 Found 335 hits with Last Name = 'dupont' and Initial = 'm'
Found 335 hits with Last Name = 'dupont' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537072

(CHEMBL440072)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N Show InChI InChI=1S/C63H88N16O16S2/c1-34(66)53(84)69-30-51(83)70-48-32-96-97-33-49(63(94)95)78-60(91)47(31-80)77-62(93)52(35(2)81)79-55(86)42(22-12-14-24-65)71-58(89)45(27-38-29-68-40-20-10-9-19-39(38)40)75-57(88)44(26-37-17-7-4-8-18-37)73-56(87)43(25-36-15-5-3-6-16-36)74-59(90)46(28-50(67)82)76-54(85)41(72-61(48)92)21-11-13-23-64/h3-10,15-20,29,34-35,41-49,52,68,80-81H,11-14,21-28,30-33,64-66H2,1-2H3,(H2,67,82)(H,69,84)(H,70,83)(H,71,89)(H,72,92)(H,73,87)(H,74,90)(H,75,88)(H,76,85)(H,77,93)(H,78,91)(H,79,86)(H,94,95)/t34-,35+,41+,42-,43+,44-,45+,46-,47-,48+,49-,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537063

(CHEMBL4590517)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(O)=O |r,t:17,19| Show InChI InChI=1S/C83H108ClN13O21S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)95-83)45(2)71-82(5,118-71)66(38-68(101)97(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)96(6)67(100)29-31-119-120-43-61(79(109)110)93-77(107)60-42-122-121-41-59(91-72(102)54(86)33-48-19-11-10-12-20-48)76(106)89-57(34-49-25-27-52(99)28-26-49)74(104)90-58(37-51-40-87-55-22-14-13-21-53(51)55)75(105)88-56(23-15-16-30-85)73(103)94-70(47(4)98)78(108)92-60/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,87,98-99,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H,88,105)(H,89,106)(H,90,104)(H,91,102)(H,92,108)(H,93,107)(H,94,103)(H,95,112)(H,109,110)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537069
(CHEMBL4584764)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC(C)(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C85H113ClN14O20S4/c1-45-20-19-26-65(117-11)85(115)41-64(118-82(114)98-85)46(2)72-84(7,120-72)66(40-68(104)100(9)62-37-51(34-45)38-63(116-10)69(62)86)119-81(113)47(3)99(8)67(103)31-33-121-124-83(5,6)71(73(89)105)97-79(111)61-44-123-122-43-60(94-74(106)55(88)35-49-21-13-12-14-22-49)78(110)92-58(36-50-27-29-53(102)30-28-50)76(108)93-59(39-52-42-90-56-24-16-15-23-54(52)56)77(109)91-57(25-17-18-32-87)75(107)96-70(48(4)101)80(112)95-61/h12-16,19-24,26-30,37-38,42,46-48,55,57-61,64-66,70-72,90,101-102,115H,17-18,25,31-36,39-41,43-44,87-88H2,1-11H3,(H2,89,105)(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,114)/b26-19+,45-20+/t46-,47+,48-,55-,57+,58+,59-,60+,61+,64+,65-,66+,70+,71-,72+,84-,85+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537077

