

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13122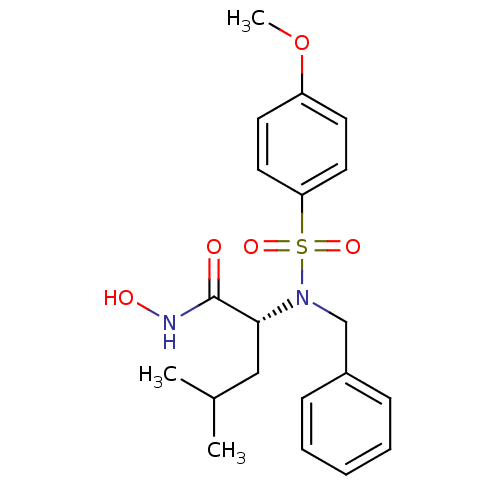 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13133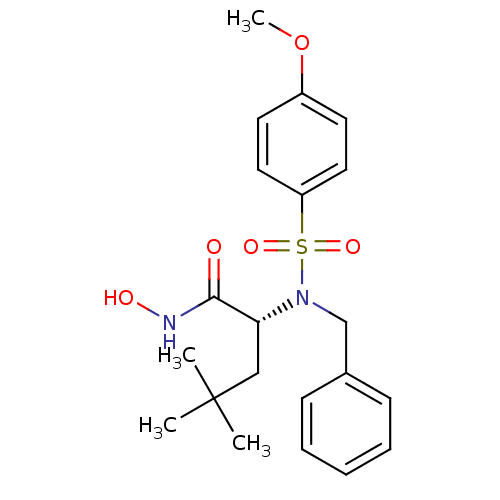 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13121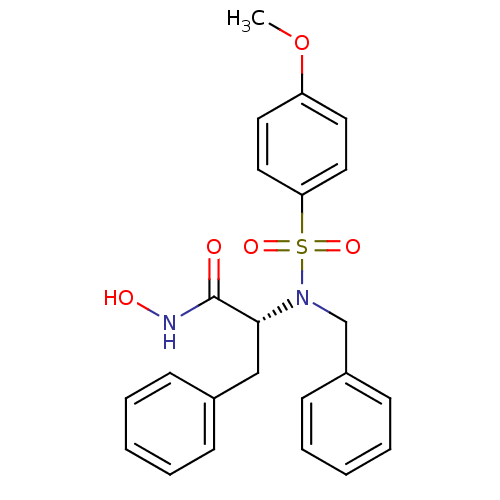 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13127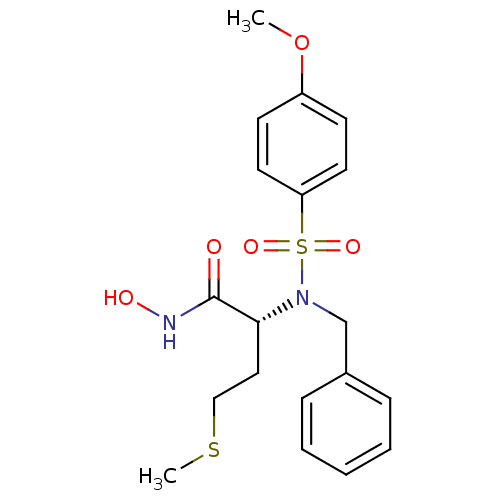 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13126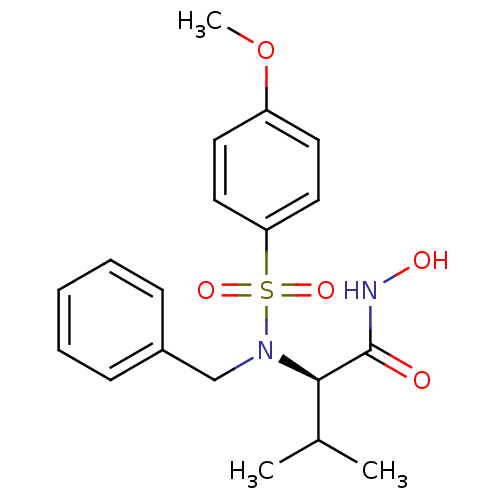 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13119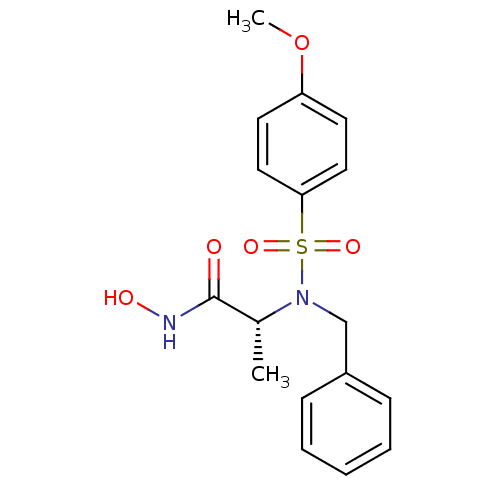 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13100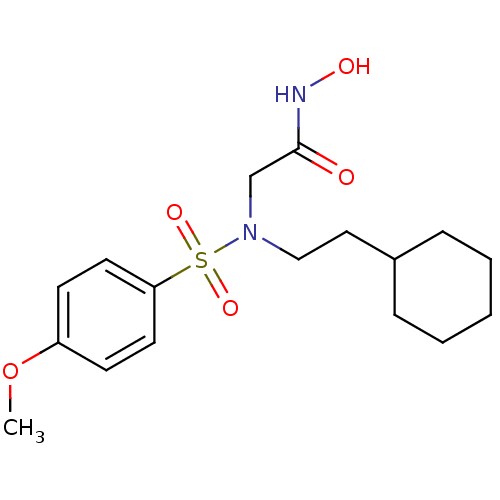 (2-[(2-cyclohexylethyl)(4-methoxybenzene)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13135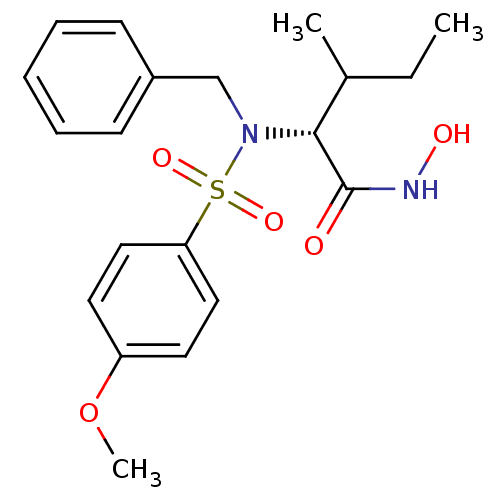 ((2R,3S)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13141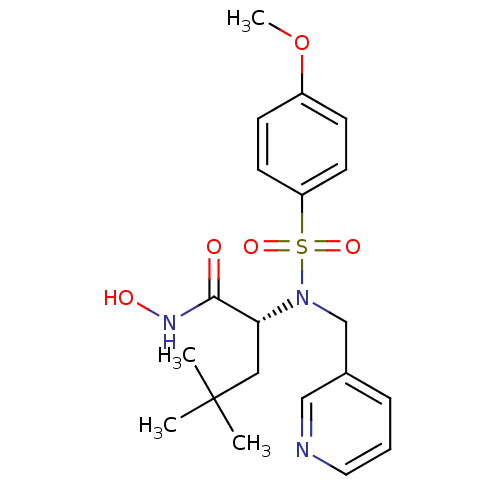 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13134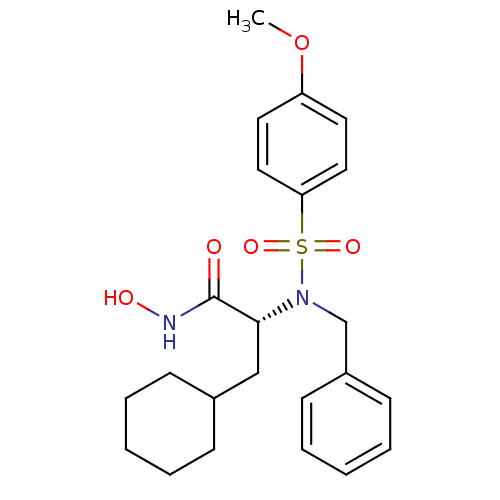 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13137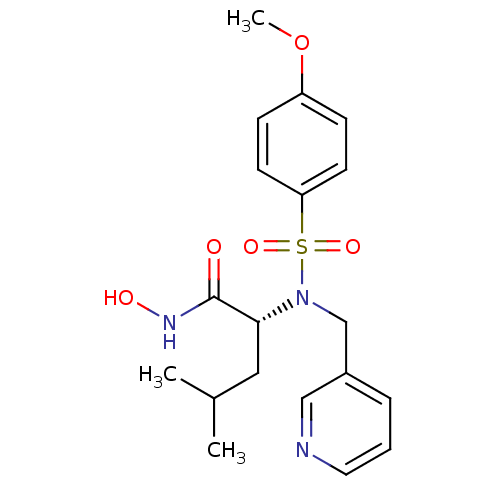 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13131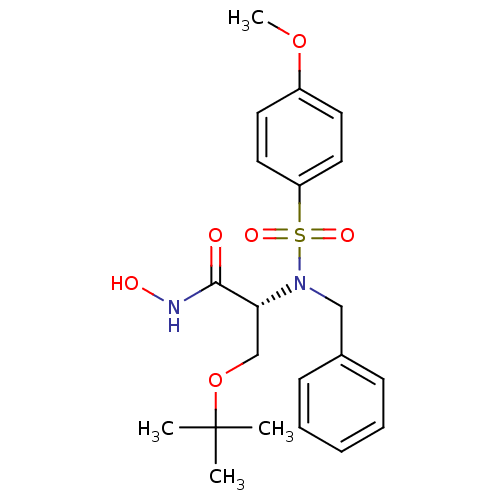 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-3-(te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13136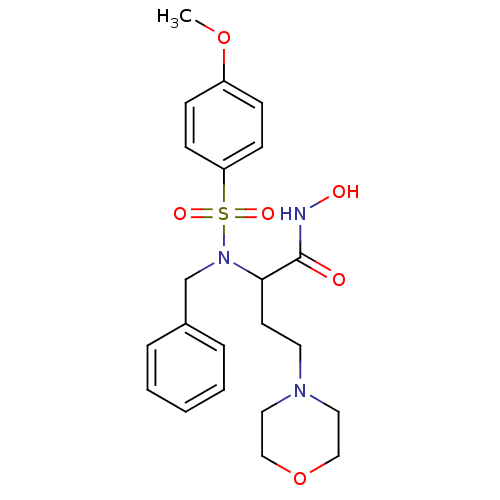 (2-[benzyl(4-methoxybenzene)sulfonamido]-N-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13088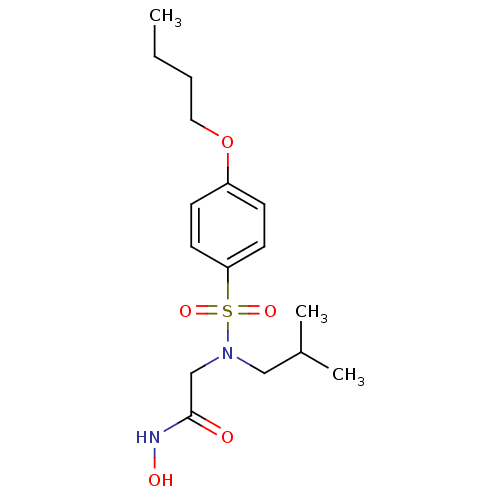 (2-[(4-butoxybenzene)(2-methylpropyl)sulfonamido]-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13099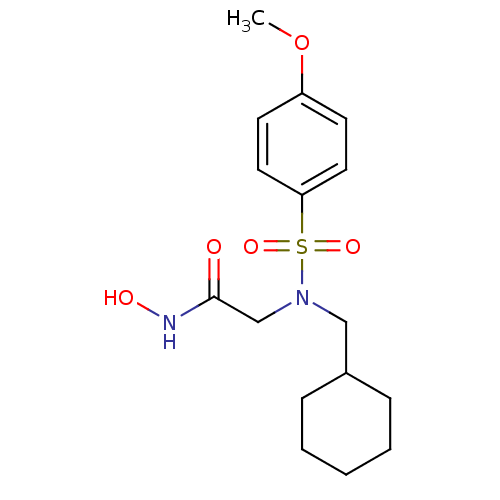 (2-[(cyclohexylmethyl)(4-methoxybenzene)sulfonamido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13091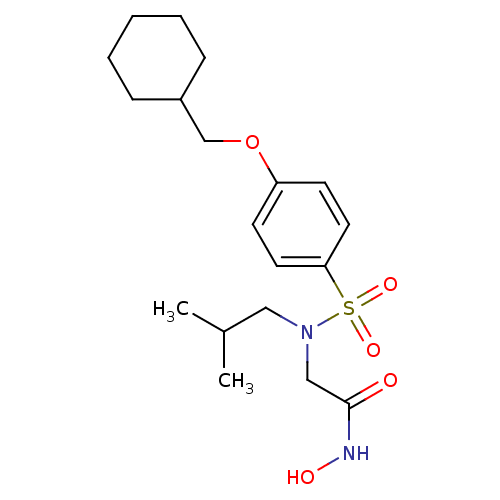 (2-{[4-(cyclohexylmethoxy)benzene](2-methylpropyl)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13090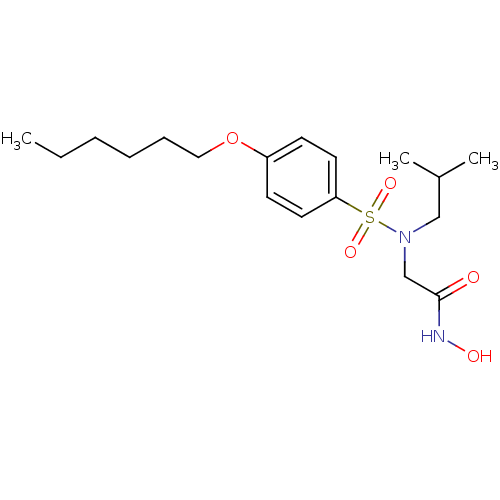 (2-{[4-(hexyloxy)benzene](2-methylpropyl)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 63 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13132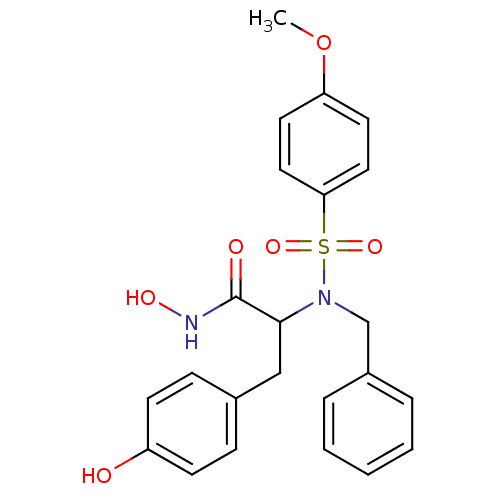 (2-[benzyl(4-methoxybenzene)sulfonamido]-N-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13095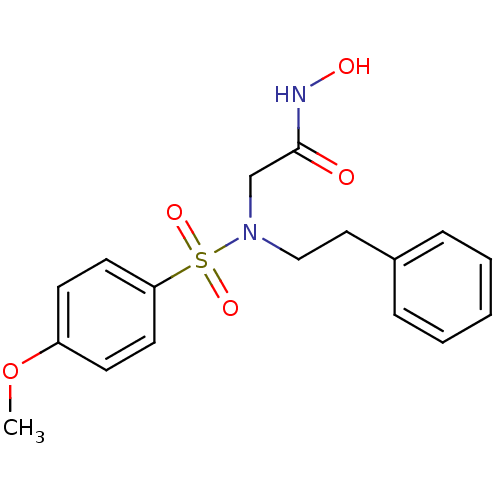 (CGS 27023A Analog 23 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 