 Found 1224 hits with Last Name = 'dow' and Initial = 'rl'
Found 1224 hits with Last Name = 'dow' and Initial = 'rl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123058
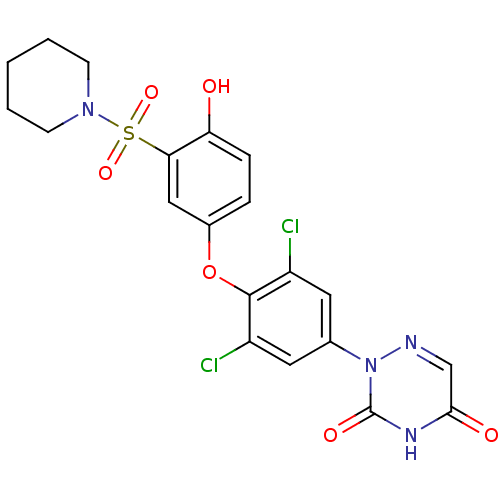
(2-(3,5-dichloro-4-(4-hydroxy-3-(piperidin-1-ylsulf...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)N1CCCCC1 Show InChI InChI=1S/C20H18Cl2N4O6S/c21-14-8-12(26-20(29)24-18(28)11-23-26)9-15(22)19(14)32-13-4-5-16(27)17(10-13)33(30,31)25-6-2-1-3-7-25/h4-5,8-11,27H,1-3,6-7H2,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388901

(CHEMBL2063237)Show SMILES CC(C)C(=O)N[C@@H]1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C23H23Cl2N3O2/c1-14(2)23(29)26-18-7-5-13-30-22-20(18)27-28(19-8-4-3-6-17(19)25)21(22)15-9-11-16(24)12-10-15/h3-4,6,8-12,14,18H,5,7,13H2,1-2H3,(H,26,29)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123046
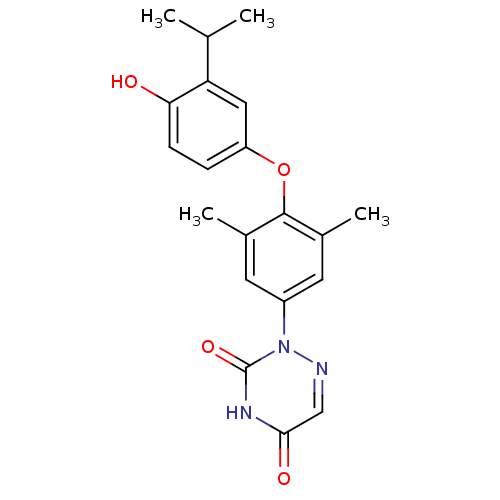
(2-[4-(4-Hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-...)Show SMILES CC(C)c1cc(Oc2c(C)cc(cc2C)-n2ncc(=O)[nH]c2=O)ccc1O Show InChI InChI=1S/C20H21N3O4/c1-11(2)16-9-15(5-6-17(16)24)27-19-12(3)7-14(8-13(19)4)23-20(26)22-18(25)10-21-23/h5-11,24H,1-4H3,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388885
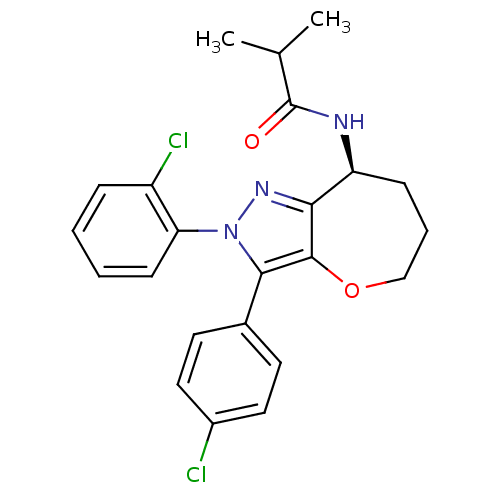
(CHEMBL2063238)Show SMILES CC(C)C(=O)N[C@H]1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C23H23Cl2N3O2/c1-14(2)23(29)26-18-7-5-13-30-22-20(18)27-28(19-8-4-3-6-17(19)25)21(22)15-9-11-16(24)12-10-15/h3-4,6,8-12,14,18H,5,7,13H2,1-2H3,(H,26,29)/t18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123044
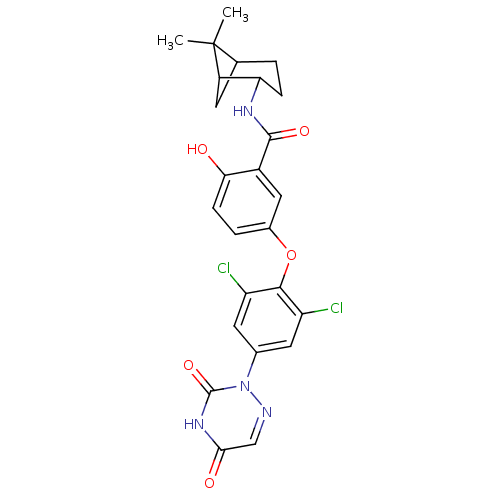
(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES CC1(C)C2CC1C(CC2)NC(=O)c1cc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)ccc1O |THB:9:6:1:4| Show InChI InChI=1S/C25H24Cl2N4O5/c1-25(2)12-3-5-19(16(25)7-12)29-23(34)15-10-14(4-6-20(15)32)36-22-17(26)8-13(9-18(22)27)31-24(35)30-21(33)11-28-31/h4,6,8-12,16,19,32H,3,5,7H2,1-2H3,(H,29,34)(H,30,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123044