(CHEMBL4550617)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCOc1ccc(C[C@@H](N)C(=O)N[C@H]2CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)cc1 |r,t:17,19| Show InChI InChI=1S/C86H114ClN13O23S4/c1-45-16-15-20-67(119-10)86(117)41-66(121-84(116)98-86)46(2)74-85(6,123-74)68(40-70(105)100(8)64-37-52(34-45)38-65(118-9)71(64)87)122-83(115)47(3)99(7)69(104)29-32-124-125-33-31-120-55-27-23-50(24-28-55)35-57(89)75(106)94-62-43-126-127-44-63(80(111)97-73(49(5)102)82(113)114)95-81(112)72(48(4)101)96-76(107)59(19-13-14-30-88)91-78(109)61(39-53-42-90-58-18-12-11-17-56(53)58)93-77(108)60(92-79(62)110)36-51-21-25-54(103)26-22-51/h11-12,15-18,20-28,37-38,42,46-49,57,59-63,66-68,72-74,90,101-103,117H,13-14,19,29-36,39-41,43-44,88-89H2,1-10H3,(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,116)(H,113,114)/b20-15+,45-16+/t46-,47+,48-,49-,57-,59+,60+,61-,62+,63+,66+,67-,68+,72+,73+,74+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537066

(CHEMBL4541310)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O |r,t:17,19| Show InChI InChI=1S/C87H116ClN13O22S4/c1-47-20-18-26-68(120-10)87(118)43-67(121-85(117)99-87)48(2)75-86(6,123-75)69(42-71(106)101(8)65-39-54(36-47)40-66(119-9)72(65)88)122-84(116)49(3)100(7)70(105)31-35-125-124-34-19-33-90-60(37-52-21-12-11-13-22-52)77(108)95-63-45-126-127-46-64(81(112)98-74(51(5)103)83(114)115)96-82(113)73(50(4)102)97-76(107)59(25-16-17-32-89)92-79(110)62(41-55-44-91-58-24-15-14-23-57(55)58)94-78(109)61(93-80(63)111)38-53-27-29-56(104)30-28-53/h11-15,18,20-24,26-30,39-40,44,48-51,59-64,67-69,73-75,90-91,102-104,118H,16-17,19,25,31-38,41-43,45-46,89H2,1-10H3,(H,92,110)(H,93,111)(H,94,109)(H,95,108)(H,96,113)(H,97,107)(H,98,112)(H,99,117)(H,114,115)/b26-18+,47-20+/t48-,49+,50-,51-,59+,60-,61+,62-,63+,64+,67+,68-,69+,73+,74+,75+,86-,87+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537076

(CHEMBL4564727)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1 |r,t:17,19| Show InChI InChI=1S/C83H110ClN13O20S4/c1-45-18-17-24-66(114-9)83(112)39-65(115-81(111)95-83)46(2)72-82(5,117-72)67(38-69(102)97(7)63-35-51(32-45)36-64(113-8)70(63)84)116-80(110)47(3)96(6)68(101)29-31-118-119-42-53(41-98)88-77(107)61-43-120-121-44-62(92-73(103)56(86)33-49-19-11-10-12-20-49)78(108)90-59(34-50-25-27-54(100)28-26-50)75(105)91-60(37-52-40-87-57-22-14-13-21-55(52)57)76(106)89-58(23-15-16-30-85)74(104)94-71(48(4)99)79(109)93-61/h10-14,17-22,24-28,35-36,40,46-48,53,56,58-62,65-67,71-72,87,98-100,112H,15-16,23,29-34,37-39,41-44,85-86H2,1-9H3,(H,88,107)(H,89,106)(H,90,108)(H,91,105)(H,92,103)(H,93,109)(H,94,104)(H,95,111)/b24-17+,45-18+/t46-,47+,48-,53-,56-,58+,59+,60-,61+,62+,65+,66-,67+,71+,72+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537061