66 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13130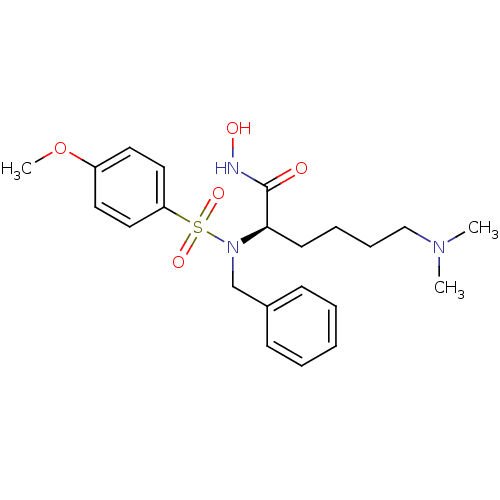 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-6-(di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM11331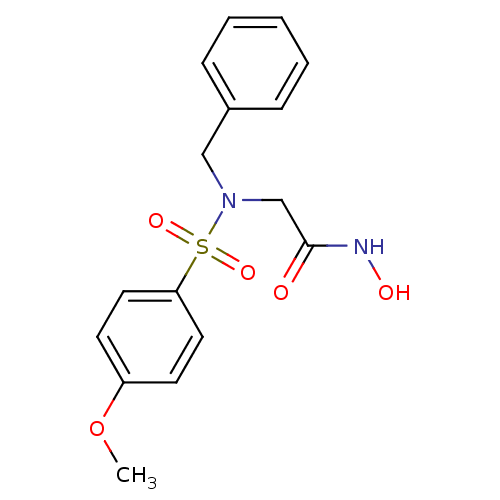 (2-[benzyl(4-methoxybenzene)sulfonamido]-N-hydroxya...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13129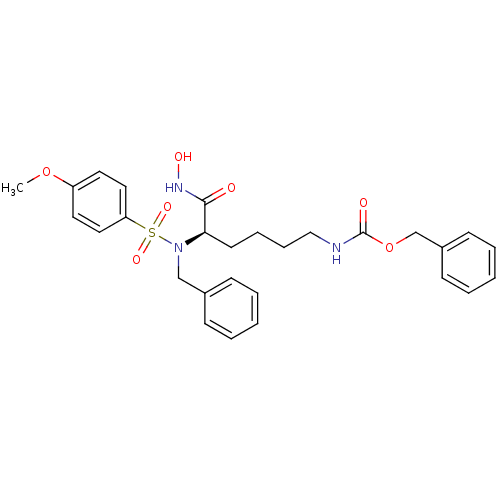 (CGS 27023A Analog 61 | benzyl N-[(5R)-5-[benzyl(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13111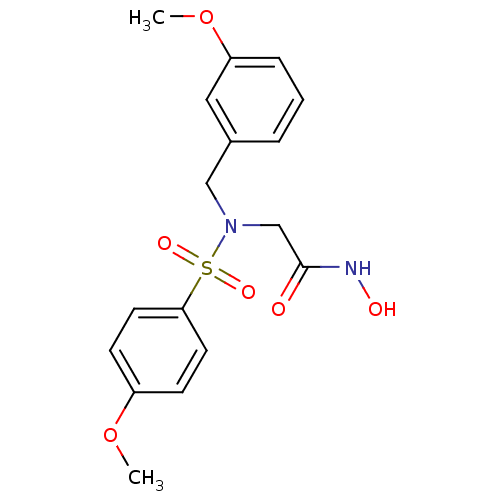 (CGS 27023A Analog 39 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13120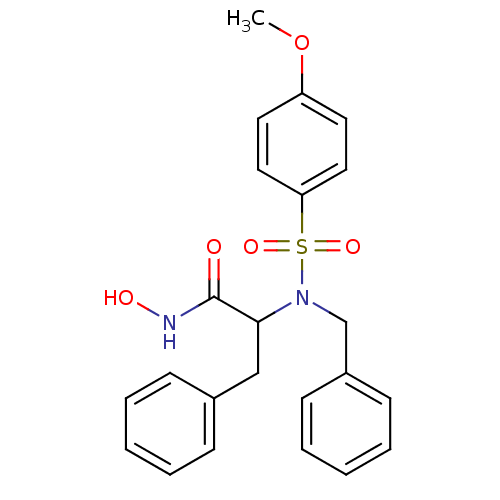 (2-[benzyl(4-methoxybenzene)sulfonamido]-N-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13089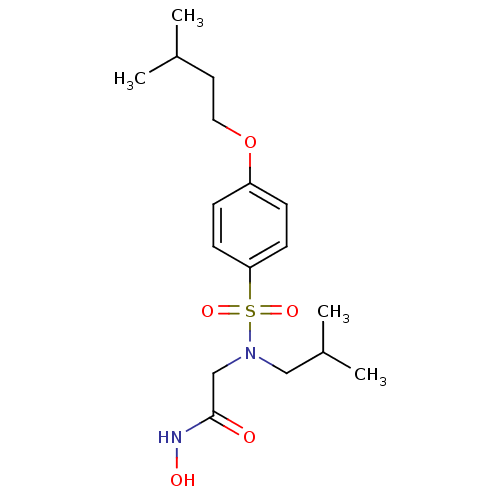 (CGS 27023A Analog 17 | N-hydroxy-2-{[4-(3-methylbu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 78 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13140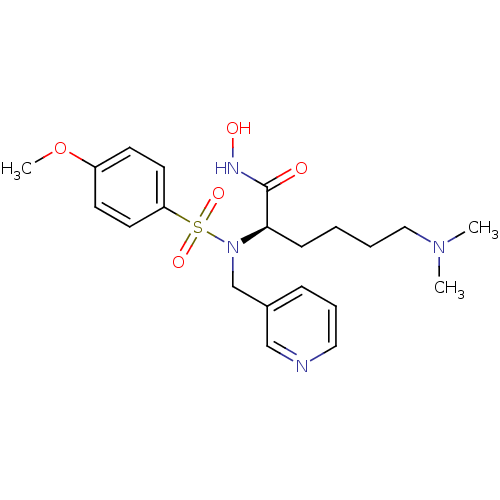 ((2R)-6-(dimethylamino)-N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13128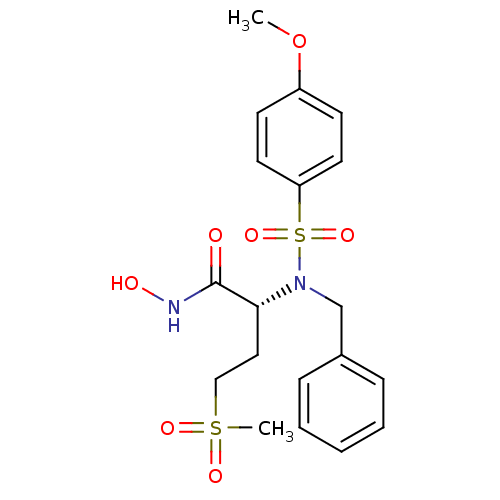 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM11337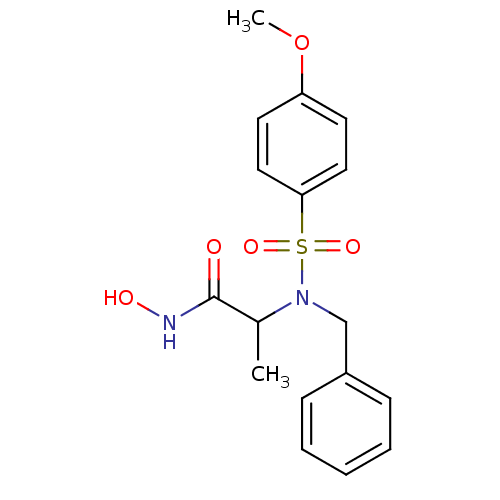 (2-[benzyl(4-methoxybenzene)sulfonamido]-N-hydroxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13115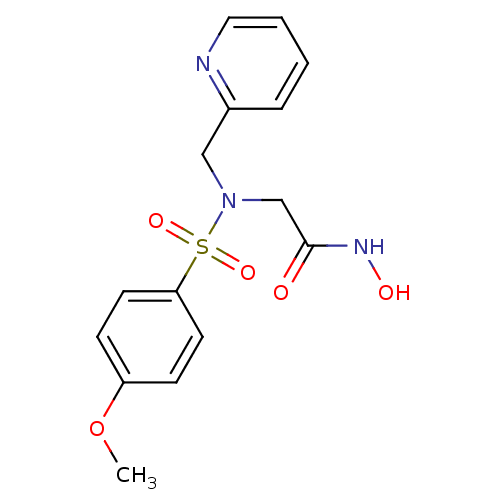 (CGS 27023A Analog 43 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13125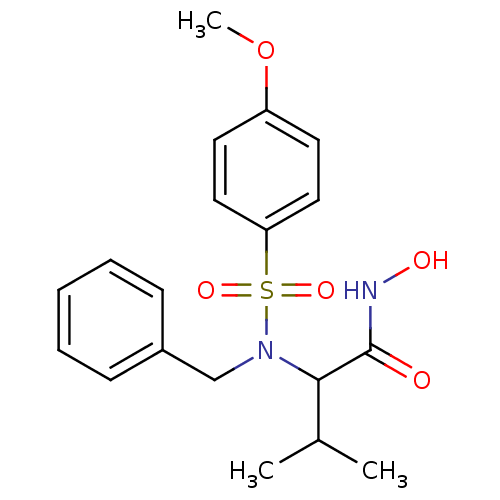 (2-[benzyl(4-methoxybenzene)sulfonamido]-N-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13104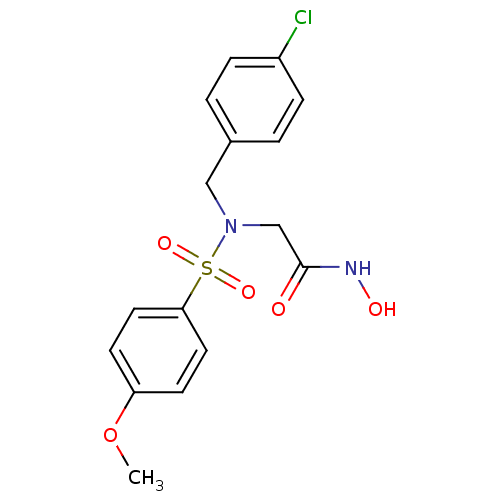 (2-{[(4-chlorophenyl)methyl](4-methoxybenzene)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13098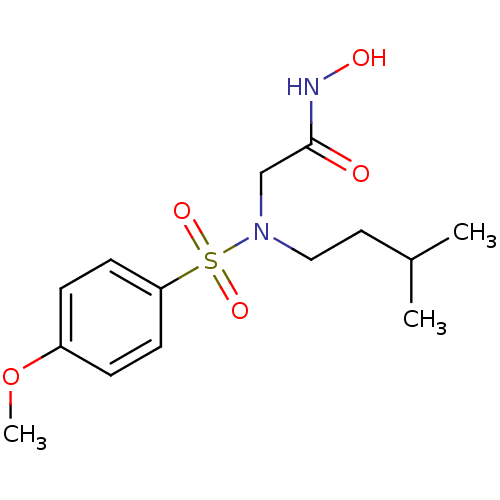 (CGS 27023A Analog 26 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13097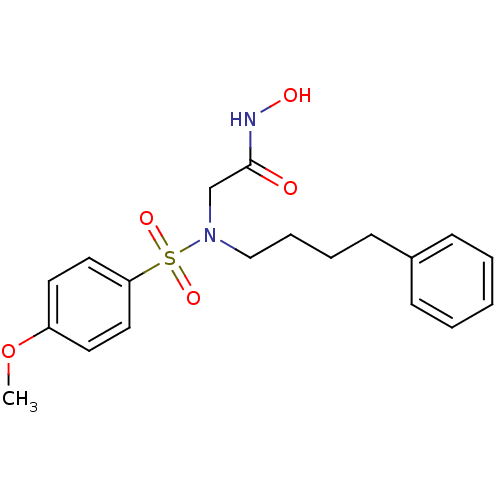 (CGS 27023A Analog 25 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13105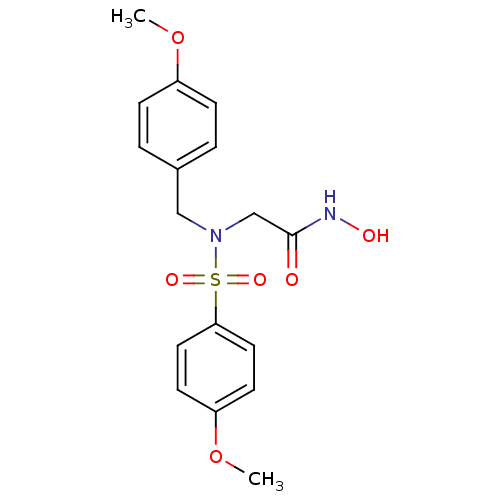 (CGS 27023A Analog 33 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13123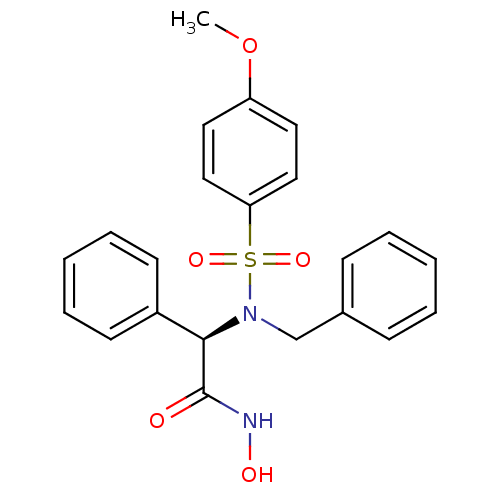 ((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13106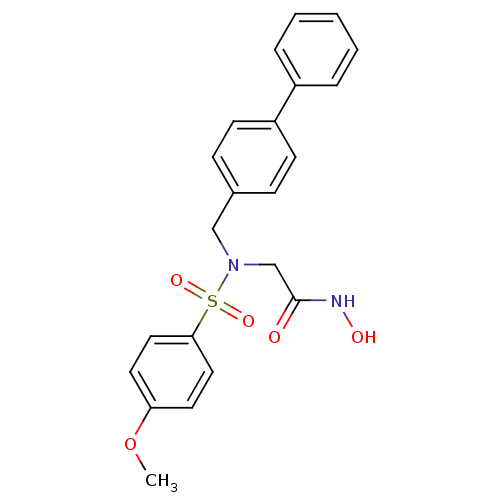 (CGS 27023A Analog 34 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13114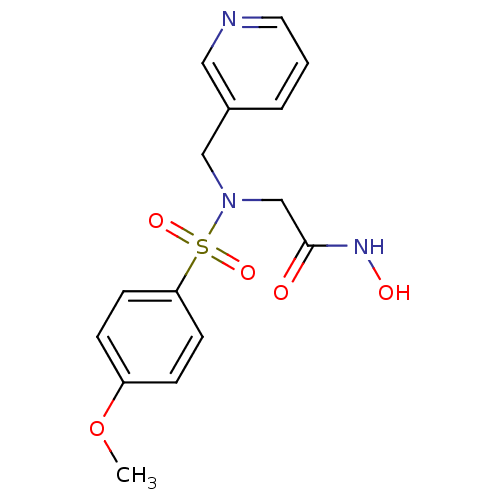 (CGS 27023A Analog 42 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13096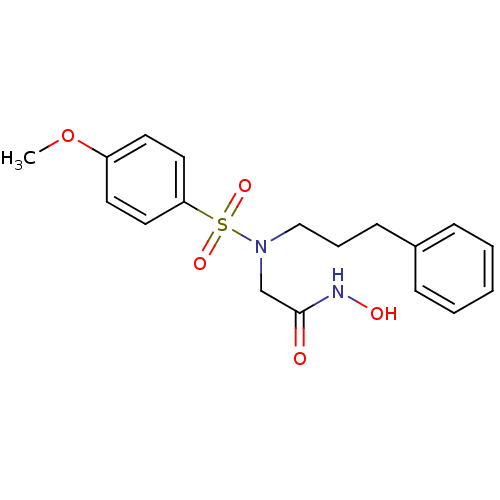 (CGS 27023A Analog 24 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13080 (CGS 27023A Analog 3 | N-hydroxy-2-[(4-methoxybenze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 133 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13116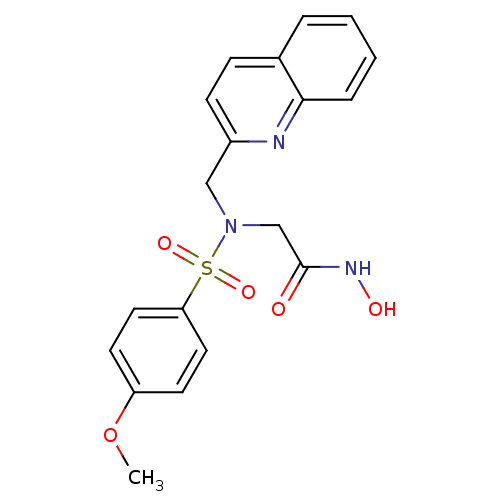 (CGS 27023A Analog 44 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13118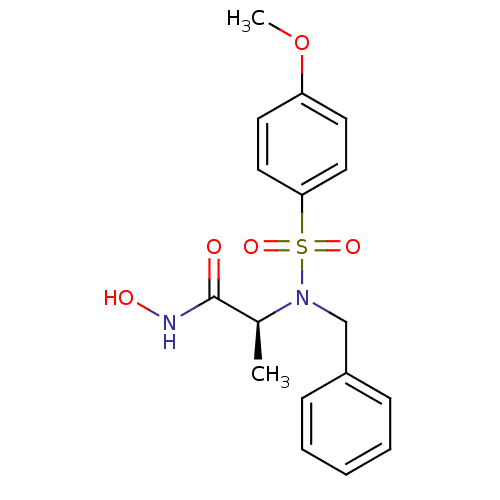 ((2S)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13112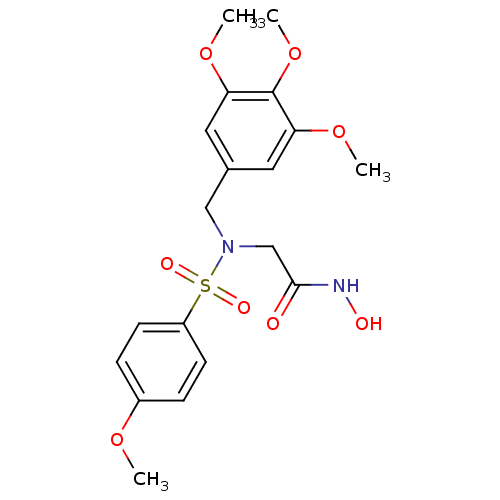 (CGS 27023A Analog 40 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13113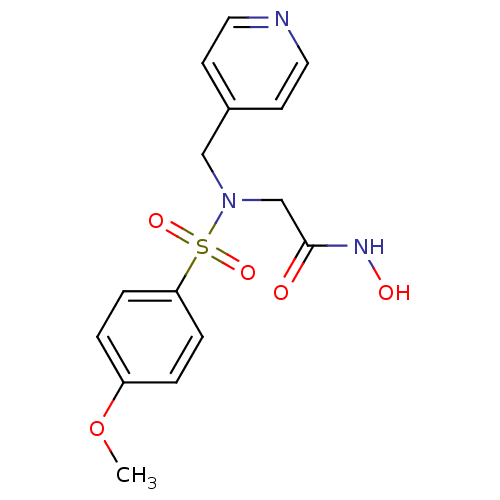 (CGS 