(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES CC1(C)C2CC1C(CC2)NC(=O)c1cc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)ccc1O |THB:9:6:1:4| Show InChI InChI=1S/C25H24Cl2N4O5/c1-25(2)12-3-5-19(16(25)7-12)29-23(34)15-10-14(4-6-20(15)32)36-22-17(26)8-13(9-18(22)27)31-24(35)30-21(33)11-28-31/h4,6,8-12,16,19,32H,3,5,7H2,1-2H3,(H,29,34)(H,30,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388902
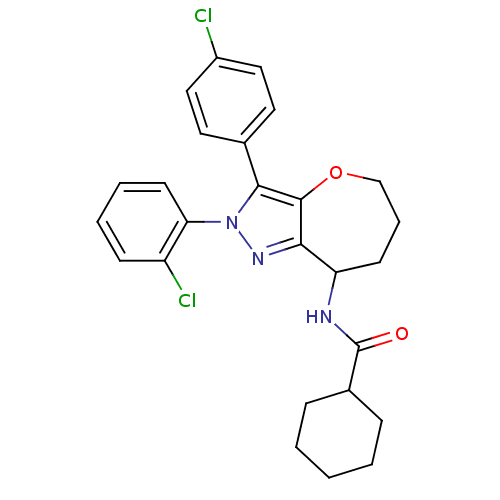
(CHEMBL2063239)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3CCCCC3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C26H27Cl2N3O2/c27-19-14-12-17(13-15-19)24-25-23(30-31(24)22-11-5-4-9-20(22)28)21(10-6-16-33-25)29-26(32)18-7-2-1-3-8-18/h4-5,9,11-15,18,21H,1-3,6-8,10,16H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388887
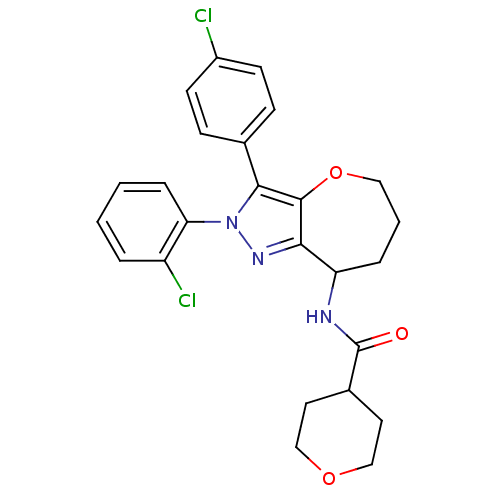
(CHEMBL2063240)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3CCOCC3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C25H25Cl2N3O3/c26-18-9-7-16(8-10-18)23-24-22(29-30(23)21-6-2-1-4-19(21)27)20(5-3-13-33-24)28-25(31)17-11-14-32-15-12-17/h1-2,4,6-10,17,20H,3,5,11-15H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123045
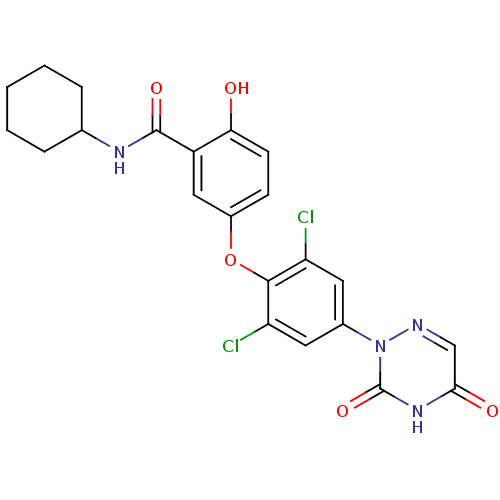
(CHEMBL413699 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)NC1CCCCC1 Show InChI InChI=1S/C22H20Cl2N4O5/c23-16-8-13(28-22(32)27-19(30)11-25-28)9-17(24)20(16)33-14-6-7-18(29)15(10-14)21(31)26-12-4-2-1-3-5-12/h6-12,29H,1-5H2,(H,26,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388893
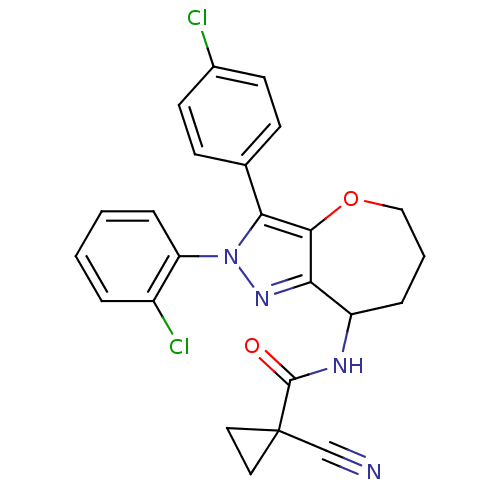
(CHEMBL2063246)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3(CC3)C#N)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C24H20Cl2N4O2/c25-16-9-7-15(8-10-16)21-22-20(29-30(21)19-6-2-1-4-17(19)26)18(5-3-13-32-22)28-23(31)24(14-27)11-12-24/h1-2,4,6-10,18H,3,5,11-13H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388896

(CHEMBL2063249)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)c3cnon3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C22H17Cl2N5O3/c23-14-9-7-13(8-10-14)20-21-19(27-29(20)18-6-2-1-4-15(18)24)16(5-3-11-31-21)26-22(30)17-12-25-32-28-17/h1-2,4,6-10,12,16H,3,5,11H2,(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388887

(CHEMBL2063240)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3CCOCC3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C25H25Cl2N3O3/c26-18-9-7-16(8-10-18)23-24-22(29-30(23)21-6-2-1-4-19(21)27)20(5-3-13-33-24)28-25(31)17-11-14-32-15-12-17/h1-2,4,6-10,17,20H,3,5,11-15H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50301739
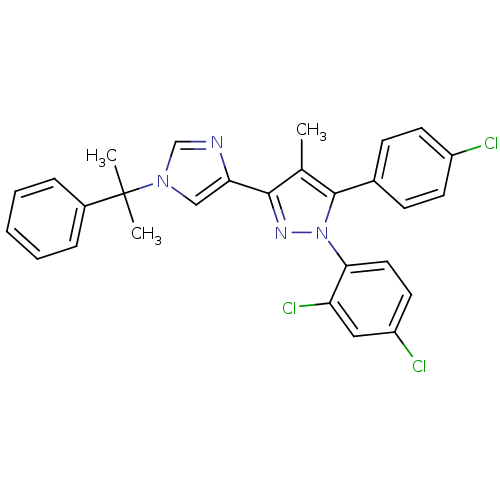
(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)-c1cn(cn1)C(C)(C)c1ccccc1 Show InChI InChI=1S/C28H23Cl3N4/c1-18-26(24-16-34(17-32-24)28(2,3)20-7-5-4-6-8-20)33-35(25-14-13-22(30)15-23(25)31)27(18)19-9-11-21(29)12-10-19/h4-17H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor transfected in CHO-K1cells by GTPgamma[35S] binding assay |
Bioorg Med Chem Lett 19: 5351-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.130
BindingDB Entry DOI: 10.7270/Q2KP827S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50176411
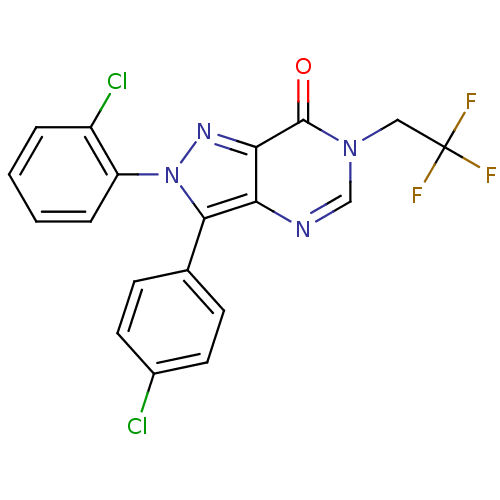
(2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...)Show SMILES FC(F)(F)Cn1cnc2c(-c3ccc(Cl)cc3)n(nc2c1=O)-c1ccccc1Cl Show InChI InChI=1S/C19H11Cl2F3N4O/c20-12-7-5-11(6-8-12)17-15-16(18(29)27(10-25-15)9-19(22,23)24)26-28(17)14-4-2-1-3-13(14)21/h1-8,10H,9H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories
Curated by ChEMBL
| Assay Description
Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay |
Bioorg Med Chem Lett 16: 731-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.019
BindingDB Entry DOI: 10.7270/Q2TM79PM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50301747
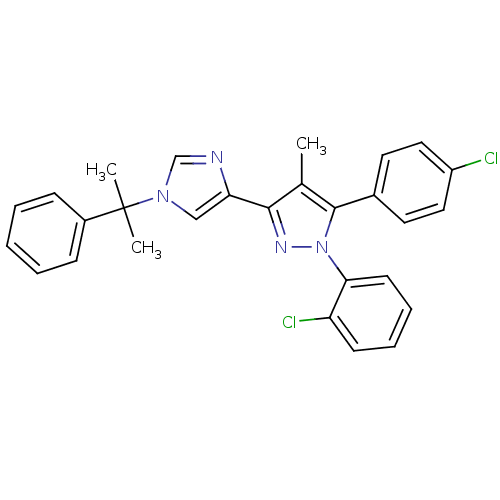
(1-(2-chlorophenyl)-5-(4-chlorophenyl)-4-methyl-3-(...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)-c1cn(cn1)C(C)(C)c1ccccc1 Show InChI InChI=1S/C28H24Cl2N4/c1-19-26(24-17-33(18-31-24)28(2,3)21-9-5-4-6-10-21)32-34(25-12-8-7-11-23(25)30)27(19)20-13-15-22(29)16-14-20/h4-18H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor transfected in CHO-K1cells by GTPgamma[35S] binding assay |
Bioorg Med Chem Lett 19: 5351-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.130
BindingDB Entry DOI: 10.7270/Q2KP827S |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123054
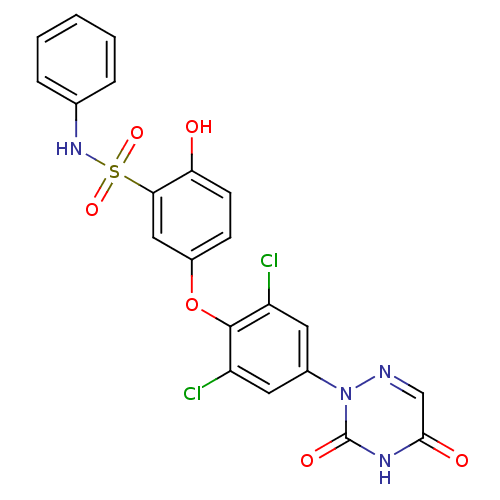
(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)Nc1ccccc1 Show InChI InChI=1S/C21H14Cl2N4O6S/c22-15-8-13(27-21(30)25-19(29)11-24-27)9-16(23)20(15)33-14-6-7-17(28)18(10-14)34(31,32)26-12-4-2-1-3-5-12/h1-11,26,28H,(H,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50176407
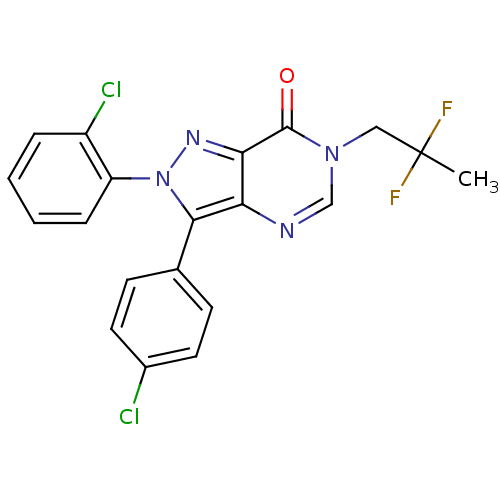
(2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...)Show SMILES CC(F)(F)Cn1cnc2c(-c3ccc(Cl)cc3)n(nc2c1=O)-c1ccccc1Cl Show InChI InChI=1S/C20H14Cl2F2N4O/c1-20(23,24)10-27-11-25-16-17(19(27)29)26-28(15-5-3-2-4-14(15)22)18(16)12-6-8-13(21)9-7-12/h2-9,11H,10H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories
Curated by ChEMBL
| Assay Description
Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay |
Bioorg Med Chem Lett 16: 731-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.019
BindingDB Entry DOI: 10.7270/Q2TM79PM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388892
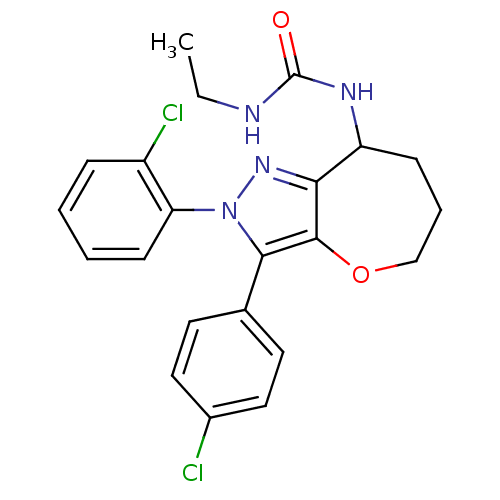
(CHEMBL2063245)Show SMILES CCNC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C22H22Cl2N4O2/c1-2-25-22(29)26-17-7-5-13-30-21-19(17)27-28(18-8-4-3-6-16(18)24)20(21)14-9-11-15(23)12-10-14/h3-4,6,8-12,17H,2,5,7,13H2,1H3,(H2,25,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388900
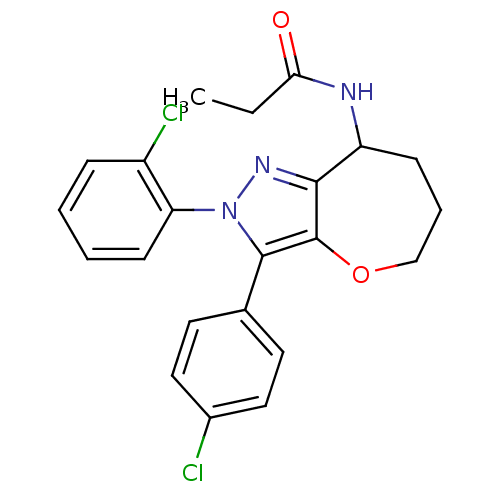
(CHEMBL2063235)Show SMILES CCC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C22H21Cl2N3O2/c1-2-19(28)25-17-7-5-13-29-22-20(17)26-27(18-8-4-3-6-16(18)24)21(22)14-9-11-15(23)12-10-14/h3-4,6,8-12,17H,2,5,7,13H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388892