(CHEMBL4527856)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C83H109ClN14O20S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)96-83)45(2)71-82(5,118-71)66(38-68(102)98(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)97(6)67(101)29-31-119-120-41-59(72(87)103)92-78(109)61-43-122-121-42-60(93-73(104)54(86)33-48-19-11-10-12-20-48)77(108)90-57(34-49-25-27-52(100)28-26-49)75(106)91-58(37-51-40-88-55-22-14-13-21-53(51)55)76(107)89-56(23-15-16-30-85)74(105)95-70(47(4)99)79(110)94-61/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,88,99-100,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H2,87,103)(H,89,107)(H,90,108)(H,91,106)(H,92,109)(H,93,104)(H,94,110)(H,95,105)(H,96,112)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537068
(CHEMBL4592483)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCNC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1 |r,t:17,19| Show InChI InChI=1S/C82H108ClN13O19S4/c1-45-18-17-24-65(112-9)82(110)41-64(113-80(109)94-82)46(2)71-81(5,115-71)66(40-68(100)96(7)62-37-51(34-45)38-63(111-8)69(62)83)114-79(108)47(3)95(6)67(99)29-32-116-117-33-31-86-73(102)60-43-118-119-44-61(91-72(101)55(85)35-49-19-11-10-12-20-49)77(106)89-58(36-50-25-27-53(98)28-26-50)75(104)90-59(39-52-42-87-56-22-14-13-21-54(52)56)76(105)88-57(23-15-16-30-84)74(103)93-70(48(4)97)78(107)92-60/h10-14,17-22,24-28,37-38,42,46-48,55,57-61,64-66,70-71,87,97-98,110H,15-16,23,29-36,39-41,43-44,84-85H2,1-9H3,(H,86,102)(H,88,105)(H,89,106)(H,90,104)(H,91,101)(H,92,107)(H,93,103)(H,94,109)/b24-17+,45-18+/t46-,47+,48-,55-,57+,58+,59-,60+,61+,64+,65-,66+,70+,71+,81-,82+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537070

(CHEMBL4581874)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCC(C)(C)SSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C86H115ClN14O20S4/c1-46-20-19-26-67(118-11)86(116)41-66(119-83(115)99-86)47(2)73-85(7,121-73)68(40-70(105)101(9)64-37-52(34-46)38-65(117-10)71(64)87)120-82(114)48(3)100(8)69(104)31-32-84(5,6)125-124-43-61(74(90)106)95-80(112)63-45-123-122-44-62(96-75(107)56(89)35-50-21-13-12-14-22-50)79(111)93-59(36-51-27-29-54(103)30-28-51)77(109)94-60(39-53-42-91-57-24-16-15-23-55(53)57)78(110)92-58(25-17-18-33-88)76(108)98-72(49(4)102)81(113)97-63/h12-16,19-24,26-30,37-38,42,47-49,56,58-63,66-68,72-73,91,102-103,116H,17-18,25,31-36,39-41,43-45,88-89H2,1-11H3,(H2,90,106)(H,92,110)(H,93,111)(H,94,109)(H,95,112)(H,96,107)(H,97,113)(H,98,108)(H,99,115)/b26-19+,46-20+/t47-,48+,49-,56-,58+,59+,60-,61+,62+,63+,66+,67-,68+,72+,73+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537074

(CHEMBL4556000)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCC(=O)NCCCC[C@@H]1N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C84H111ClN12O19S2/c1-48-21-20-28-67(113-10)84(111)46-66(114-82(110)94-84)49(2)74-83(5,116-74)68(45-71(102)96(7)64-42-54(39-48)43-65(112-9)72(64)85)115-81(109)50(3)95(6)70(101)34-38-118-117-37-33-69(100)87-36-19-17-27-63-78(106)91-60(40-53-29-31-56(99)32-30-53)76(104)90-61(44-55-47-88-58-25-15-14-24-57(55)58)77(105)89-59(26-16-18-35-86)75(103)93-73(51(4)98)79(107)92-62(80(108)97(63)8)41-52-22-12-11-13-23-52/h11-15,20-25,28-32,42-43,47,49-51,59-63,66-68,73-74,88,98-99,111H,16-19,26-27,33-41,44-46,86H2,1-10H3,(H,87,100)(H,89,105)(H,90,104)(H,91,106)(H,92,107)(H,93,103)(H,94,110)/b28-20+,48-21+/t49-,50+,51-,59+,60+,61-,62+,63+,66+,67-,68+,73+,74+,83-,84+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537067
(CHEMBL4532058)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCNC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C86H115ClN14O21S4/c1-46-19-18-25-67(119-10)86(117)42-66(120-84(116)99-86)47(2)74-85(6,122-74)68(41-70(106)101(8)64-38-53(35-46)39-65(118-9)71(64)87)121-83(115)48(3)100(7)69(105)30-33-123-124-34-32-90-81(113)72(49(4)102)97-80(112)63-45-126-125-44-62(95-75(107)57(89)36-51-20-12-11-13-21-51)79(111)93-60(37-52-26-28-55(104)29-27-52)77(109)94-61(40-54-43-91-58-23-15-14-22-56(54)58)78(110)92-59(24-16-17-31-88)76(108)98-73(50(5)103)82(114)96-63/h11-15,18-23,25-29,38-39,43,47-50,57,59-63,66-68,72-74,91,102-104,117H,16-17,24,30-37,40-42,44-45,88-89H2,1-10H3,(H,90,113)(H,92,110)(H,93,111)(H,94,109)(H,95,107)(H,96,114)(H,97,112)(H,98,108)(H,99,116)/b25-18+,46-19+/t47-,48+,49-,50-,57-,59+,60+,61-,62+,63+,66+,67-,68+,72+,73+,74+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537062

(CHEMBL4549303)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C87H118ClN13O20S4/c1-49-23-21-31-70(118-10)87(116)44-69(119-85(115)99-87)50(2)76-86(6,121-76)71(43-73(106)101(8)67-40-56(37-49)41-68(117-9)74(67)88)120-84(114)51(3)100(7)72(105)32-36-123-122-35-22-34-90-61(38-54-24-13-11-14-25-54)78(108)96-65-47-124-125-48-66(82(112)95-64(46-102)52(4)103)97-83(113)75(53(5)104)98-77(107)60(30-19-20-33-89)92-80(110)63(42-57-45-91-59-29-18-17-28-58(57)59)94-79(109)62(93-81(65)111)39-55-26-15-12-16-27-55/h11-18,21,23-29,31,40-41,45,50-53,60-66,69-71,75-76,90-91,102-104,116H,19-20,22,30,32-39,42-44,46-48,89H2,1-10H3,(H,92,110)(H,93,111)(H,94,109)(H,95,112)(H,96,108)(H,97,113)(H,98,107)(H,99,115)/b31-21+,49-23+/t50-,51+,52-,53-,60+,61-,62+,63-,64-,65+,66+,69+,70-,71+,75+,76+,86-,87+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537075

(CHEMBL4548228)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSC1CC(=O)N(CC(=O)NC[C@H](NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](N)Cc3ccccc3)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@H](Cc3c[nH]c4ccccc34)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N2)C(N)=O)C1=O |r,t:17,19| Show InChI