27023A Analog 41 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13109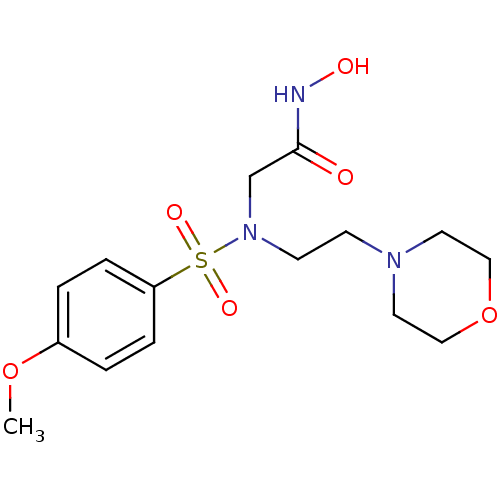 (CGS 27023A Analog 37 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13107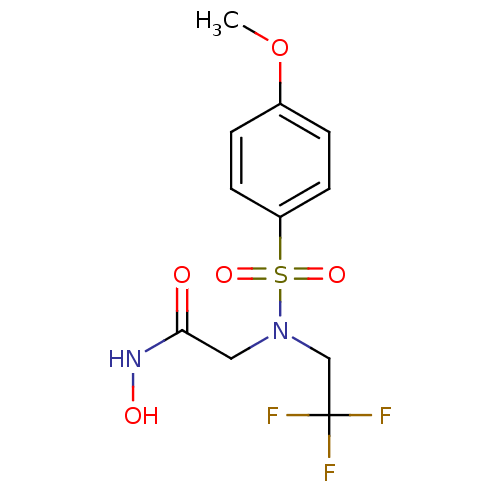 (CGS 27023A Analog 35 | N-hydroxy-2-[(4-methoxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM13092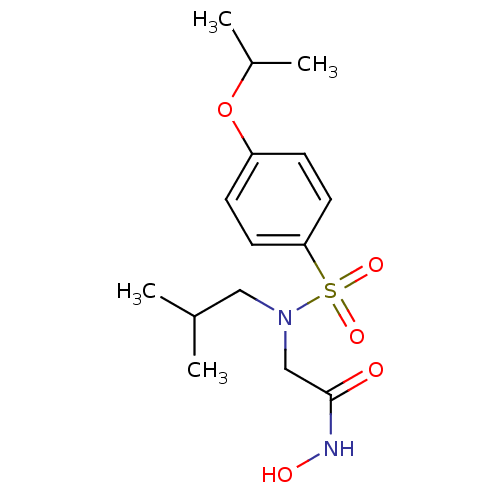 (CGS 27023A Analog 20 | N-hydroxy-2-[(2-methylpropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 336 | -38.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis Pharmaceuticals | Assay Description Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, ... | J Med Chem 40: 2525-32 (1997) Article DOI: 10.1021/jm960871c BindingDB Entry DOI: 10.7270/Q2MW2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |