

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477604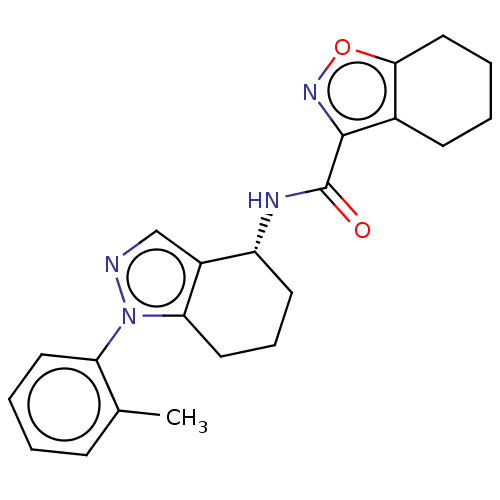 ((R)óN-(1-(2-methylphenyl)-4,5,6,7-tetrahydro-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477601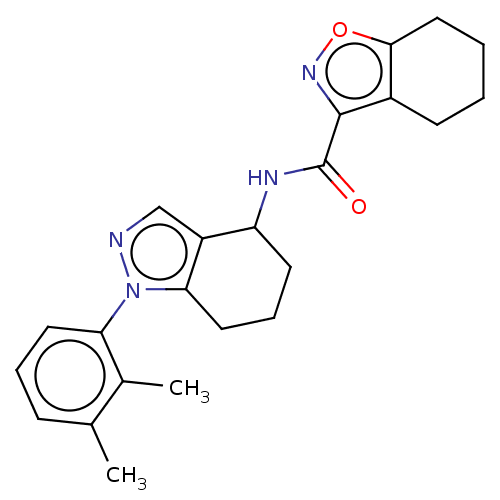 (N-[1-(2,3-dimethylphenyl)-4,5,6,7-tetrahydro-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477599 (N-[1-(2-methylphenyl)-4,5,6,7-tetrahydro-1H-indazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477616 ((R)óN-(1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477618 ((R)óN-(1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477586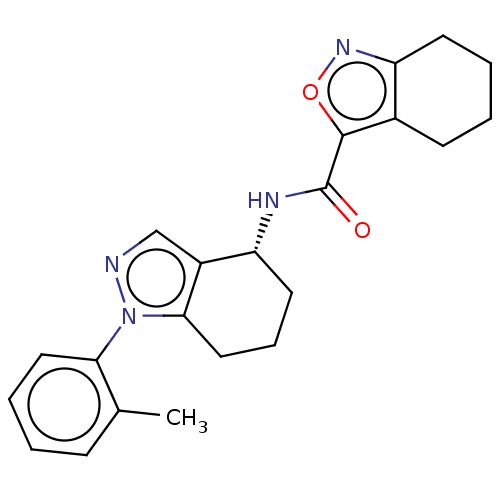 (N-[(4R)-1-(2-methylphenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477600 (N-[1-(3-methylphenyl)-4,5,6,7-tetrahydro-1H-indazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477584 (N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477583 (N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477622 (Propan-2-yl-3-[(4R)-4-(4,5,6,7-tetrahydro-1,2-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477595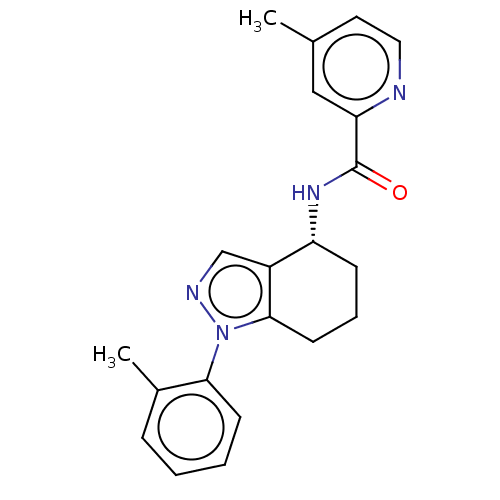 (4-methyl-N-[(4R)-1-(2-methylphenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477619 ((R)óN-(1-(2-fluoro-5-methylphenyl)-4,5,6,7-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477598 (N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477609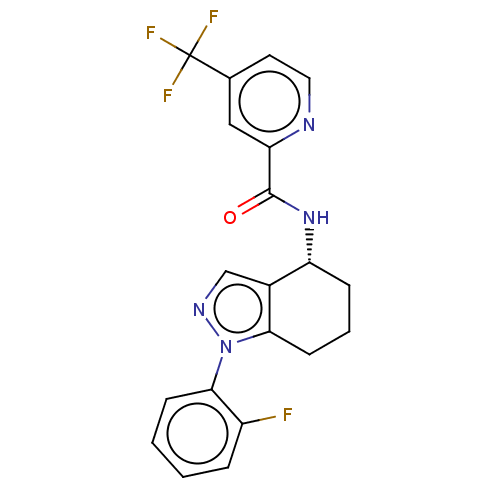 (N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477602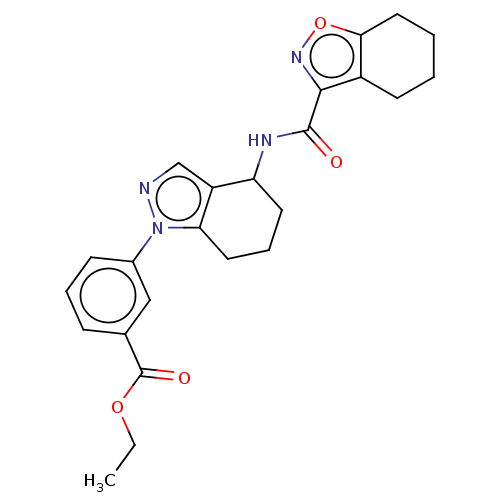 (Ethyl 3-[4-(4,5,6,7-tetrahydro-1,2-benzoxazole-3-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477606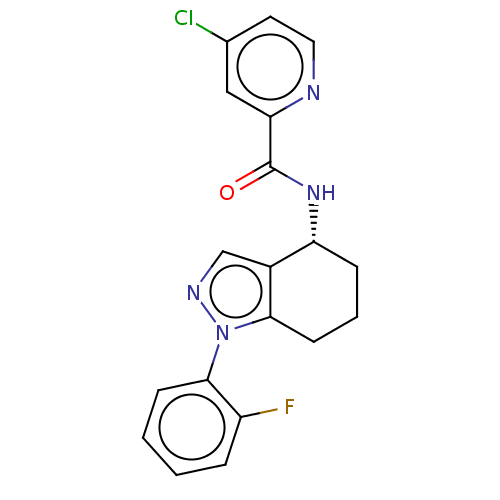 (4-chloro-N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477621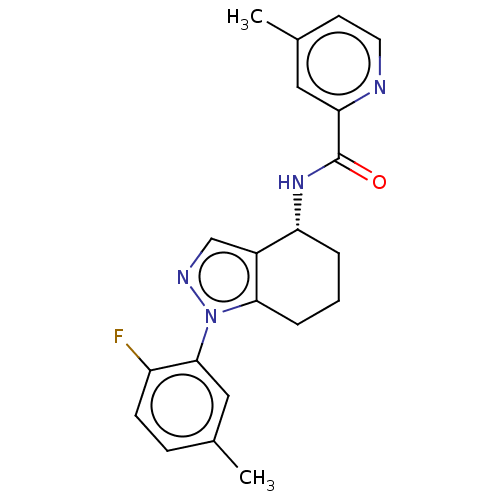 (N-[(4R)-1-(2-fluoro-5-methylphenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477592 (N-(1-phenyl-4,5,6,7-tetrahydro-1H-indazol-4-yl)-4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477579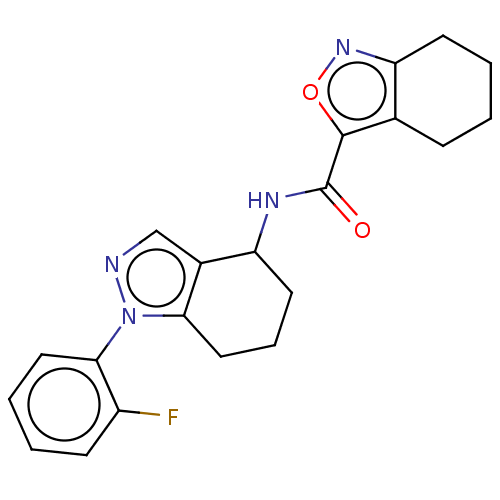 (N-(1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-indazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay A: DHODH enzyme assays were performed with 6 nM recombinant human DHODH (purified essentially as described by Walse et. al., Biochemistry, 47, ... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477614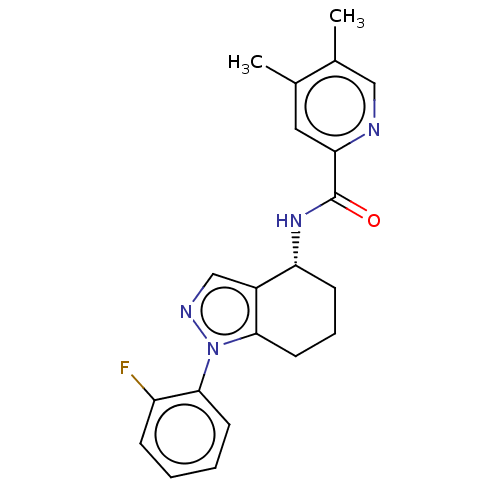 (N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477607 (4-bromo-N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM350085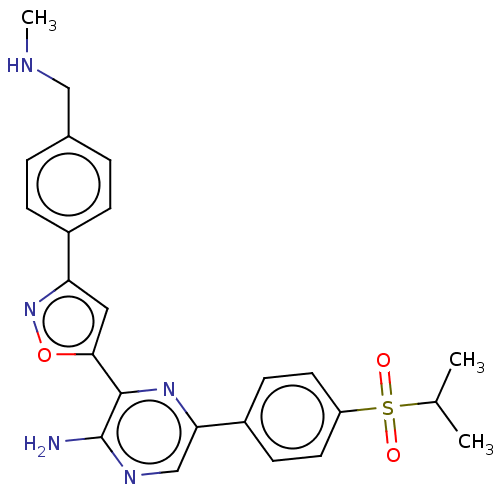 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477612 ((R)óN-(1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477617 (4-fluoro-N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477594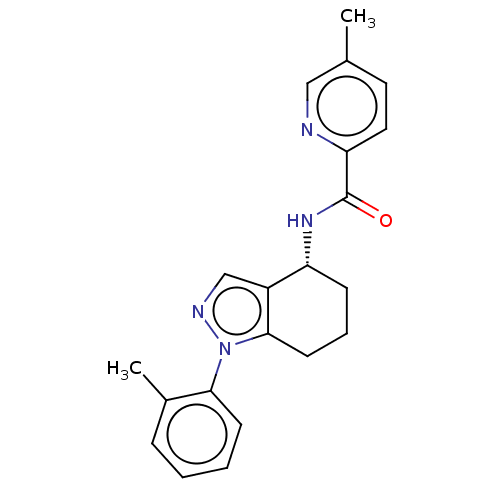 (5-methyl-N-[(4R)-1-(2-methylphenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477611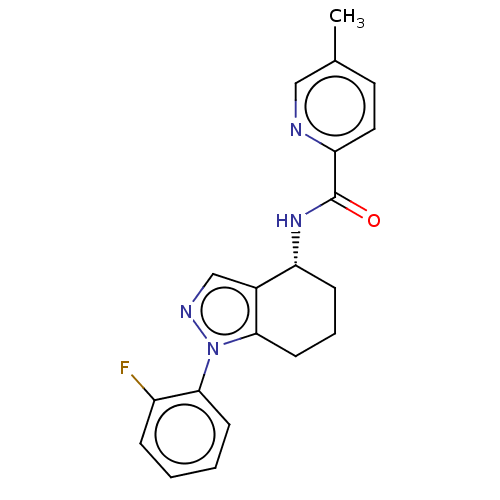 (N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477603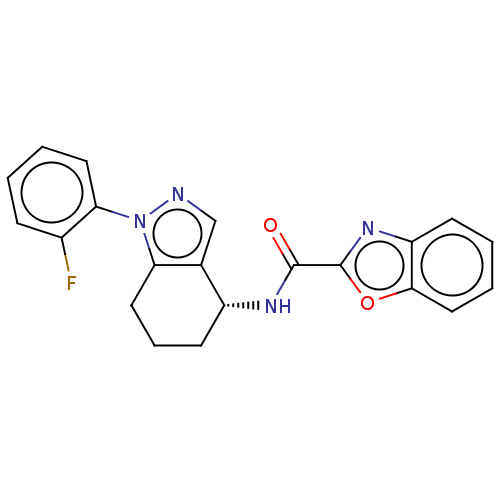 (N-[(4R)-1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477596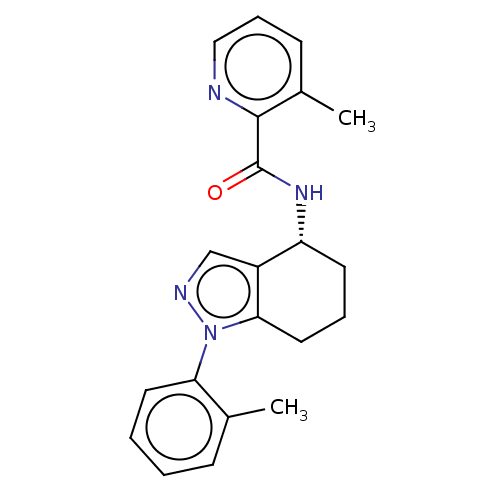 (3-methyl-N-[(4R)-1-(2-methylphenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477597 (N-(1-phenyl-4,5,6,7-tetrahydro-1H-indazol-4-yl)-4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477620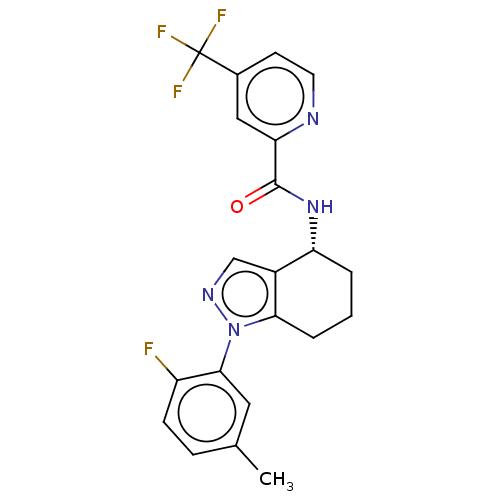 (N-[(4R)-1-(2-fluoro-5-methylphenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477587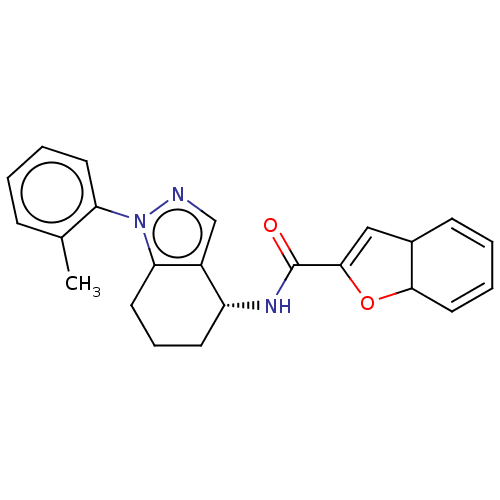 (N-[(4R)-1-(2-methylphenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477589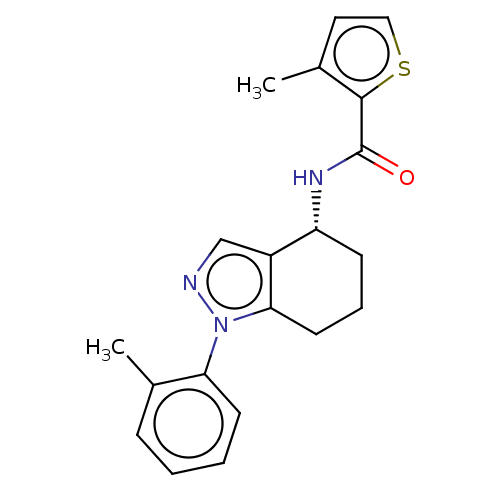 (3-methyl-N-[(4R)-1-(2-methylphenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477574 ((R)óN-(1-(2-fluorophenyl)-4,5,6,7-tetrahydro-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay A: DHODH enzyme assays were performed with 6 nM recombinant human DHODH (purified essentially as described by Walse et. al., Biochemistry, 47, ... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339398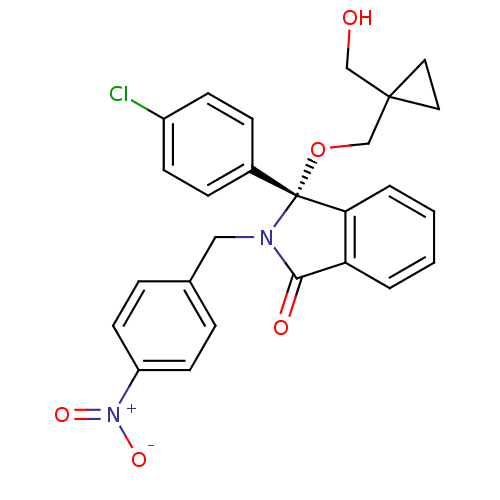 ((+)-R-3-(4-Chlorophenyl)-3-(1-hydroxymethylcyclopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477623 (US10889564, Example 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339369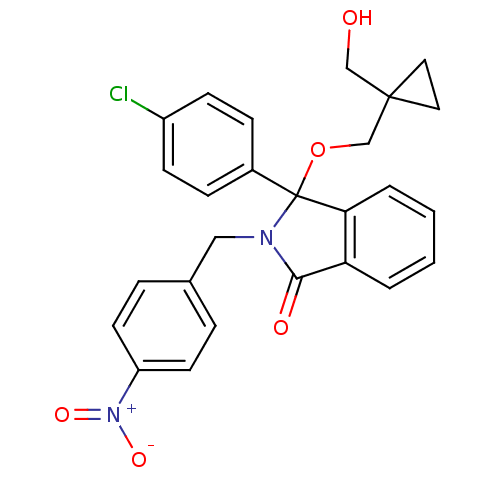 ((+/-)-3-(4-chlorophenyl)-3-((1-(hydroxymethyl)cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477576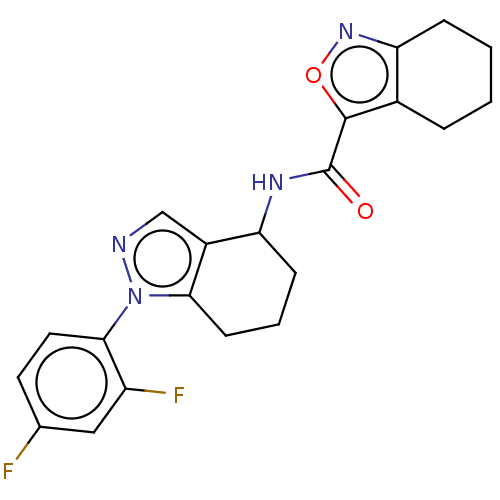 (N-(1-(2,4-difluorophenyl)-4,5,6,7-tetrahydro-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay A: DHODH enzyme assays were performed with 6 nM recombinant human DHODH (purified essentially as described by Walse et. al., Biochemistry, 47, ... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339371 ((+/-)-trans-3-(4-Chlorophenyl)-3-(4-hydroxycyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477590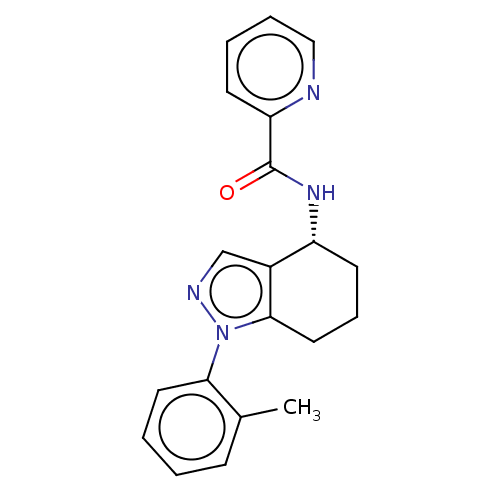 (N-[(4R)-1-(2-methylphenyl)-4,5,6,7-tetrahydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339355 ((+/-)-3-(4-Chlorophenyl)-3-(4-hydroxybutoxy)-2-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339365 ((+/-)-trans-3-(4-Chlorophenyl)-3-(5-hydroxycyclooc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339370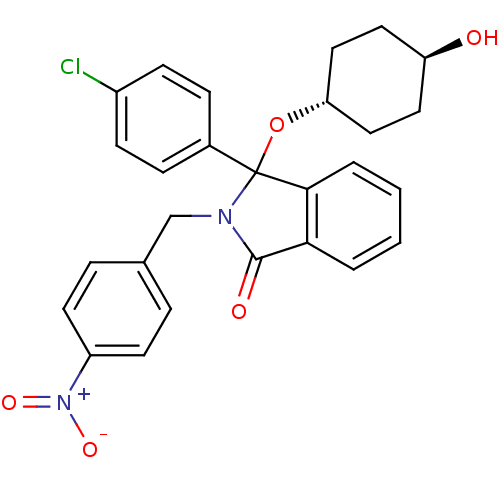 ((+/-)-trans-3-(4-Chlorophenyl)-3-(4-hydroxycyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339368 ((+/-)-3-(4-Chlorophenyl)-3-(3-hydroxy-2,2-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339353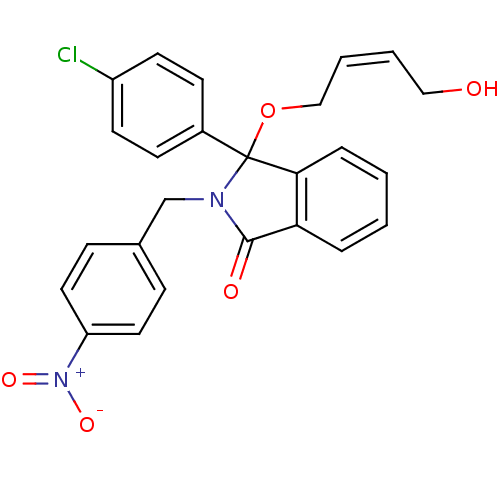 ((+/-)-(Z)-3-(4-Chlorophenyl)-3-(4-hydroxybut-2-eny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339437 ((+/-)-3-(4-chlorophenyl)-3-((cis)-3-hydroxycyclope...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477613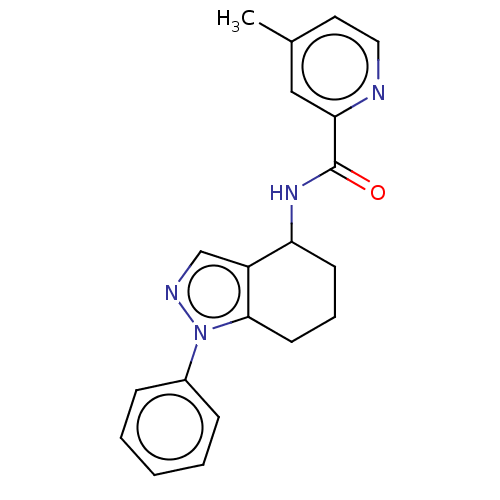 (4-methyl-N-(1-phenyl-4,5,6,7-tetrahydro-1H-indazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 441 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339354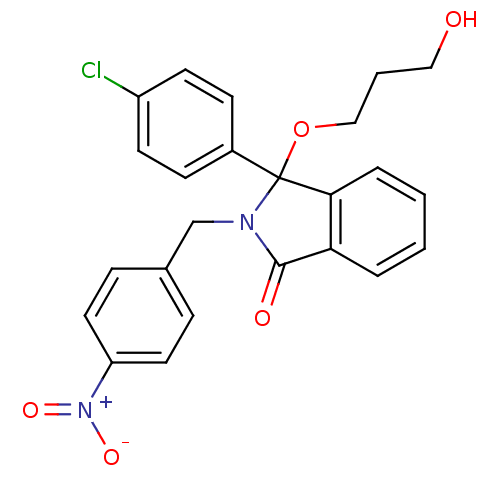 ((+/-)-3-(4-Chlorophenyl)-3-(3-hydroxypropoxy)-2-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM477588 (3-methyl-N-[(4R)-1-(2-methylphenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
Genase Therapeutics B.V. US Patent | Assay Description Assay B: Example compounds as described herein were dispensed with an acoustic liquid handler, Echo 550 (Labcyte) to a maximum volume of 50 nl per we... | US Patent US10889564 (2021) BindingDB Entry DOI: 10.7270/Q2N019MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50339361 ((+/-)-3-(4-Bromophenyl)-3-(4-hydroxybutoxy)-2-(4-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of Mdm2 -p53 protein interaction by ELISA | J Med Chem 54: 1233-43 (2011) Article DOI: 10.1021/jm1011929 BindingDB Entry DOI: 10.7270/Q29K4BHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 150 total ) | Next | Last >> |