InChI=1S/C89H115ClN16O23S3/c1-46-18-17-24-68(126-9)89(124)40-66(127-87(123)103-89)47(2)76-88(5,129-76)69(39-72(111)105(7)64-35-52(32-46)36-65(125-8)74(64)90)128-86(122)48(3)104(6)71(110)29-31-130-67-38-73(112)106(85(67)121)43-70(109)95-42-61(77(93)113)99-83(119)63-45-132-131-44-62(100-78(114)56(92)33-50-19-11-10-12-20-50)82(118)97-59(34-51-25-27-54(108)28-26-51)80(116)98-60(37-53-41-94-57-22-14-13-21-55(53)57)81(117)96-58(23-15-16-30-91)79(115)102-75(49(4)107)84(120)101-63/h10-14,17-22,24-28,35-36,41,47-49,56,58-63,66-69,75-76,94,107-108,124H,15-16,23,29-34,37-40,42-45,91-92H2,1-9H3,(H2,93,113)(H,95,109)(H,96,117)(H,97,118)(H,98,116)(H,99,119)(H,100,114)(H,101,120)(H,102,115)(H,103,123)/b24-17+,46-18+/t47-,48+,49-,56-,58+,59+,60-,61+,62+,63+,66+,67?,68-,69+,75+,76+,88-,89+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537064
(CHEMBL4563111)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSC1CC(=O)N(CCCNC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CCCCCN2C(=O)CC(SCCCN[C@H](Cc3ccccc3)C(=O)N[C@H]3CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](Cc4ccccc4)NC3=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O)C2=O)C1=O |r,t:17,19| Show InChI InChI=1S/C123H167ClN20O29S4/c1-70(2)52-85(109(155)130-65-100(149)127-46-30-49-144-105(154)61-96(119(144)165)175-51-44-102(151)141(9)73(5)120(166)172-98-62-103(152)142(10)92-57-79(58-93(169-11)106(92)124)53-71(3)32-29-42-97(170-12)123(168)63-94(171-121(167)140-123)72(4)108-122(98,8)173-108)133-112(158)86(55-77-35-19-14-20-36-77)131-101(150)66-129-99(148)43-23-16-28-48-143-104(153)60-95(118(143)164)174-50-31-47-126-84(54-76-33-17-13-18-34-76)111(157)137-90-68-176-177-69-91(116(162)136-89(67-145)74(6)146)138-117(163)107(75(7)147)139-110(156)83(41-26-27-45-125)132-114(160)88(59-80-64-128-82-40-25-24-39-81(80)82)135-113(159)87(134-115(90)161)56-78-37-21-15-22-38-78/h13-15,17-22,24-25,29,32-40,42,57-58,64,70,72-75,83-91,94-98,107-108,126,128,145-147,168H,16,23,26-28,30-31,41,43-56,59-63,65-69,125H2,1-12H3,(H,127,149)(H,129,148)(H,130,155)(H,131,150)(H,132,160)(H,133,158)(H,134,161)(H,135,159)(H,136,162)(H,137,157)(H,138,163)(H,139,156)(H,140,167)/b42-29+,71-32+/t72-,73+,74-,75-,83+,84-,85+,86+,87+,88-,89-,90+,91+,94+,95?,96?,97-,98+,107+,108+,122-,123+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537071

(CHEMBL4581646)Show SMILES [H][C@]1(CCCN1C(=O)C[C@@H](OC)[C@H]([C@@H](C)CC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O)C(C)C)[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1 |r| Show InChI InChI=1S/C90H132N16O19S4/c1-14-52(6)76(71(123-12)45-72(109)106-39-25-33-70(106)78(124-13)53(7)80(112)95-54(8)77(110)58-28-19-16-20-29-58)104(10)89(121)73(50(2)3)102-88(120)75(51(4)5)105(11)90(122)125-40-41-126-127-47-67(79(93)111)99-86(118)69-49-129-128-48-68(100-81(113)62(92)42-56-26-17-15-18-27-56)85(117)97-65(43-57-34-36-60(108)37-35-57)83(115)98-66(44-59-46-94-63-31-22-21-30-61(59)63)84(116)96-64(32-23-24-38-91)82(114)103-74(55(9)107)87(119)101-69/h15-22,26-31,34-37,46,50-55,62,64-71,73-78,94,107-108,110H,14,23-25,32-33,38-45,47-49,91-92H2,1-13H3,(H2,93,111)(H,95,112)(H,96,116)(H,97,117)(H,98,115)(H,99,118)(H,100,113)(H,101,119)(H,102,120)(H,103,114)/t52-,53+,54+,55+,62+,64-,65-,66+,67-,68-,69-,70-,71+,73-,74-,75-,76-,77+,78+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537065
(CHEMBL4537192)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,t:17,19| Show InChI InChI=1S/C95H122ClN15O19S4/c1-53(2)82-91(122)107-73(89(120)103-68(84(98)115)45-60-49-100-65-27-16-14-25-63(60)65)52-134-133-51-72(90(121)104-70(42-58-31-33-62(112)34-32-58)87(118)105-71(46-61-50-101-66-28-17-15-26-64(61)66)88(119)102-67(85(116)108-82)29-18-19-36-97)106-86(117)69(41-57-23-12-11-13-24-57)99-37-21-38-131-132-39-35-79(113)110(7)56(5)92(123)129-78-47-80(114)111(8)74-43-59(44-75(126-9)81(74)96)40-54(3)22-20-30-77(127-10)95(125)48-76(128-93(124)109-95)55(4)83-94(78,6)130-83/h11-17,20,22-28,30-34,43-44,49-50,53,55-56,67-73,76-78,82-83,99-101,112,125H,18-19,21,29,35-42,45-48,51-52,97H2,1-10H3,(H2,98,115)(H,102,119)(H,103,120)(H,104,121)(H,105,118)(H,106,117)(H,107,122)(H,108,116)(H,109,124)/b30-20+,54-22+/t55-,56+,67+,68+,69-,70+,71-,72+,73+,76+,77-,78+,82+,83+,94-,95+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537078

(CHEMBL4577466)Show SMILES [H][C@]([C@@H](C)CC)([C@@H](CC(=O)N1CCC[C@@]1([H])[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1)OC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O)C(C)C |r| Show InChI InChI=1S/C94H141N15O19S4/c1-15-57(6)81(76(126-13)50-77(113)109-43-29-40-75(109)83(127-14)58(7)84(115)98-59(8)82(114)64-35-23-18-24-36-64)107(11)93(124)78(55(2)3)105-92(123)80(56(4)5)108(12)94(125)128-44-46-130-129-45-30-42-96-69(47-62-31-19-16-20-32-62)86(117)103-73-53-131-132-54-74(90(121)102-72(52-110)60(9)111)104-91(122)79(61(10)112)106-85(116)68(39-27-28-41-95)99-88(119)71(49-65-51-97-67-38-26-25-37-66(65)67)101-87(118)70(100-89(73)120)48-63-33-21-17-22-34-63/h16-26,31-38,51,55-61,68-76,78-83,96-97,110-112,114H,15,27-30,39-50,52-54,95H2,1-14H3,(H,98,115)(H,99,119)(H,100,120)(H,101,118)(H,102,121)(H,103,117)(H,104,122)(H,105,123)(H,106,116)/t57-,58+,59+,60+,61+,68-,69+,70-,71+,72+,73-,74-,75-,76+,78-,79-,80-,81-,82+,83+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537073
(CHEMBL4534477)Show SMILES [H][C@@]12C[C@H](OC)[C@@]3(C)C(=O)[C@H](OC)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](OC(=O)OCCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O)[C@@H](NC(=O)OC(C)(C)C)c1ccccc1 |r,c:13| Show InChI