(CHEMBL2063245)Show SMILES CCNC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C22H22Cl2N4O2/c1-2-25-22(29)26-17-7-5-13-30-21-19(17)27-28(18-8-4-3-6-16(18)24)20(21)14-9-11-15(23)12-10-14/h3-4,6,8-12,17H,2,5,7,13H2,1H3,(H2,25,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388891
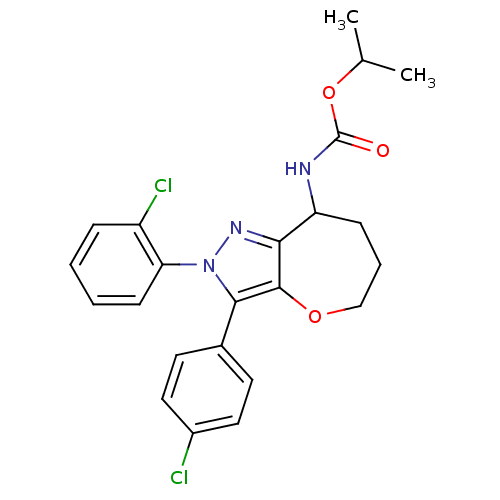
(CHEMBL2063244)Show SMILES CC(C)OC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C23H23Cl2N3O3/c1-14(2)31-23(29)26-18-7-5-13-30-22-20(18)27-28(19-8-4-3-6-17(19)25)21(22)15-9-11-16(24)12-10-15/h3-4,6,8-12,14,18H,5,7,13H2,1-2H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388893

(CHEMBL2063246)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3(CC3)C#N)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C24H20Cl2N4O2/c25-16-9-7-15(8-10-16)21-22-20(29-30(21)19-6-2-1-4-17(19)26)18(5-3-13-32-22)28-23(31)24(14-27)11-12-24/h1-2,4,6-10,18H,3,5,11-13H2,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123064
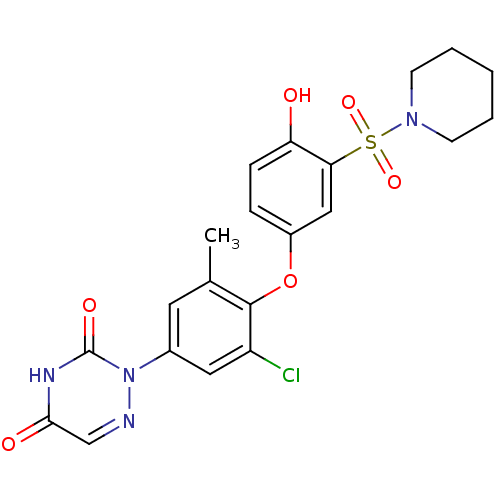
(2-(3-chloro-4-(4-hydroxy-3-(piperidin-1-ylsulfonyl...)Show SMILES Cc1cc(cc(Cl)c1Oc1ccc(O)c(c1)S(=O)(=O)N1CCCCC1)-n1ncc(=O)[nH]c1=O Show InChI InChI=1S/C21H21ClN4O6S/c1-13-9-14(26-21(29)24-19(28)12-23-26)10-16(22)20(13)32-15-5-6-17(27)18(11-15)33(30,31)25-7-3-2-4-8-25/h5-6,9-12,27H,2-4,7-8H2,1H3,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388901

(CHEMBL2063237)Show SMILES CC(C)C(=O)N[C@@H]1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C23H23Cl2N3O2/c1-14(2)23(29)26-18-7-5-13-30-22-20(18)27-28(19-8-4-3-6-17(19)25)21(22)15-9-11-16(24)12-10-15/h3-4,6,8-12,14,18H,5,7,13H2,1-2H3,(H,26,29)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388885

(CHEMBL2063238)Show SMILES CC(C)C(=O)N[C@H]1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C23H23Cl2N3O2/c1-14(2)23(29)26-18-7-5-13-30-22-20(18)27-28(19-8-4-3-6-17(19)25)21(22)15-9-11-16(24)12-10-15/h3-4,6,8-12,14,18H,5,7,13H2,1-2H3,(H,26,29)/t18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50176407

(2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...)Show SMILES CC(F)(F)Cn1cnc2c(-c3ccc(Cl)cc3)n(nc2c1=O)-c1ccccc1Cl Show InChI InChI=1S/C20H14Cl2F2N4O/c1-20(23,24)10-27-11-25-16-17(19(27)29)26-28(15-5-3-2-4-14(15)22)18(16)12-6-8-13(21)9-7-12/h2-9,11H,10H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 731-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.019
BindingDB Entry DOI: 10.7270/Q2TM79PM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388895

(CHEMBL2063248)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)c3ccno3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C23H18Cl2N4O3/c24-15-9-7-14(8-10-15)21-22-20(28-29(21)18-6-2-1-4-16(18)25)17(5-3-13-31-22)27-23(30)19-11-12-26-32-19/h1-2,4,6-12,17H,3,5,13H2,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123063
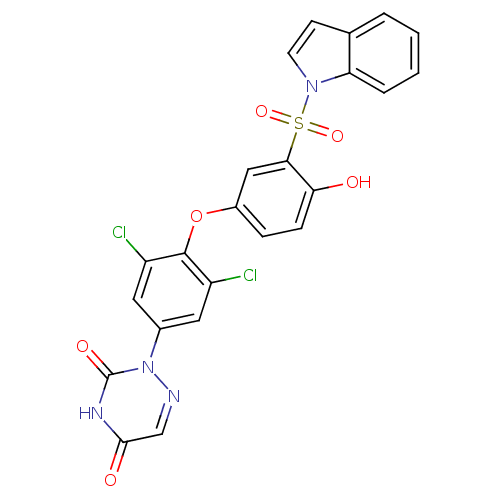
(2-{3,5-Dichloro-4-[4-hydroxy-3-(indole-1-sulfonyl)...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)n1ccc2ccccc12 Show InChI InChI=1S/C23H14Cl2N4O6S/c24-16-9-14(29-23(32)27-21(31)12-26-29)10-17(25)22(16)35-15-5-6-19(30)20(11-15)36(33,34)28-8-7-13-3-1-2-4-18(13)28/h1-12,30H,(H,27,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388890
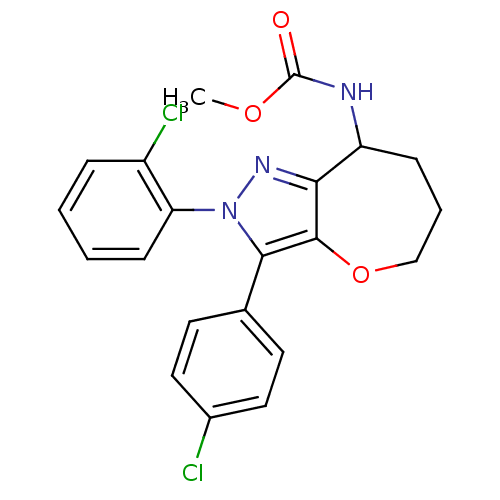
(CHEMBL2063243)Show SMILES COC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C21H19Cl2N3O3/c1-28-21(27)24-16-6-4-12-29-20-18(16)25-26(17-7-3-2-5-15(17)23)19(20)13-8-10-14(22)11-9-13/h2-3,5,7-11,16H,4,6,12H2,1H3,(H,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388894
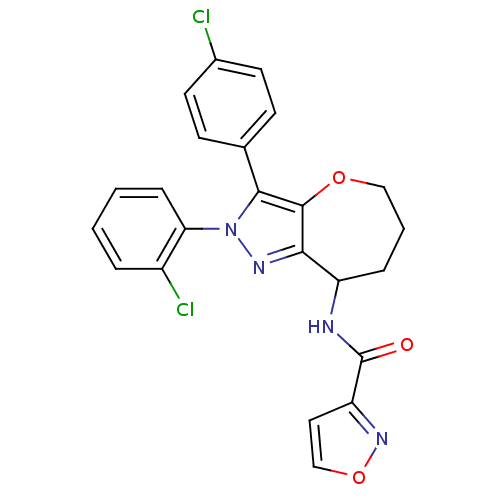
(CHEMBL2063247)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)c3ccon3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C23H18Cl2N4O3/c24-15-9-7-14(8-10-15)21-22-20(27-29(21)19-6-2-1-4-16(19)25)17(5-3-12-31-22)26-23(30)18-11-13-32-28-18/h1-2,4,6-11,13,17H,3,5,12H2,(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388891

(CHEMBL2063244)Show SMILES CC(C)OC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C23H23Cl2N3O3/c1-14(2)31-23(29)26-18-7-5-13-30-22-20(18)27-28(19-8-4-3-6-17(19)25)21(22)15-9-11-16(24)12-10-15/h3-4,6,8-12,14,18H,5,7,13H2,1-2H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123052
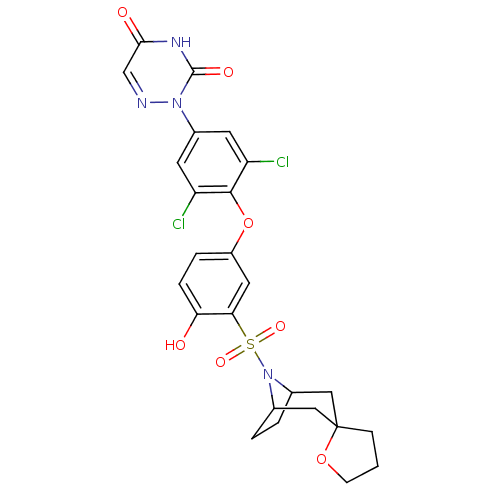
(2-[4-(3-{8-azaspiro[bicyclo[3.2.1]octane-3,2'-oxol...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)N1C2CCC1CC1(CCCO1)C2 |TLB:34:33:27:29.30,THB:24:27:33.38.32:29.30,37:33:27:29.30| Show InChI InChI=1S/C25H24Cl2N4O7S/c26-18-8-16(30-24(34)29-22(33)13-28-30)9-19(27)23(18)38-17-4-5-20(32)21(10-17)39(35,36)31-14-2-3-15(31)12-25(11-14)6-1-7-37-25/h4-5,8-10,13-15,32H,1-3,6-7,11-12H2,(H,29,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50176411

(2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...)Show SMILES FC(F)(F)Cn1cnc2c(-c3ccc(Cl)cc3)n(nc2c1=O)-c1ccccc1Cl Show InChI InChI=1S/C19H11Cl2F3N4O/c20-12-7-5-11(6-8-12)17-15-16(18(29)27(10-25-15)9-19(22,23)24)26-28(17)14-4-2-1-3-13(14)21/h1-8,10H,9H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 731-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.019
BindingDB Entry DOI: 10.7270/Q2TM79PM |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123061
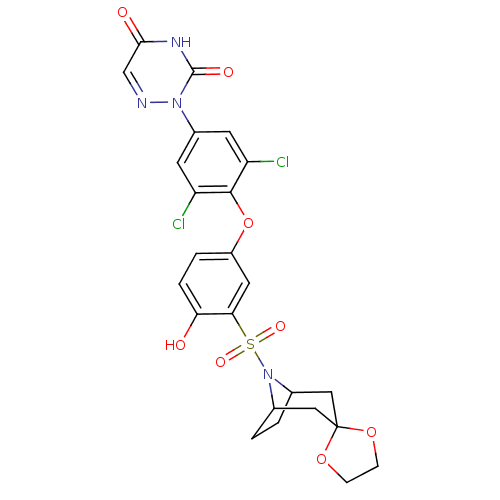
(2-[4-(3-{8-azaspiro[bicyclo[3.2.1]octane-3,2'-[1,3...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)N1C2CCC1CC1(C2)OCCO1 |THB:24:27:33.32.34:29.30| Show InChI InChI=1S/C24H22Cl2N4O8S/c25-17-7-15(29-23(33)28-21(32)12-27-29)8-18(26)22(17)38-16-3-4-19(31)20(9-16)39(34,35)30-13-1-2-14(30)11-24(10-13)36-5-6-37-24/h3-4,7-9,12-14,31H,1-2,5-6,10-11H2,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123059
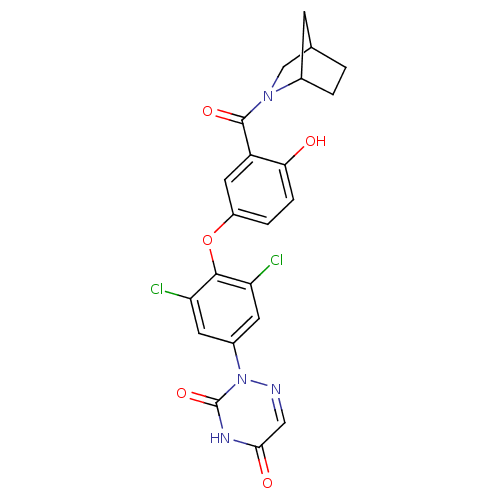
(2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)N1CC2CCC1C2 Show InChI InChI=1S/C22H18Cl2N4O5/c23-16-6-13(28-22(32)26-19(30)9-25-28)7-17(24)20(16)33-14-3-4-18(29)15(8-14)21(31)27-10-11-1-2-12(27)5-11/h3-4,6-9,11-12,29H,1-2,5,10H2,(H,26,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123059

(2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)N1CC2CCC1C2 Show InChI InChI=1S/C22H18Cl2N4O5/c23-16-6-13(28-22(32)26-19(30)9-25-28)7-17(24)20(16)33-14-3-4-18(29)15(8-14)21(31)27-10-11-1-2-12(27)5-11/h3-4,6-9,11-12,29H,1-2,5,10H2,(H,26,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50304781
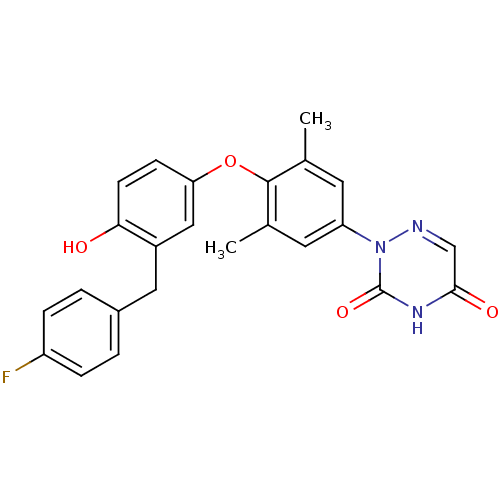
(2-(4-(3-(4-fluorobenzyl)-4-hydroxyphenoxy)-3,5-dim...)Show SMILES Cc1cc(cc(C)c1Oc1ccc(O)c(Cc2ccc(F)cc2)c1)-n1ncc(=O)[nH]c1=O Show InChI InChI=1S/C24H20FN3O4/c1-14-9-19(28-24(31)27-22(30)13-26-28)10-15(2)23(14)32-20-7-8-21(29)17(12-20)11-16-3-5-18(25)6-4-16/h3-10,12-13,29H,11H2,1-2H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thyroid receptor beta |
Bioorg Med Chem Lett 20: 306-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.109
BindingDB Entry DOI: 10.7270/Q2J38SNP |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123057
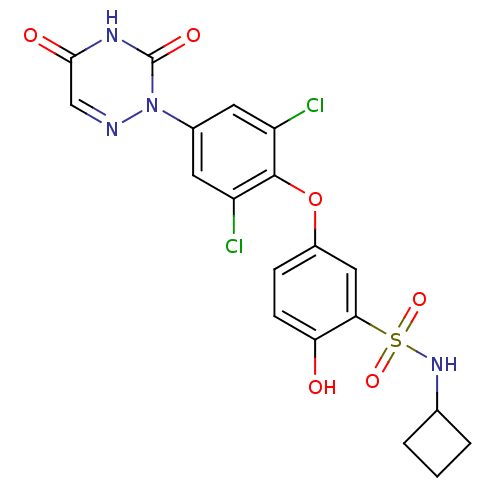
(CHEMBL124039 | N-Cyclobutyl-5-[2,6-dichloro-4-(3,5...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)NC1CCC1 Show InChI InChI=1S/C19H16Cl2N4O6S/c20-13-6-11(25-19(28)23-17(27)9-22-25)7-14(21)18(13)31-12-4-5-15(26)16(8-12)32(29,30)24-10-2-1-3-10/h4-10,24,26H,1-3H2,(H,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388900

(CHEMBL2063235)Show SMILES CCC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C22H21Cl2N3O2/c1-2-19(28)25-17-7-5-13-29-22-20(17)26-27(18-8-4-3-6-16(18)24)21(22)14-9-11-15(23)12-10-14/h3-4,6,8-12,17H,2,5,7,13H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123056
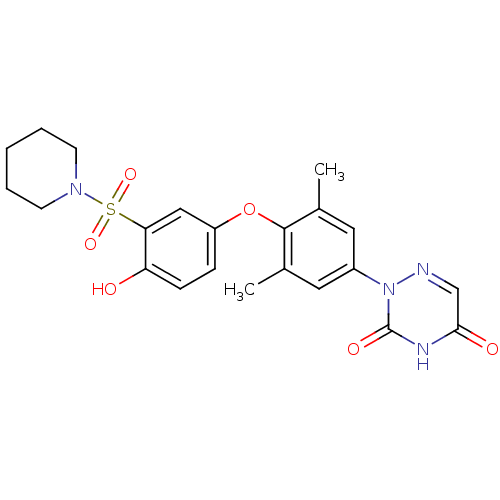
(2-(4-(4-hydroxy-3-(piperidin-1-ylsulfonyl)phenoxy)...)Show SMILES Cc1cc(cc(C)c1Oc1ccc(O)c(c1)S(=O)(=O)N1CCCCC1)-n1ncc(=O)[nH]c1=O Show InChI InChI=1S/C22H24N4O6S/c1-14-10-16(26-22(29)24-20(28)13-23-26)11-15(2)21(14)32-17-6-7-18(27)19(12-17)33(30,31)25-8-4-3-5-9-25/h6-7,10-13,27H,3-5,8-9H2,1-2H3,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123062
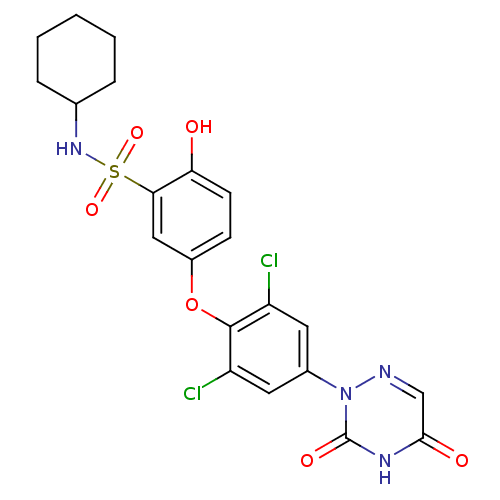
(CHEMBL340158 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)NC1CCCCC1 Show InChI InChI=1S/C21H20Cl2N4O6S/c22-15-8-13(27-21(30)25-19(29)11-24-27)9-16(23)20(15)33-14-6-7-17(28)18(10-14)34(31,32)26-12-4-2-1-3-5-12/h6-12,26,28H,1-5H2,(H,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50304778
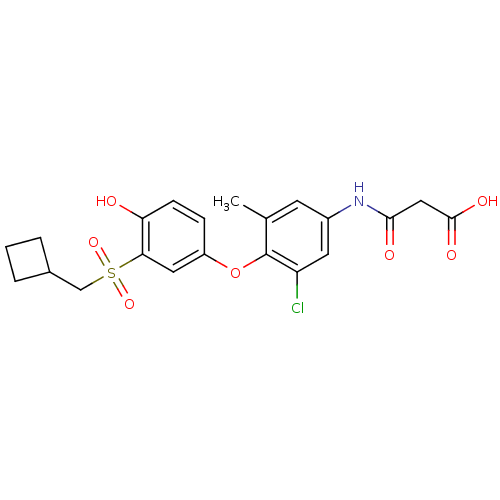
(3-(3-chloro-4-(3-(cyclobutylmethylsulfonyl)-4-hydr...)Show SMILES Cc1cc(NC(=O)CC(O)=O)cc(Cl)c1Oc1ccc(O)c(c1)S(=O)(=O)CC1CCC1 Show InChI InChI=1S/C21H22ClNO7S/c1-12-7-14(23-19(25)10-20(26)27)8-16(22)21(12)30-15-5-6-17(24)18(9-15)31(28,29)11-13-3-2-4-13/h5-9,13,24H,2-4,10-11H2,1H3,(H,23,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thyroid receptor beta |
Bioorg Med Chem Lett 20: 306-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.109
BindingDB Entry DOI: 10.7270/Q2J38SNP |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50304777
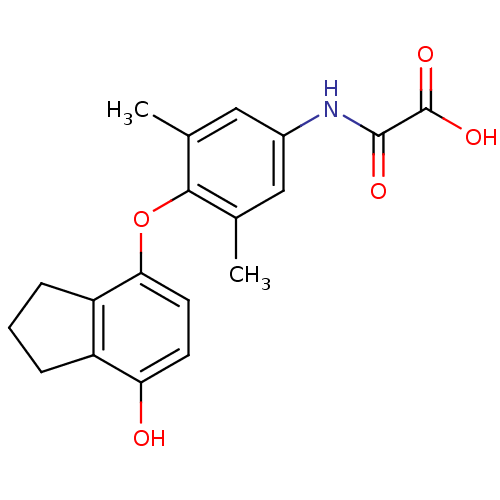
(2-(4-(7-hydroxy-2,3-dihydro-1H-inden-4-yloxy)-3,5-...)Show SMILES Cc1cc(NC(=O)C(O)=O)cc(C)c1Oc1ccc(O)c2CCCc12 Show InChI InChI=1S/C19H19NO5/c1-10-8-12(20-18(22)19(23)24)9-11(2)17(10)25-16-7-6-15(21)13-4-3-5-14(13)16/h6-9,21H,3-5H2,1-2H3,(H,20,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thyroid receptor beta |
Bioorg Med Chem Lett 20: 306-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.109
BindingDB Entry DOI: 10.7270/Q2J38SNP |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50304780
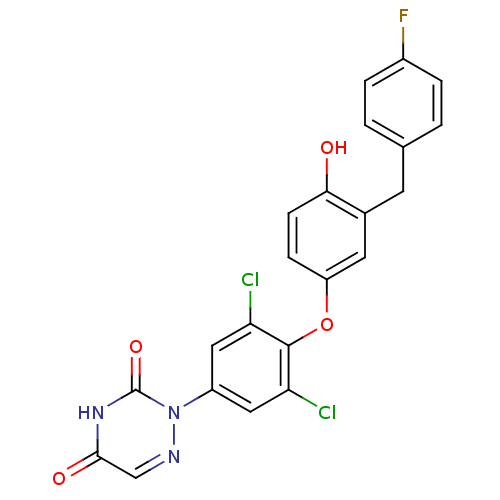
(2-(3,5-dichloro-4-(3-(4-fluorobenzyl)-4-hydroxyphe...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1Cc1ccc(F)cc1 Show InChI InChI=1S/C22H14Cl2FN3O4/c23-17-9-15(28-22(31)27-20(30)11-26-28)10-18(24)21(17)32-16-5-6-19(29)13(8-16)7-12-1-3-14(25)4-2-12/h1-6,8-11,29H,7H2,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thyroid receptor beta |
Bioorg Med Chem Lett 20: 306-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.109
BindingDB Entry DOI: 10.7270/Q2J38SNP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388897
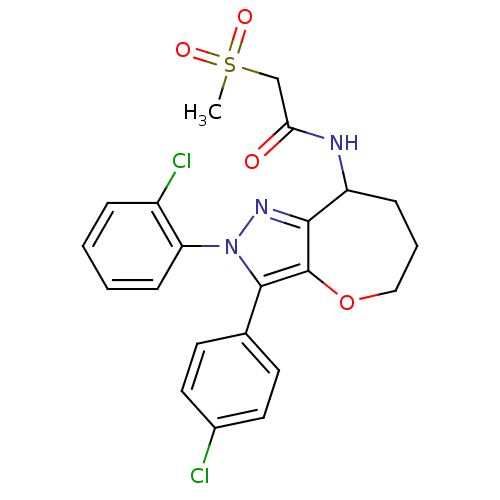
(CHEMBL2063250)Show SMILES CS(=O)(=O)CC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C22H21Cl2N3O4S/c1-32(29,30)13-19(28)25-17-6-4-12-31-22-20(17)26-27(18-7-3-2-5-16(18)24)21(22)14-8-10-15(23)11-9-14/h2-3,5,7-11,17H,4,6,12-13H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388895

(CHEMBL2063248)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)c3ccno3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C23H18Cl2N4O3/c24-15-9-7-14(8-10-15)21-22-20(28-29(21)18-6-2-1-4-16(18)25)17(5-3-13-31-22)27-23(30)19-11-12-26-32-19/h1-2,4,6-12,17H,3,5,13H2,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388889
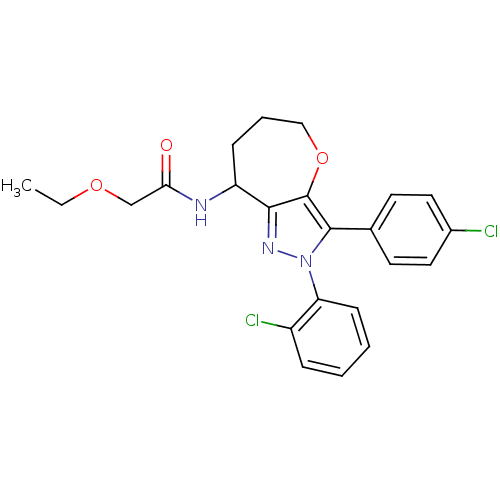
(CHEMBL2063242)Show SMILES CCOCC(=O)NC1CCCOc2c1nn(c2-c1ccc(Cl)cc1)-c1ccccc1Cl Show InChI InChI=1S/C23H23Cl2N3O3/c1-2-30-14-20(29)26-18-7-5-13-31-23-21(18)27-28(19-8-4-3-6-17(19)25)22(23)15-9-11-16(24)12-10-15/h3-4,6,8-12,18H,2,5,7,13-14H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123059

(2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)N1CC2CCC1C2 Show InChI InChI=1S/C22H18Cl2N4O5/c23-16-6-13(28-22(32)26-19(30)9-25-28)7-17(24)20(16)33-14-3-4-18(29)15(8-14)21(31)27-10-11-1-2-12(27)5-11/h3-4,6-9,11-12,29H,1-2,5,10H2,(H,26,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50388902

(CHEMBL2063239)Show SMILES Clc1ccc(cc1)-c1c2OCCCC(NC(=O)C3CCCCC3)c2nn1-c1ccccc1Cl Show InChI InChI=1S/C26H27Cl2N3O2/c27-19-14-12-17(13-15-19)24-25-23(30-31(24)22-11-5-4-9-20(22)28)21(10-6-16-33-25)29-26(32)18-7-2-1-3-8-18/h4-5,9,11-15,18,21H,1-3,6-8,10,16H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A form human CB1 receptor expressed in HEK293 cells after 60 mins by beta counting |
ACS Med Chem Lett 3: 397-401 (2012)
Article DOI: 10.1021/ml3000325
BindingDB Entry DOI: 10.7270/Q22N53BD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data