 Found 2658 hits with Last Name = 'du' and Initial = 'f'
Found 2658 hits with Last Name = 'du' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50399614
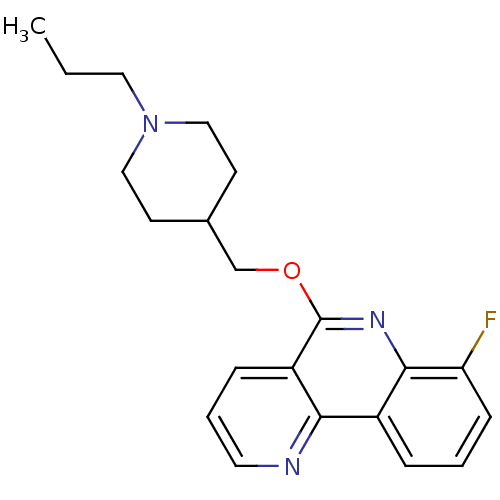
(CHEMBL2181170)Show InChI InChI=1S/C21H24FN3O/c1-2-11-25-12-8-15(9-13-25)14-26-21-17-6-4-10-23-19(17)16-5-3-7-18(22)20(16)24-21/h3-7,10,15H,2,8-9,11-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human 5HT4R by Cerep protocol based assay |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50399614

(CHEMBL2181170)Show InChI InChI=1S/C21H24FN3O/c1-2-11-25-12-8-15(9-13-25)14-26-21-17-6-4-10-23-19(17)16-5-3-7-18(22)20(16)24-21/h3-7,10,15H,2,8-9,11-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human 5HT4R by Cerep protocol based assay |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485941

(CHEMBL2181004)Show SMILES COc1ccc(CN[C@H]2CCc3cc(OC)c(OC)c(OC)c3-c3ccc(OC)c(=O)cc23)cc1 |r| Show InChI InChI=1S/C28H31NO6/c1-31-19-9-6-17(7-10-19)16-29-22-12-8-18-14-25(33-3)27(34-4)28(35-5)26(18)20-11-13-24(32-2)23(30)15-21(20)22/h6-7,9-11,13-15,22,29H,8,12,16H2,1-5H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485945

(CHEMBL2181003)Show SMILES COc1cc2CC[C@H](NCc3ccc(cc3)[N+]([O-])=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28N2O7/c1-33-23-12-10-19-20(14-22(23)30)21(28-15-16-5-8-18(9-6-16)29(31)32)11-7-17-13-24(34-2)26(35-3)27(36-4)25(17)19/h5-6,8-10,12-14,21,28H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485942

(CHEMBL2181002)Show SMILES COc1cc2CC[C@H](NCc3cc(F)c(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H26F3NO5/c1-33-22-8-6-16-17(12-21(22)32)20(31-13-14-9-18(28)25(30)19(29)10-14)7-5-15-11-23(34-2)26(35-3)27(36-4)24(15)16/h6,8-12,20,31H,5,7,13H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598740
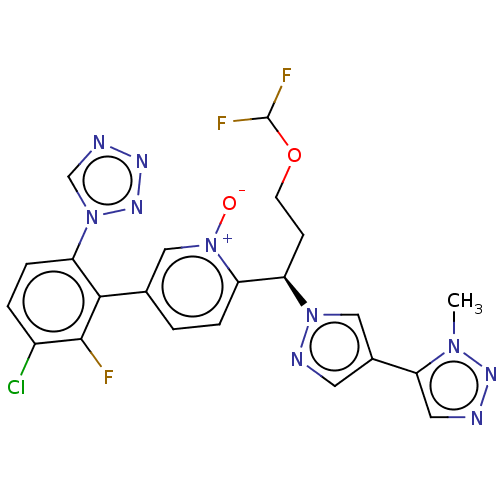
(CHEMBL5175227)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485950
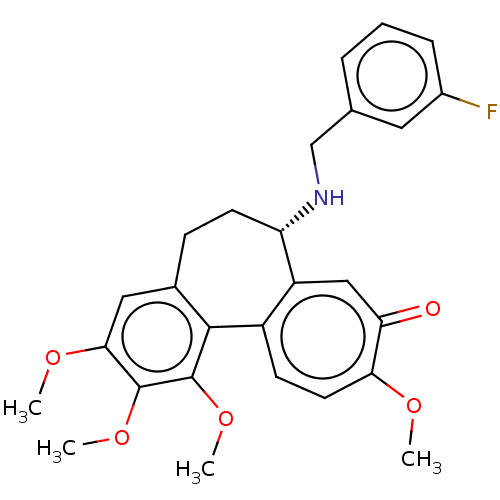
(CHEMBL2181009)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-11-9-19-20(14-22(23)30)21(29-15-16-6-5-7-18(28)12-16)10-8-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-7,9,11-14,21,29H,8,10,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485944