InChI=1S/C100H131N11O26S4/c1-56-74(50-100(128)84(135-92(124)63-36-23-16-24-37-63)82-98(10,83(116)80(130-12)77(56)97(100,8)9)75(129-11)49-76-99(82,55-132-76)136-59(4)115)133-93(125)81(79(62-34-21-15-22-35-62)111-94(126)137-96(5,6)7)134-95(127)131-43-45-139-138-44-29-42-102-68(46-60-30-17-13-18-31-60)86(118)108-72-53-140-141-54-73(90(122)107-71(52-112)57(2)113)109-91(123)78(58(3)114)110-85(117)67(40-27-28-41-101)104-88(120)70(48-64-51-103-66-39-26-25-38-65(64)66)106-87(119)69(105-89(72)121)47-61-32-19-14-20-33-61/h13-26,30-39,51,57-58,67-76,78-82,84,102-103,112-114,128H,27-29,40-50,52-55,101H2,1-12H3,(H,104,120)(H,105,121)(H,106,119)(H,107,122)(H,108,118)(H,109,123)(H,110,117)(H,111,126)/t57-,58-,67+,68-,69+,70-,71-,72+,73+,74+,75+,76-,78+,79+,80-,81-,82+,84+,98-,99+,100-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537060
(CHEMBL4575530)Show SMILES [H][C@@]12C[C@H](OC)[C@@]3(C)C(=O)[C@H](OC)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](OC(=O)OCCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O)[C@@H](NC(=O)OC(C)(C)C)c1ccccc1 |r,c:13| Show InChI InChI=1S/C96H122N12O26S4/c1-51-69(45-96(125)79(132-88(121)57-29-19-14-20-30-57)77-94(9,78(112)75(127-11)72(51)93(96,7)8)70(126-10)44-71-95(77,50-129-71)133-53(3)110)130-89(122)76(74(56-27-17-13-18-28-56)108-90(123)134-92(4,5)6)131-91(124)128-39-40-135-136-47-66(80(99)113)104-86(119)68-49-138-137-48-67(105-81(114)61(98)41-54-25-15-12-16-26-54)85(118)102-64(42-55-34-36-59(111)37-35-55)83(116)103-65(43-58-46-100-62-32-22-21-31-60(58)62)84(117)101-63(33-23-24-38-97)82(115)107-73(52(2)109)87(120)106-68/h12-22,25-32,34-37,46,52,61,63-71,73-77,79,100,109,111,125H,23-24,33,38-45,47-50,97-98H2,1-11H3,(H2,99,113)(H,101,117)(H,102,118)(H,103,116)(H,104,119)(H,105,114)(H,106,120)(H,107,115)(H,108,123)/t52-,61-,63+,64+,65-,66+,67+,68+,69+,70+,71-,73+,74+,75-,76-,77+,79+,94-,95+,96-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537079
(CHEMBL4587303)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCNC(=O)NCCCCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C90H123ClN16O21S4/c1-50-22-21-28-71(125-9)90(123)45-70(126-88(122)105-90)51(2)77-89(5,128-77)72(44-74(111)107(7)68-41-56(38-50)42-69(124-8)75(68)91)127-86(120)52(3)106(6)73(110)33-37-129-130-47-65(78(94)112)101-84(118)67-49-132-131-48-66(102-79(113)60(93)39-54-23-13-12-14-24-54)83(117)99-63(40-55-29-31-58(109)32-30-55)81(115)100-64(43-57-46-97-61-26-16-15-25-59(57)61)82(116)98-62(80(114)104-76(53(4)108)85(119)103-67)27-17-20-36-96-87(121)95-35-19-11-10-18-34-92/h12-16,21-26,28-32,41-42,46,51-53,60,62-67,70-72,76-77,97,108-109,123H,10-11,17-20,27,33-40,43-45,47-49,92-93H2,1-9H3,(H2,94,112)(H,98,116)(H,99,117)(H,100,115)(H,101,118)(H,102,113)(H,103,119)(H,104,114)(H,105,122)(H2,95,96,121)/b28-21+,50-22+/t51-,52+,53-,60-,62+,63+,64-,65+,66+,67+,70+,71-,72+,76+,77+,89-,90+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396302
(CHEMBL2172485)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cncnc1 Show InChI InChI=1S/C18H15N11/c1-10-23-16(27-18(19)24-10)15-17(25-13-4-5-22-28-13)26-14-3-2-11(8-29(14)15)12-6-20-9-21-7-12/h2-9H,1H3,(H2,22,25,28)(H2,19,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396298
