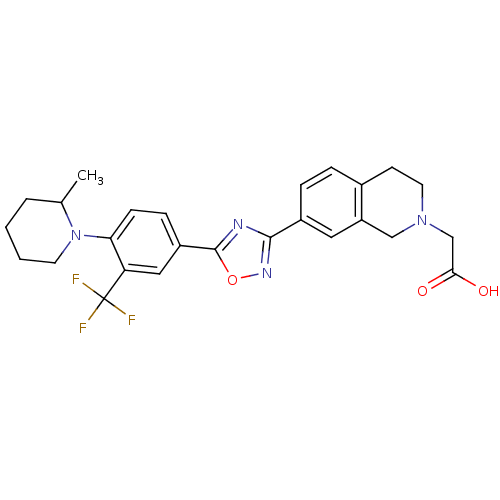 (US8741923, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410618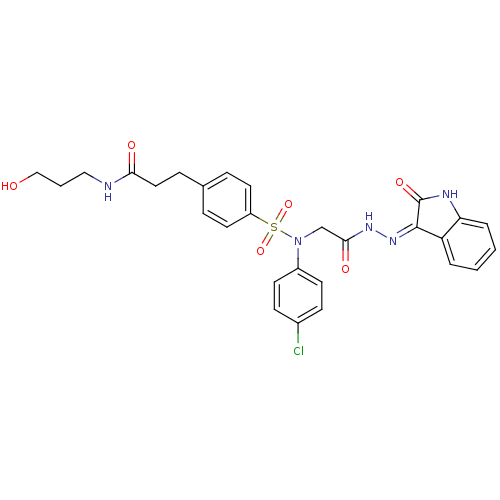 (CHEMBL2113181) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410616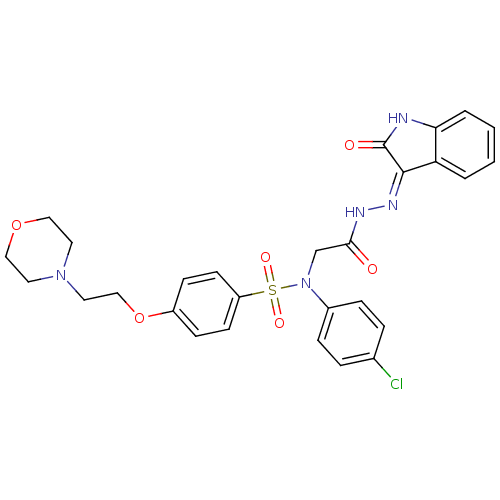 (CHEMBL2113185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123456 (US8741923, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123467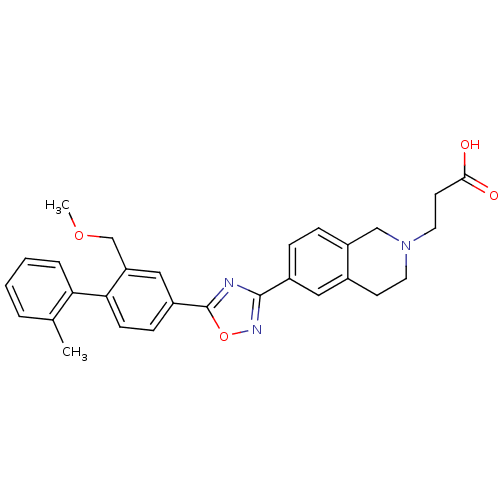 (US8741923, 40) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410625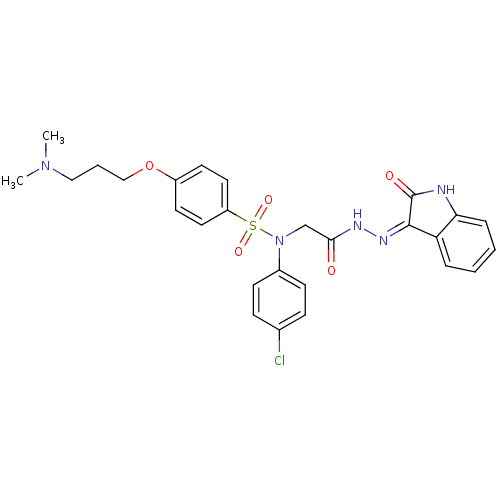 (CHEMBL2113201) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410634 (CHEMBL2113189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM86210 (CAS_50-56-6 | NSC_439302 | Oxytocin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410622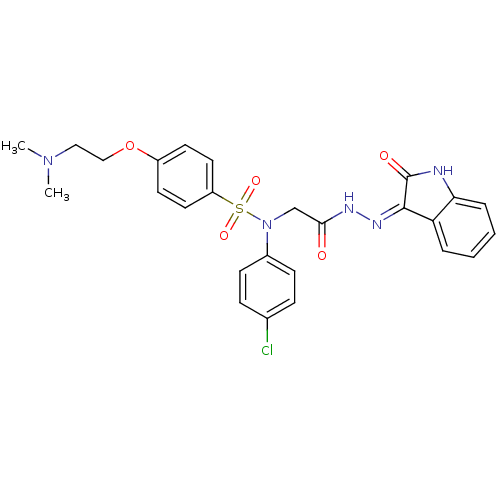 (CHEMBL2113203) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410634 (CHEMBL2113189) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123453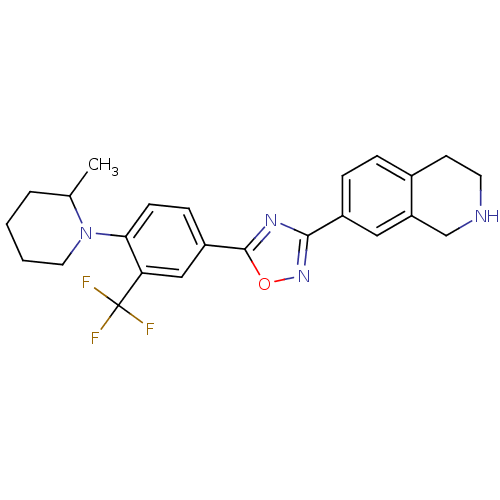 (US8741923, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410619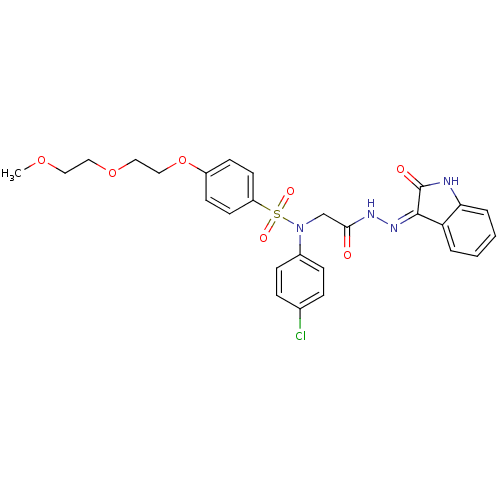 (CHEMBL2113208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123459 (US8741923, 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 3 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM86209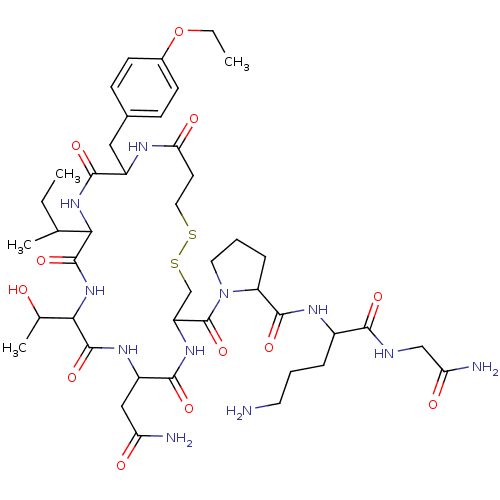 (Atosiban | CAS_90779-69-4 | NSC_0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410640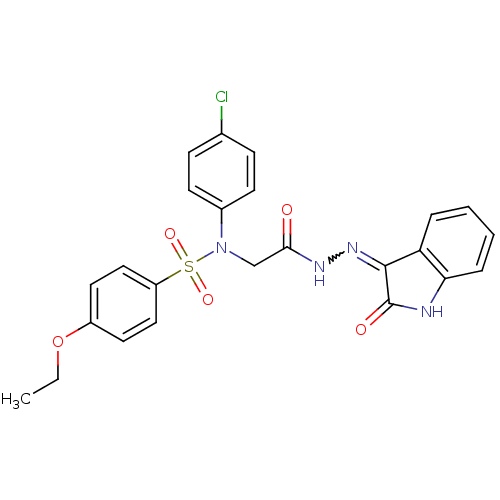 (CHEMBL2113198) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123454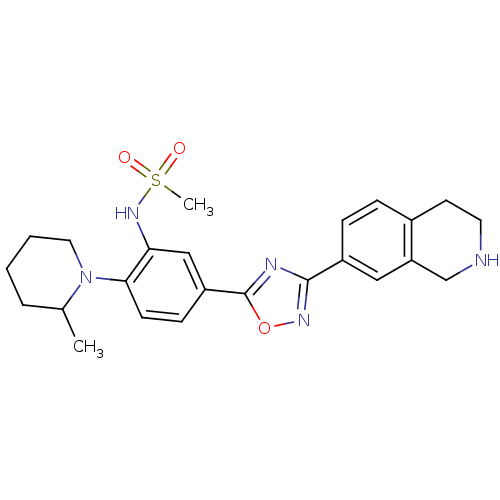 (US8741923, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410623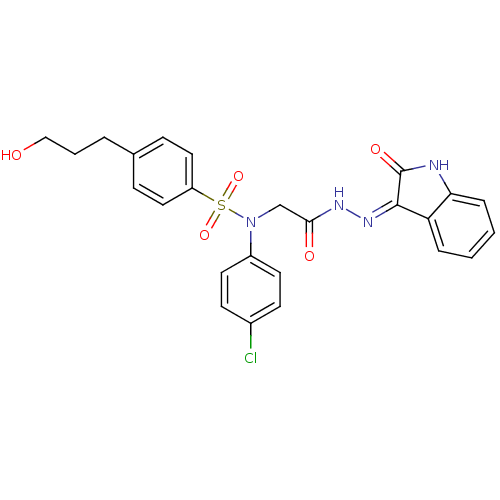 (CHEMBL2113179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123461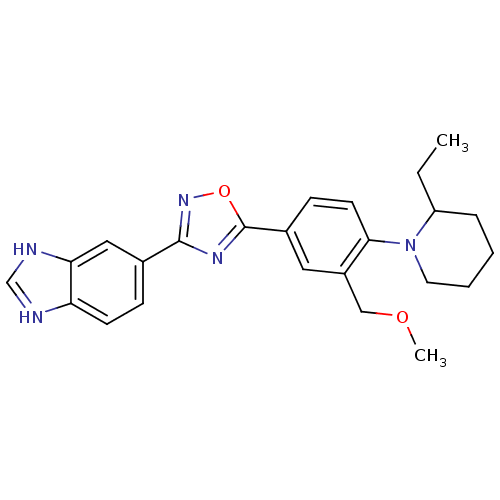 (US8741923, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.26 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410625 (CHEMBL2113201) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123449 (US8741923, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123452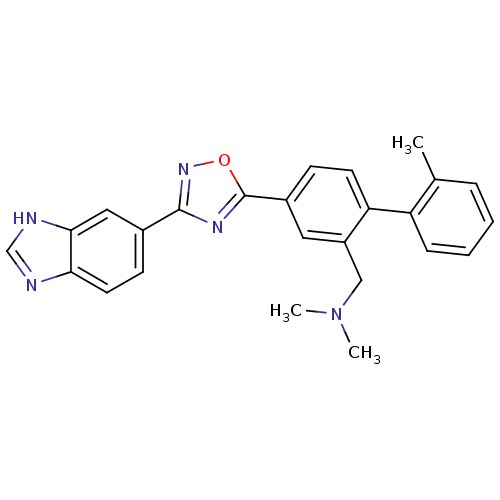 (US8741923, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123451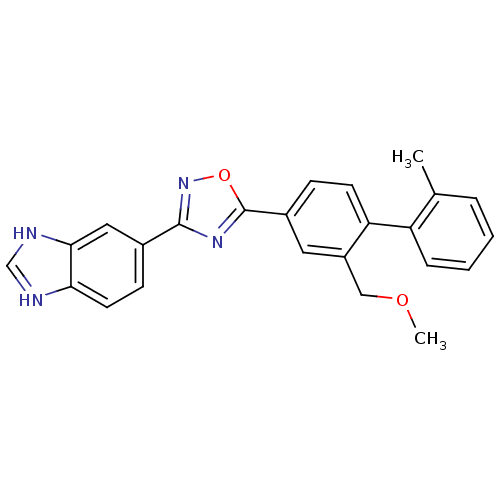 (US8741923, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410621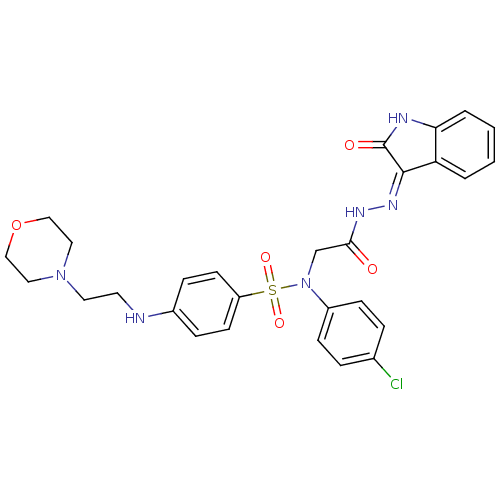 (CHEMBL2113177) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410635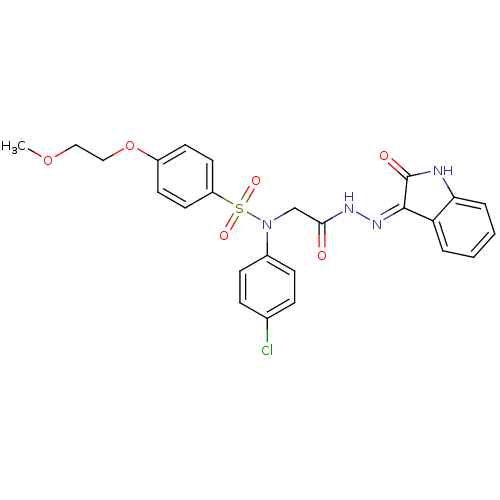 (CHEMBL2113211) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410616 (CHEMBL2113185) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410638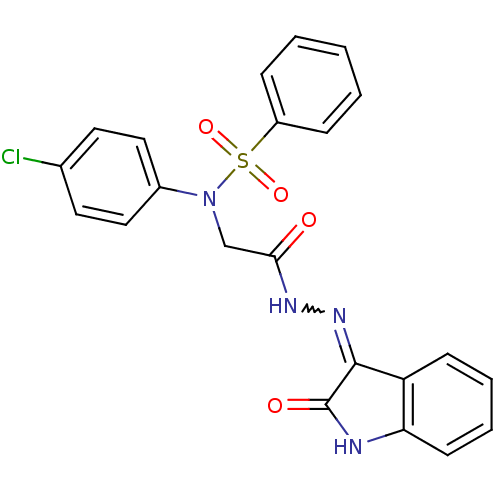 (CHEMBL2113207) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410630 (CHEMBL2113209) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM123460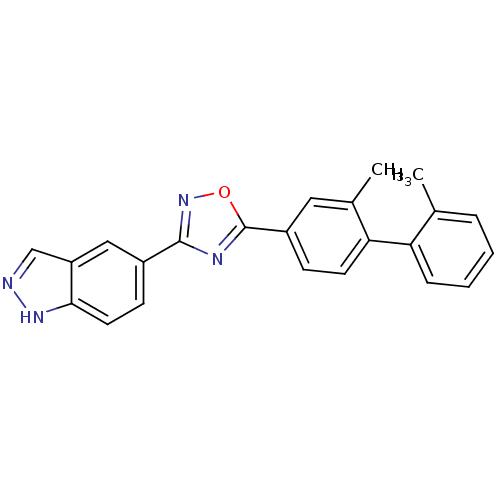 (US8741923, 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA US Patent | Assay Description Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... | US Patent US8741923 (2014) BindingDB Entry DOI: 10.7270/Q2PK0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410619 (CHEMBL2113208) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178153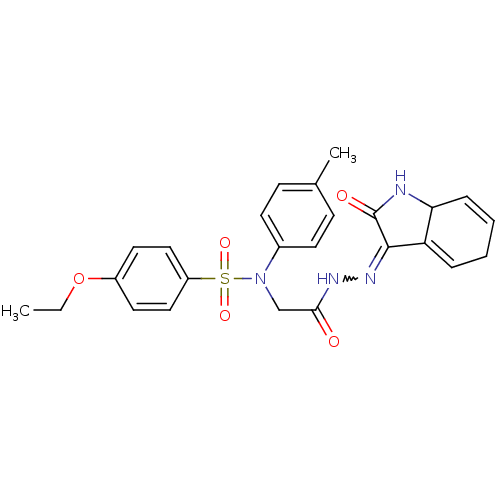 (4-ethoxy-N-(4-methylphenyl)-N-{2-oxo-2-[2-(2-oxo-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178186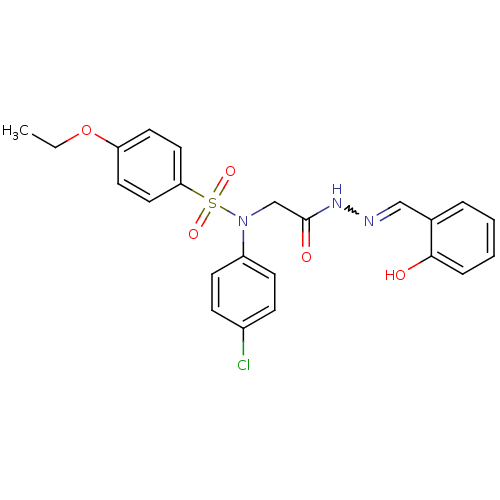 (CHEMBL372122 | N-(4-chlorophenyl)-4-ethoxy-N-{2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410622 (CHEMBL2113203) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326713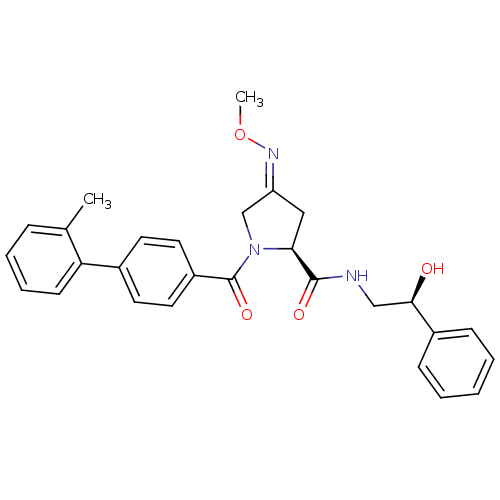 ((2S,4Z)-N-[(2S)-2-hydroxy-2-phenylethyl]-4-(methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178209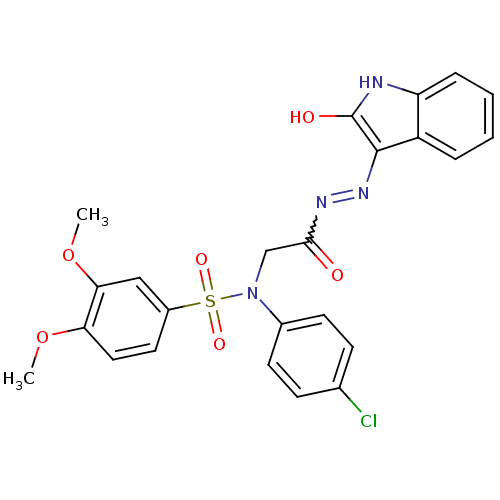 (CHEMBL371223 | N-(4-chlorophenyl)-3,4-dimethoxy-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410637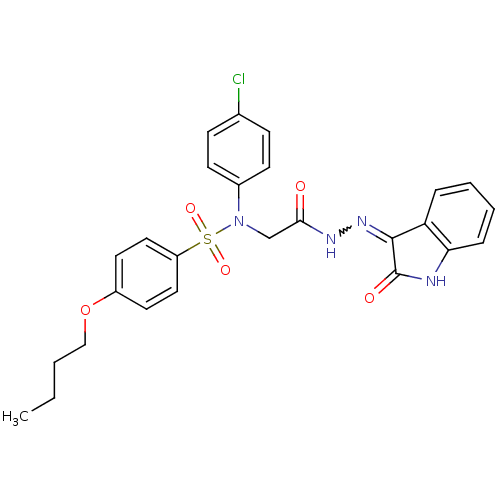 (CHEMBL2113210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410624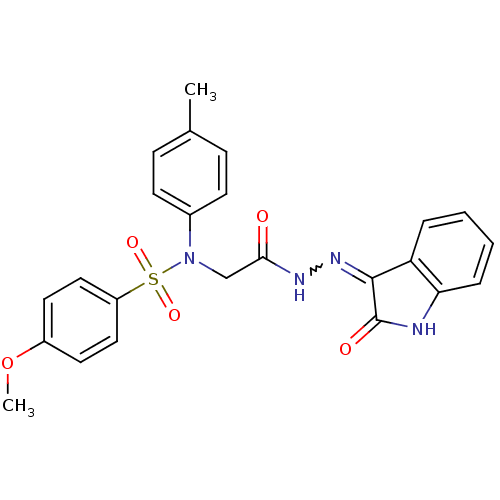 (CHEMBL2113215) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410628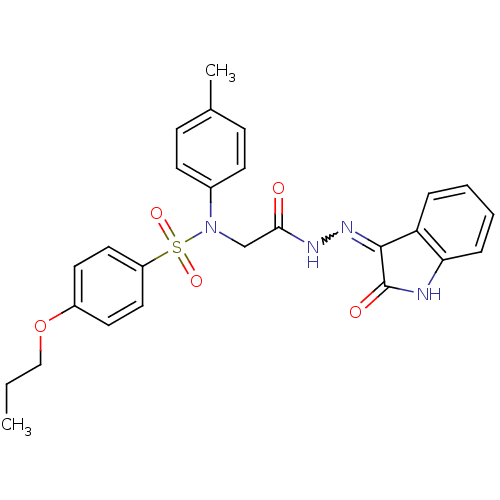 (CHEMBL2113216) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410621 (CHEMBL2113177) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50178153 (4-ethoxy-N-(4-methylphenyl)-N-{2-oxo-2-[2-(2-oxo-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM86209 (Atosiban | CAS_90779-69-4 | NSC_0) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 285 total ) | Next | Last >> |