

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM27960 ((2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -50.9 | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Intervet Innovation GmbH | Assay Description In the binding assays, the results were expressed as percent inhibition of the control radioligand specific binding. The IC50 values (concentration c... | J Med Chem 52: 1773-7 (2009) Article DOI: 10.1021/jm801211c BindingDB Entry DOI: 10.7270/Q2H993HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM27959 (4-(1-hydroxy-2-{[4-(4-hydroxyphenyl)butan-2-yl]ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 180 | -38.1 | 330 | n/a | 9.10 | n/a | n/a | 7.4 | 22 |
Intervet Innovation GmbH | Assay Description In the binding assays, the results were expressed as percent inhibition of the control radioligand specific binding. The IC50 values (concentration c... | J Med Chem 52: 1773-7 (2009) Article DOI: 10.1021/jm801211c BindingDB Entry DOI: 10.7270/Q2H993HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM27955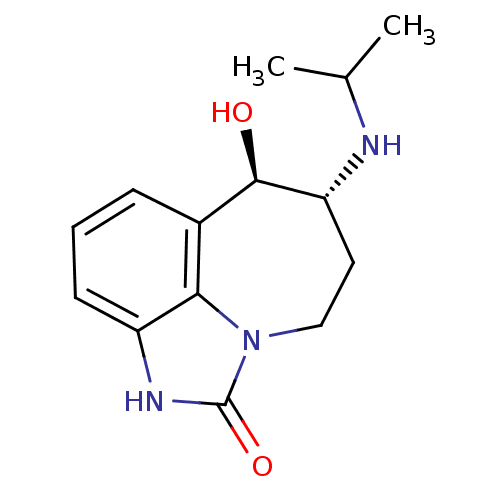 ((-)-Zilpaterol | (9R,10R)-9-hydroxy-10-(propan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 320 | -36.7 | 620 | n/a | 8.70 | n/a | n/a | 7.4 | 22 |
Intervet Innovation GmbH | Assay Description In the binding assays, the results were expressed as percent inhibition of the control radioligand specific binding. The IC50 values (concentration c... | J Med Chem 52: 1773-7 (2009) Article DOI: 10.1021/jm801211c BindingDB Entry DOI: 10.7270/Q2H993HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25769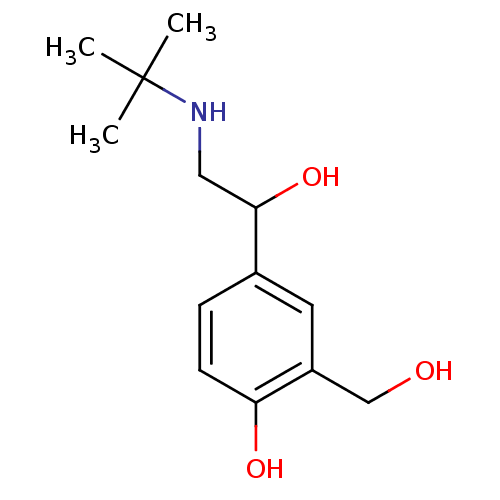 (4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 510 | -35.6 | 980 | n/a | 19 | n/a | n/a | 7.4 | 22 |
Intervet Innovation GmbH | Assay Description In the binding assays, the results were expressed as percent inhibition of the control radioligand specific binding. The IC50 values (concentration c... | J Med Chem 52: 1773-7 (2009) Article DOI: 10.1021/jm801211c BindingDB Entry DOI: 10.7270/Q2H993HR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM27958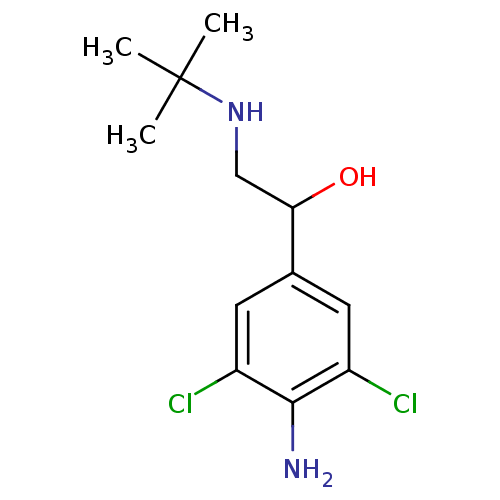 (1-(4-amino-3,5-dichlorophenyl)-2-(tert-butylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 570 | -35.3 | 1.30E+3 | n/a | 6.20 | n/a | n/a | 7.4 | 22 |
Intervet Innovation GmbH | Assay Description In the binding assays, the results were expressed as percent inhibition of the control radioligand specific binding. The IC50 values (concentration c... | J Med Chem 52: 1773-7 (2009) Article DOI: 10.1021/jm801211c BindingDB Entry DOI: 10.7270/Q2H993HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM27956 ((rac)-Zilpaterol | 9-hydroxy-10-(propan-2-ylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 580 | -35.2 | 1.10E+3 | n/a | 13 | n/a | n/a | 7.4 | 22 |
Intervet Innovation GmbH | Assay Description In the binding assays, the results were expressed as percent inhibition of the control radioligand specific binding. The IC50 values (concentration c... | J Med Chem 52: 1773-7 (2009) Article DOI: 10.1021/jm801211c BindingDB Entry DOI: 10.7270/Q2H993HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM27957 ((+)-Zilpaterol | (9S,10S)-9-hydroxy-10-(propan-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 7.00E+3 | -29.1 | 1.70E+4 | n/a | 1.40E+5 | n/a | n/a | 7.4 | 22 |
Intervet Innovation GmbH | Assay Description In the binding assays, the results were expressed as percent inhibition of the control radioligand specific binding. The IC50 values (concentration c... | J Med Chem 52: 1773-7 (2009) Article DOI: 10.1021/jm801211c BindingDB Entry DOI: 10.7270/Q2H993HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM27955 ((-)-Zilpaterol | (9R,10R)-9-hydroxy-10-(propan-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+5 | -22.0 | 3.20E+5 | n/a | >1.00E+6 | n/a | n/a | 7.4 | 22 |
Intervet Innovation GmbH | Assay Description In the binding assays, the results were expressed as percent inhibition of the control radioligand specific binding. The IC50 values (concentration c... | J Med Chem 52: 1773-7 (2009) Article DOI: 10.1021/jm801211c BindingDB Entry DOI: 10.7270/Q2H993HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM27957 ((+)-Zilpaterol | (9S,10S)-9-hydroxy-10-(propan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.90E+3 | n/a | n/a | 7.4 | 22 |
Intervet Innovation GmbH | Assay Description In the binding assays, the results were expressed as percent inhibition of the control radioligand specific binding. The IC50 values (concentration c... | J Med Chem 52: 1773-7 (2009) Article DOI: 10.1021/jm801211c BindingDB Entry DOI: 10.7270/Q2H993HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||