(CHEMBL2181006)Show SMILES COc1cc2CC[C@H](NCc3ccc(Cl)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28ClNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598738
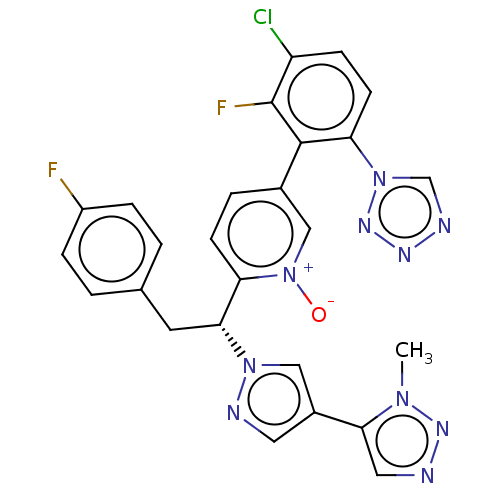
(CHEMBL5204065)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598739

(CHEMBL5188215)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485943
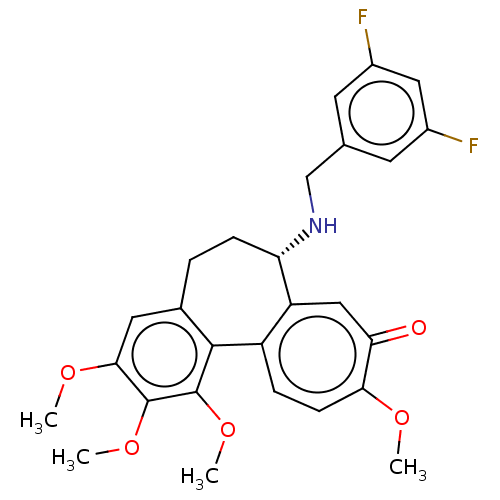
(CHEMBL2181001)Show SMILES COc1cc2CC[C@H](NCc3cc(F)cc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-8-6-19-20(13-22(23)31)21(30-14-15-9-17(28)12-18(29)10-15)7-5-16-11-24(33-2)26(34-3)27(35-4)25(16)19/h6,8-13,21,30H,5,7,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485946
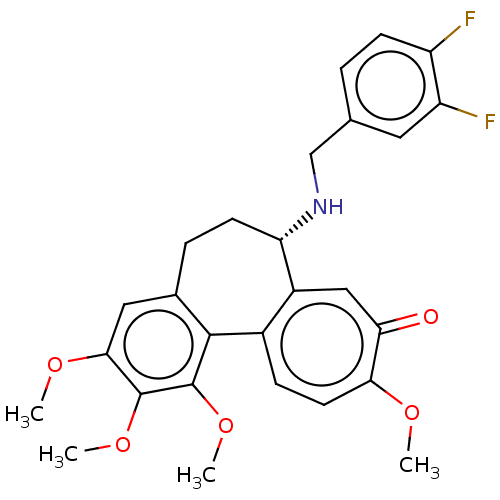
(CHEMBL2181000)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-10-7-17-18(13-22(23)31)21(30-14-15-5-8-19(28)20(29)11-15)9-6-16-12-24(33-2)26(34-3)27(35-4)25(16)17/h5,7-8,10-13,21,30H,6,9,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598724

(CHEMBL5170592)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta chain
(Sus scrofa) | BDBM50485947
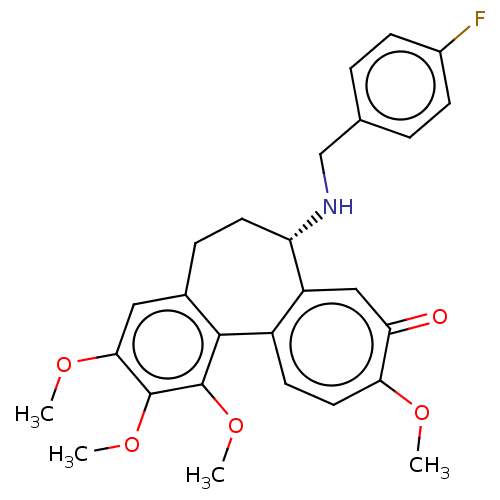
(CHEMBL2181008)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485949

(CHEMBL2180999)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3F)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-22-11-9-17-18(13-21(22)31)20(30-14-16-6-5-7-19(28)25(16)29)10-8-15-12-23(33-2)26(34-3)27(35-4)24(15)17/h5-7,9,11-13,20,30H,8,10,14H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598737
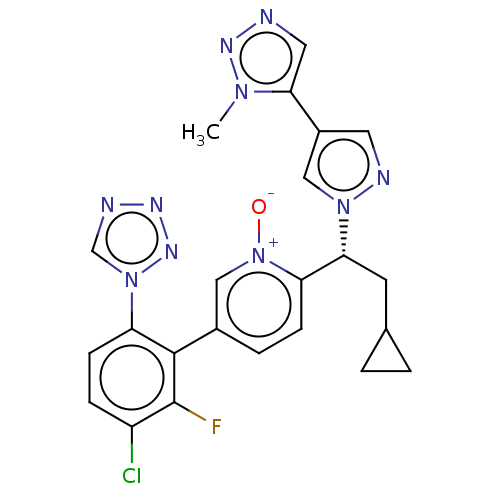
(CHEMBL5205631)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598741

(CHEMBL5204894)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50399621
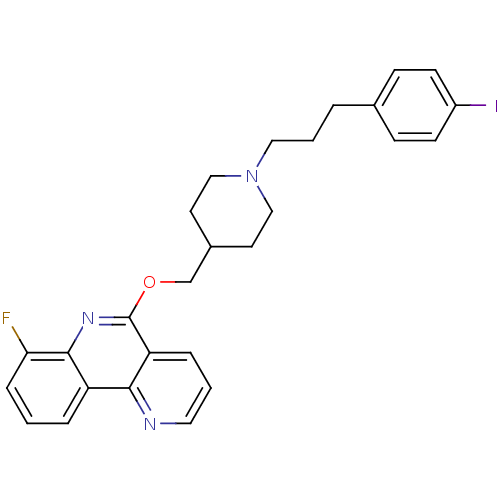
(CHEMBL2181166)Show SMILES Fc1cccc2c1nc(OCC1CCN(CCCc3ccc(I)cc3)CC1)c1cccnc21 Show InChI InChI=1S/C27H27FIN3O/c28-24-7-1-5-22-25-23(6-2-14-30-25)27(31-26(22)24)33-18-20-12-16-32(17-13-20)15-3-4-19-8-10-21(29)11-9-19/h1-2,5-11,14,20H,3-4,12-13,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human 5HT4R by Cerep protocol based assay |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598725

(CHEMBL5185397)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnc([nH]1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598743
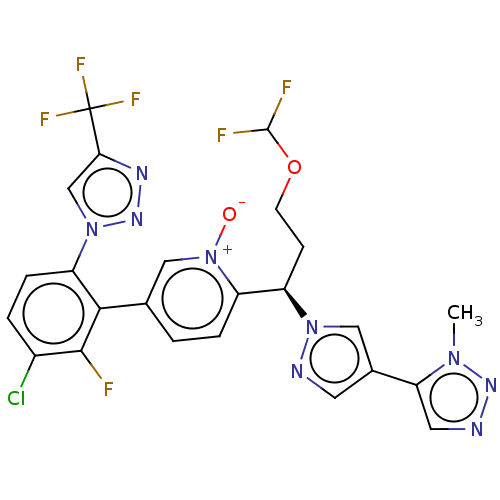
(CHEMBL5178223)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50399622
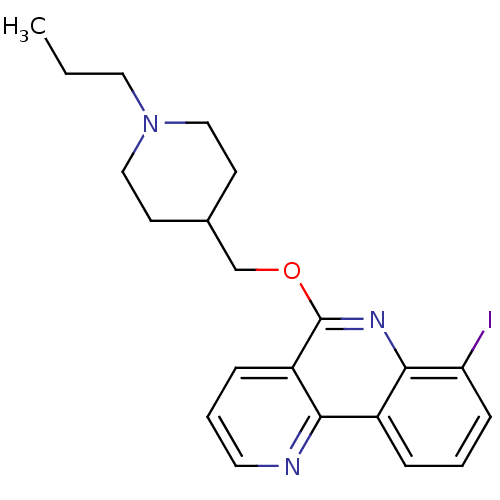
(CHEMBL2181168)Show InChI InChI=1S/C21H24IN3O/c1-2-11-25-12-8-15(9-13-25)14-26-21-17-6-4-10-23-19(17)16-5-3-7-18(22)20(16)24-21/h3-7,10,15H,2,8-9,11-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human 5HT4R by Cerep protocol based assay |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485948
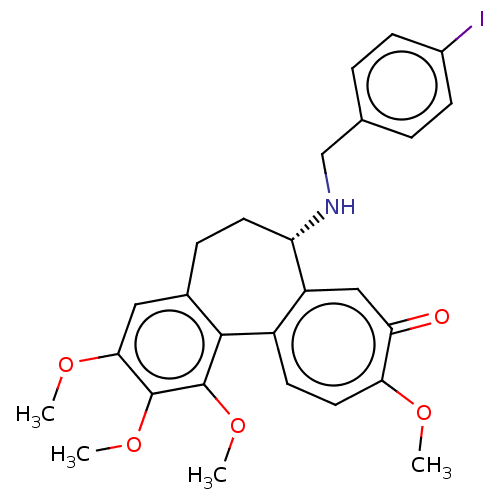
(CHEMBL2181007)Show SMILES COc1cc2CC[C@H](NCc3ccc(I)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28INO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598745
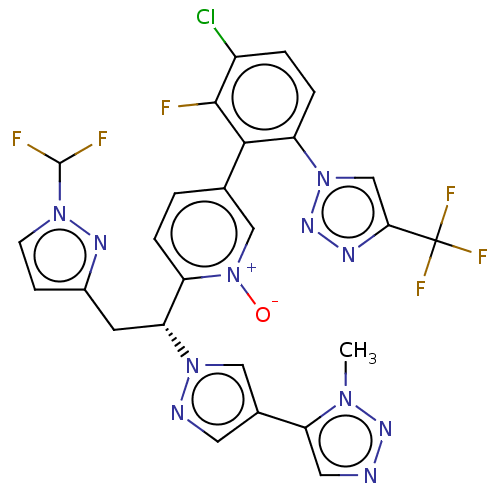
(CHEMBL5198823)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccn(n1)C(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598734
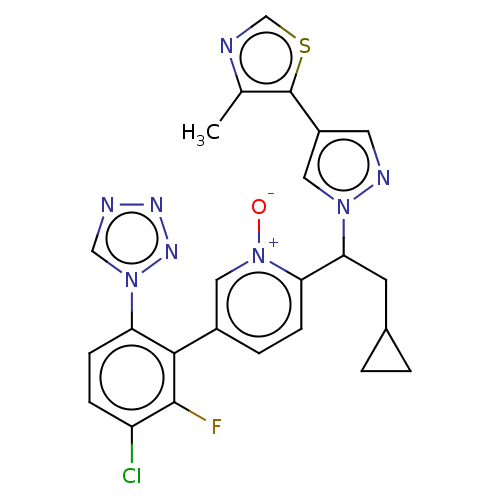
(CHEMBL5197480)Show SMILES Cc1ncsc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta chain
(Sus scrofa) | BDBM50485940

(CHEMBL2181005)Show SMILES COc1cc2CC[C@H](NCc3ccc(Br)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28BrNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598736

(CHEMBL5208095)Show SMILES Cn1cncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598744
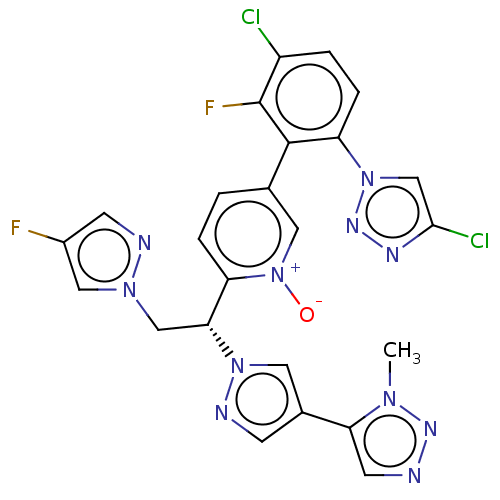
(CHEMBL5190323)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cn1cc(F)cn1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598732
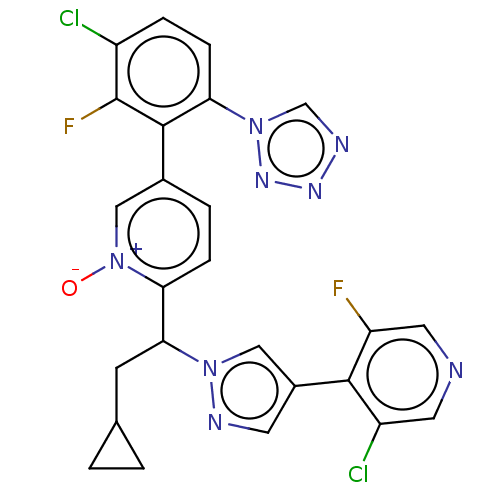
(CHEMBL5192284)Show SMILES [O-][n+]1cc(ccc1C(CC1CC1)n1cc(cn1)-c1c(F)cncc1Cl)-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50331549
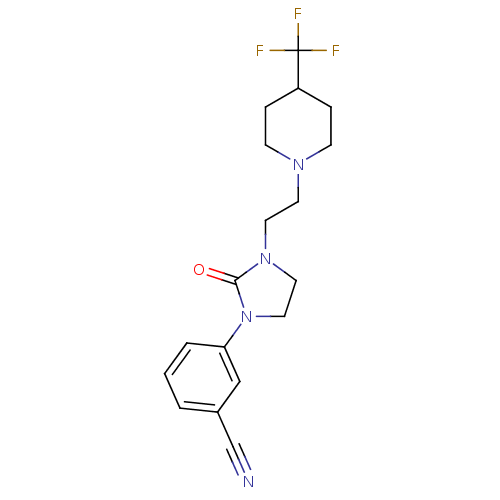
(CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...)Show SMILES FC(F)(F)C1CCN(CCN2CCN(C2=O)c2cccc(c2)C#N)CC1 Show InChI InChI=1S/C18H21F3N4O/c19-18(20,21)15-4-6-23(7-5-15)8-9-24-10-11-25(17(24)26)16-3-1-2-14(12-16)13-22/h1-3,12,15H,4-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D3 receptor by filtration binding assay |
Bioorg Med Chem Lett 19: 4011-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.028
BindingDB Entry DOI: 10.7270/Q2J67J55 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50331549

(CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...)Show SMILES FC(F)(F)C1CCN(CCN2CCN(C2=O)c2cccc(c2)C#N)CC1 Show InChI InChI=1S/C18H21F3N4O/c19-18(20,21)15-4-6-23(7-5-15)8-9-24-10-11-25(17(24)26)16-3-1-2-14(12-16)13-22/h1-3,12,15H,4-11H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor in rat native tissue by filtration binding assay |
Bioorg Med Chem Lett 19: 4011-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.028
BindingDB Entry DOI: 10.7270/Q2J67J55 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50432574
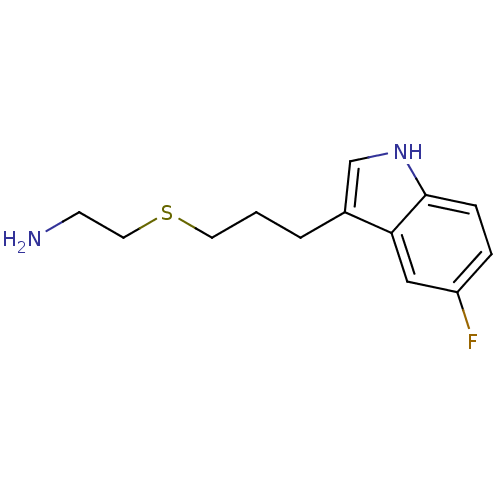
(CHEMBL2347169)Show InChI InChI=1S/C13H17FN2S/c14-11-3-4-13-12(8-11)10(9-16-13)2-1-6-17-7-5-15/h3-4,8-9,16H,1-2,5-7,15H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles
Curated by ChEMBL
| Assay Description
Inhibition of serotonin reuptake at human SERT expressed in HEK293 cells coexpressing macrophage scavenger receptor |
J Med Chem 56: 3943-58 (2013)
Article DOI: 10.1021/jm4001538
BindingDB Entry DOI: 10.7270/Q21C1Z7T |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50331549

(CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...)Show SMILES FC(F)(F)C1CCN(CCN2CCN(C2=O)c2cccc(c2)C#N)CC1 Show InChI InChI=1S/C18H21F3N4O/c19-18(20,21)15-4-6-23(7-5-15)8-9-24-10-11-25(17(24)26)16-3-1-2-14(12-16)13-22/h1-3,12,15H,4-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells by [35S]GTP-gamma-S-based scintillation spectrometry |
Bioorg Med Chem Lett 19: 4011-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.028
BindingDB Entry DOI: 10.7270/Q2J67J55 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414436
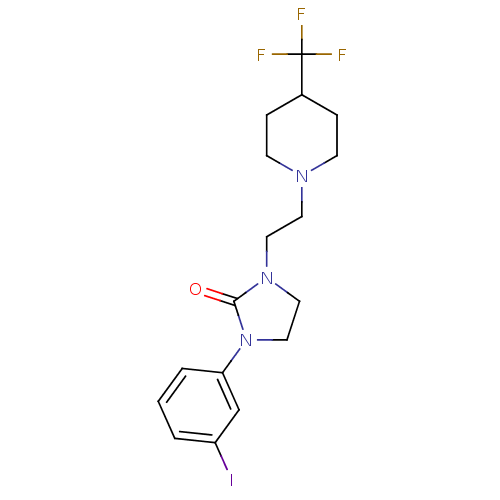
(CHEMBL560574)Show InChI InChI=1S/C17H21F3IN3O/c18-17(19,20)13-4-6-22(7-5-13)8-9-23-10-11-24(16(23)25)15-3-1-2-14(21)12-15/h1-3,12-13H,4-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells by [35S]GTP-gamma-S-based scintillation spectrometry |
Bioorg Med Chem Lett 19: 4011-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.028
BindingDB Entry DOI: 10.7270/Q2J67J55 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598742
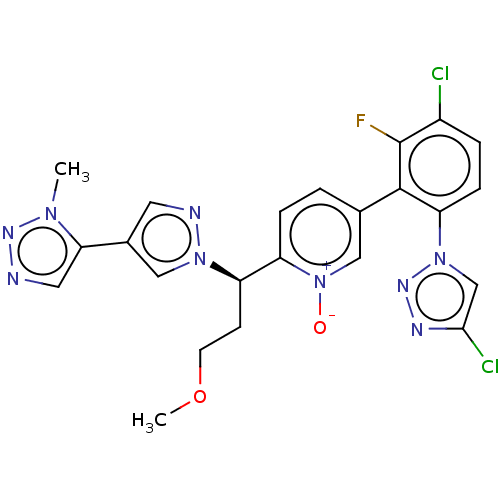
(CHEMBL5182855)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50399623

(CHEMBL2181169)Show InChI InChI=1S/C22H25IN2O/c1-2-12-25-13-10-16(11-14-25)15-26-22-19-7-4-3-6-17(19)18-8-5-9-20(23)21(18)24-22/h3-9,16H,2,10-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human 5HT4R by Cerep protocol based assay |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414406
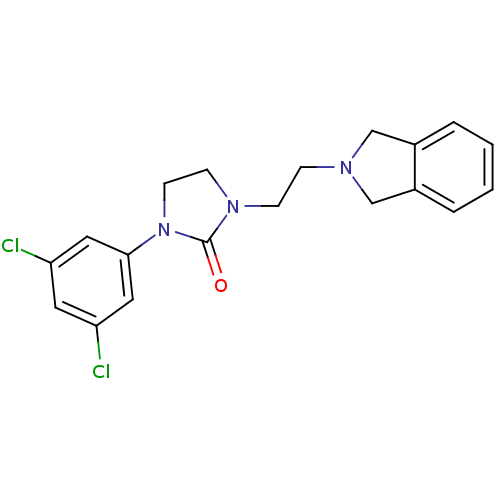
(CHEMBL561512)Show InChI InChI=1S/C19H19Cl2N3O/c20-16-9-17(21)11-18(10-16)24-8-7-23(19(24)25)6-5-22-12-14-3-1-2-4-15(14)13-22/h1-4,9-11H,5-8,12-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells by [35S]GTP-gamma-S-based scintillation spectrometry |
Bioorg Med Chem Lett 19: 4011-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.028
BindingDB Entry DOI: 10.7270/Q2J67J55 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414435

(CHEMBL569846)Show InChI InChI=1S/C17H22F3N3O2/c18-17(19,20)13-4-6-21(7-5-13)8-9-22-10-11-23(16(22)25)14-2-1-3-15(24)12-14/h1-3,12-13,24H,4-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells by [35S]GTP-gamma-S-based scintillation spectrometry |
Bioorg Med Chem Lett 19: 4011-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.028
BindingDB Entry DOI: 10.7270/Q2J67J55 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50027366
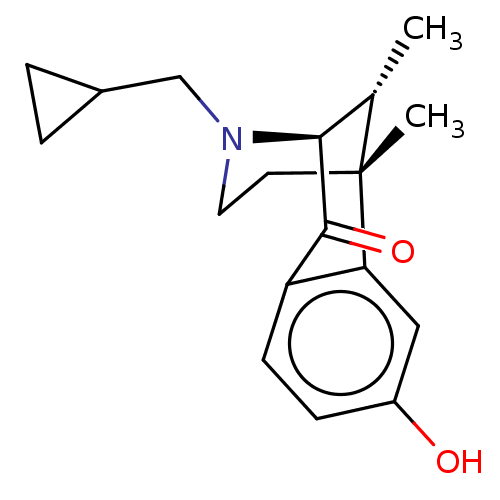
(Ketazocine | US10081602, Example Ketazocine | US10...)Show SMILES [H][C@@]12[C@H](C)[C@@](C)(CCN1CC1CC1)c1cc(O)ccc1C2=O |TLB:3:2:20.13.19:8.6.7| Show InChI InChI=1S/C18H23NO2/c1-11-16-17(21)14-6-5-13(20)9-15(14)18(11,2)7-8-19(16)10-12-3-4-12/h5-6,9,11-12,16,20H,3-4,7-8,10H2,1-2H3/t11-,16-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 1.30 | -50.7 | 5.60 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Nektar Therapeutics
US Patent
| Assay Description
Competition binding experiments were conducted by incubating membrane protein to equilibrium in triplicate in the presence of a fixed concentration o... |
US Patent US9688638 (2017)
BindingDB Entry DOI: 10.7270/Q2VM49FN |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50027366

(Ketazocine | US10081602, Example Ketazocine | US10...)Show SMILES [H][C@@]12[C@H](C)[C@@](C)(CCN1CC1CC1)c1cc(O)ccc1C2=O |TLB:3:2:20.13.19:8.6.7| Show InChI InChI=1S/C18H23NO2/c1-11-16-17(21)14-6-5-13(20)9-15(14)18(11,2)7-8-19(16)10-12-3-4-12/h5-6,9,11-12,16,20H,3-4,7-8,10H2,1-2H3/t11-,16-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nektar Therapeutics
US Patent
| Assay Description
The binding affinities of certain compounds of the present invention were evaluated using radioligand binding assays in membranes prepared from CHO-K... |
US Patent US10081602 (2018)
BindingDB Entry DOI: 10.7270/Q2N87CT7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50027366

(Ketazocine | US10081602, Example Ketazocine | US10...)Show SMILES [H][C@@]12[C@H](C)[C@@](C)(CCN1CC1CC1)c1cc(O)ccc1C2=O |TLB:3:2:20.13.19:8.6.7| Show InChI InChI=1S/C18H23NO2/c1-11-16-17(21)14-6-5-13(20)9-15(14)18(11,2)7-8-19(16)10-12-3-4-12/h5-6,9,11-12,16,20H,3-4,7-8,10H2,1-2H3/t11-,16-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nektar Therapeutics
US Patent
| Assay Description
The binding affinities of certain compounds of the present invention were evaluated using radioligand binding assays in membranes prepared from CHO-K... |
US Patent US10865186 (2020)
BindingDB Entry DOI: 10.7270/Q2GM8BDQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598729
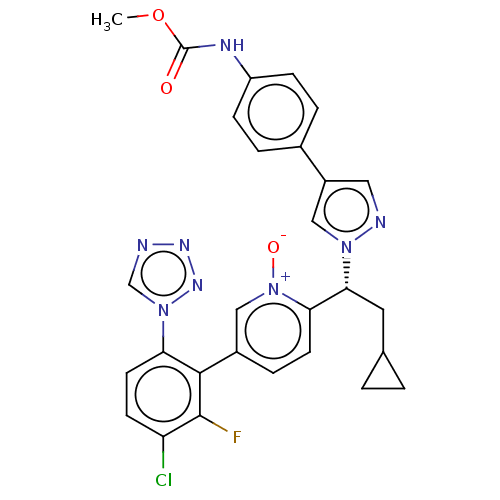
(CHEMBL5195600)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598727
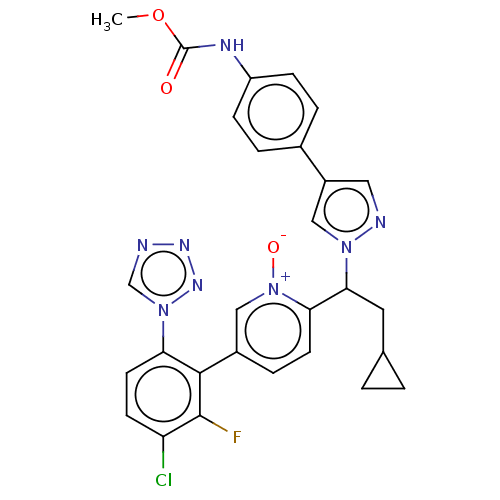
(CHEMBL5198338)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414413
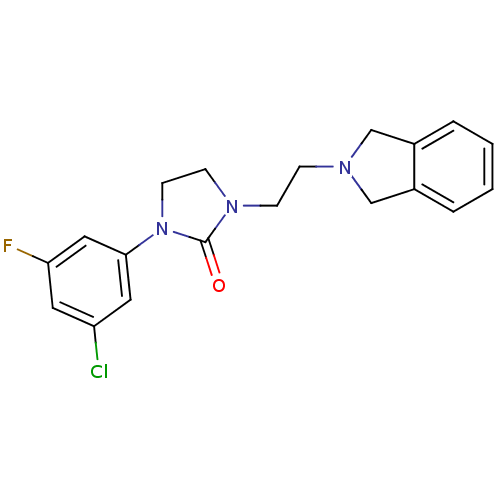
(CHEMBL556272)Show InChI InChI=1S/C19H19ClFN3O/c20-16-9-17(21)11-18(10-16)24-8-7-23(19(24)25)6-5-22-12-14-3-1-2-4-15(14)13-22/h1-4,9-11H,5-8,12-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells by [35S]GTP-gamma-S-based scintillation spectrometry |
Bioorg Med Chem Lett 19: 4011-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.028
BindingDB Entry DOI: 10.7270/Q2J67J55 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414409

(CHEMBL565017)Show SMILES FC(F)(F)Oc1cccc(c1)N1CCN(CCN2Cc3ccccc3C2)C1=O Show InChI InChI=1S/C20H20F3N3O2/c21-20(22,23)28-18-7-3-6-17(12-18)26-11-10-25(19(26)27)9-8-24-13-15-4-1-2-5-16(15)14-24/h1-7,12H,8-11,13-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells by [35S]GTP-gamma-S-based scintillation spectrometry |
Bioorg Med Chem Lett 19: 4011-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.028
BindingDB Entry DOI: 10.7270/Q2J67J55 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414437
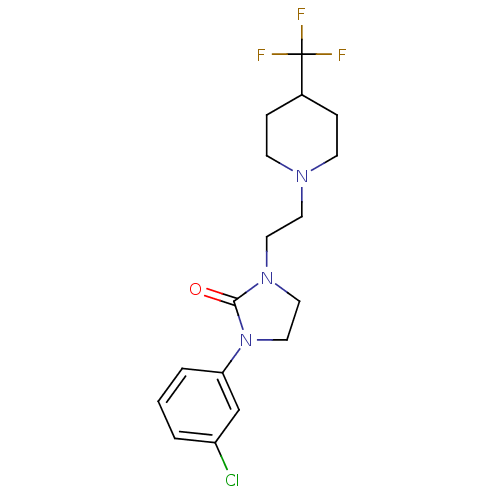
(CHEMBL562624)Show SMILES FC(F)(F)C1CCN(CCN2CCN(C2=O)c2cccc(Cl)c2)CC1 Show InChI InChI=1S/C17H21ClF3N3O/c18-14-2-1-3-15(12-14)24-11-10-23(16(24)25)9-8-22-6-4-13(5-7-22)17(19,20)21/h1-3,12-13H,4-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D3 receptor expressed in CHO cells by [35S]GTP-gamma-S-based scintillation spectrometry |
Bioorg Med Chem Lett 19: 4011-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.028
BindingDB Entry DOI: 10.7270/Q2J67J55 |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50014846
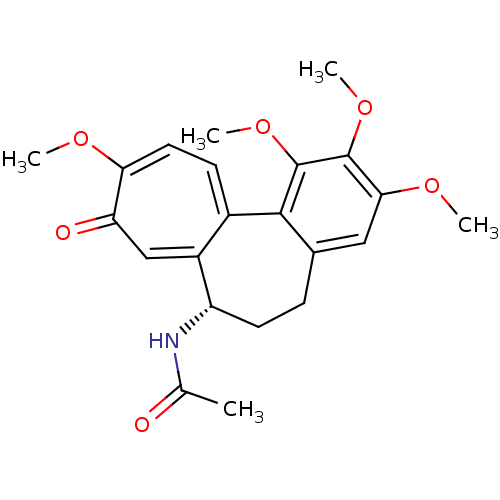
((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598735
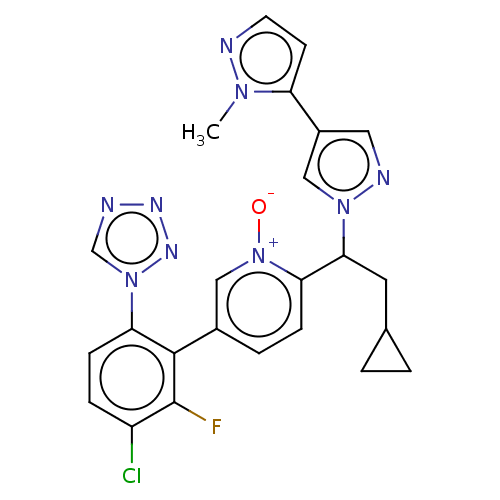
(CHEMBL5193267)Show SMILES Cn1nccc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Furin
(Homo sapiens (Human)) | BDBM50448473
(CHEMBL3126399)Show SMILES [#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C49H90N24O8/c1-28(67-41(77)33(68-29(2)74)13-7-23-62-46(54)55)39(75)69-35(14-8-24-63-47(56)57)43(79)72-37(16-10-26-65-49(60)61)45(81)73-36(15-9-25-64-48(58)59)44(80)71-34(12-4-6-22-51)42(78)70-32(11-3-5-21-50)40(76)66-27-30-17-19-31(20-18-30)38(52)53/h17-20,28,32-37H,3-16,21-27,50-51H2,1-2H3,(H3,52,53)(H,66,76)(H,67,77)(H,68,74)(H,69,75)(H,70,78)(H,71,80)(H,72,79)(H,73,81)(H4,54,55,62)(H4,56,57,63)(H4,58,59,64)(H4,60,61,65)/t28-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant furin (unknown origin) using as substrate after 60 mins |
J Med Chem 57: 29-41 (2014)
Article DOI: 10.1021/jm400633d
BindingDB Entry DOI: 10.7270/Q27M09FJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50042235
(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Angiotensin II receptor, type 1 of human hepatoma cell line PLC-PRF-5 |
J Med Chem 45: 4794-8 (2002)
BindingDB Entry DOI: 10.7270/Q2QC02TH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50399614

(CHEMBL2181170)Show InChI InChI=1S/C21H24FN3O/c1-2-11-25-12-8-15(9-13-25)14-26-21-17-6-4-10-23-19(17)16-5-3-7-18(22)20(16)24-21/h3-7,10,15H,2,8-9,11-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4R in guinea pig striatal membranes |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data