(CHEMBL2172489)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cnn(c1)C1CC1 Show InChI InChI=1S/C20H19N11/c1-11-24-18(28-20(21)25-11)17-19(26-15-6-7-22-29-15)27-16-5-2-12(9-30(16)17)13-8-23-31(10-13)14-3-4-14/h2,5-10,14H,3-4H2,1H3,(H2,22,26,29)(H2,21,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341035

(1-(4-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-b...)Show SMILES CNC(=O)Nc1ccc(Nc2nc3ccccc3n2-c2nc(C)nc(N)n2)cc1 Show InChI InChI=1S/C19H19N9O/c1-11-22-16(20)27-17(23-11)28-15-6-4-3-5-14(15)26-18(28)24-12-7-9-13(10-8-12)25-19(29)21-2/h3-10H,1-2H3,(H,24,26)(H2,21,25,29)(H2,20,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396305
(CHEMBL2172482)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cnccc1C Show InChI InChI=1S/C20H18N10/c1-11-5-7-22-9-14(11)13-3-4-16-27-19(26-15-6-8-23-29-15)17(30(16)10-13)18-24-12(2)25-20(21)28-18/h3-10H,1-2H3,(H2,23,26,29)(H2,21,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
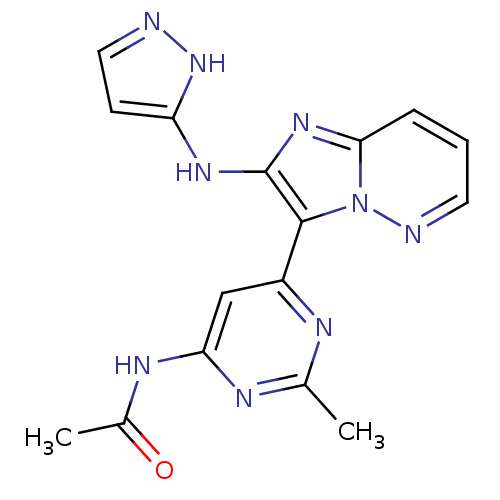
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396322
(CHEMBL2172629)Show SMILES CC(=O)Nc1cc(nc(C)n1)-c1c(Nc2ccn[nH]2)nc2cccnn12 Show InChI InChI=1S/C16H15N9O/c1-9-19-11(8-13(20-9)21-10(2)26)15-16(22-12-5-7-17-24-12)23-14-4-3-6-18-25(14)15/h3-8H,1-2H3,(H2,17,22,24)(H,19,20,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341054
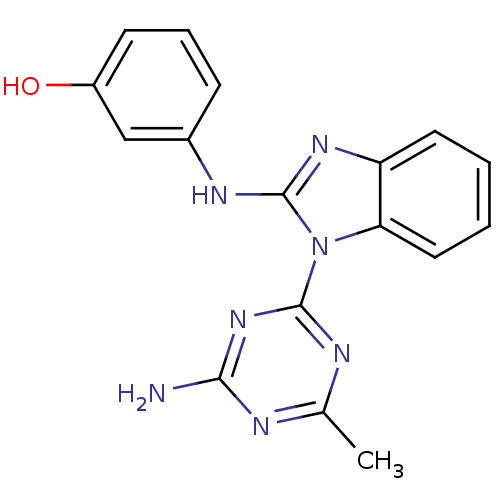
(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
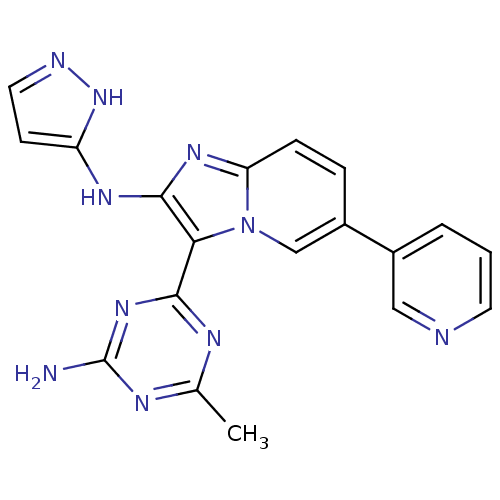
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396308
(CHEMBL2172636)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cccnc1 Show InChI InChI=1S/C19H16N10/c1-11-23-17(27-19(20)24-11)16-18(25-14-6-8-22-28-14)26-15-5-4-13(10-29(15)16)12-3-2-7-21-9-12/h2-10H,1H3,(H2,22,25,28)(H2,20,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
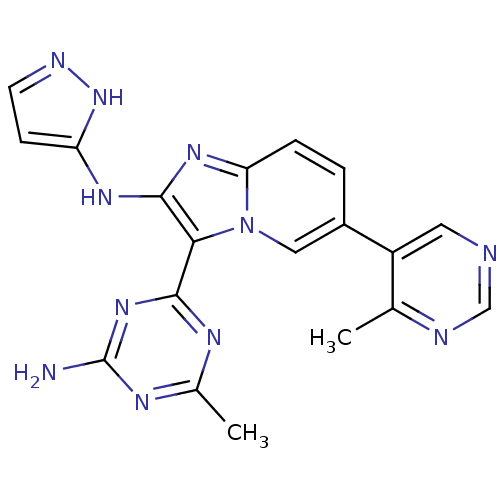
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396301
(CHEMBL2172486)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cncnc1C Show InChI InChI=1S/C19H17N11/c1-10-13(7-21-9-22-10)12-3-4-15-27-18(26-14-5-6-23-29-14)16(30(15)8-12)17-24-11(2)25-19(20)28-17/h3-9H,1-2H3,(H2,23,26,29)(H2,20,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396307
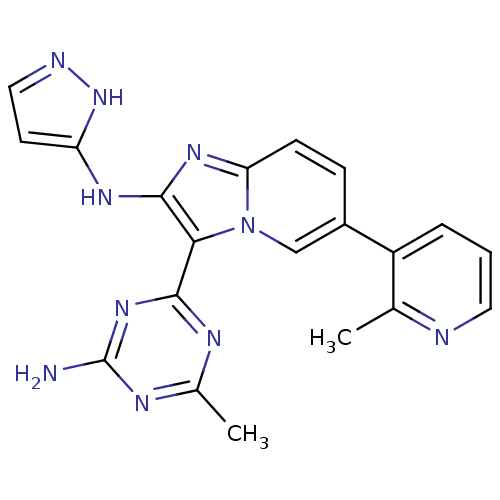
(CHEMBL2172480)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cccnc1C Show InChI InChI=1S/C20H18N10/c1-11-14(4-3-8-22-11)13-5-6-16-27-19(26-15-7-9-23-29-15)17(30(16)10-13)18-24-12(2)25-20(21)28-18/h3-10H,1-2H3,(H2,23,26,29)(H2,21,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396297
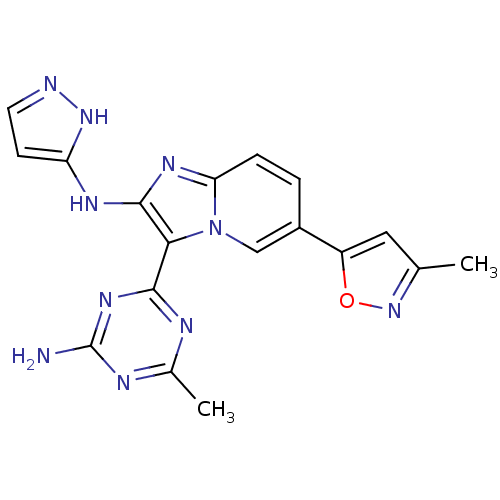
(CHEMBL2172490)Show SMILES Cc1cc(on1)-c1ccc2nc(Nc3ccn[nH]3)c(-c3nc(C)nc(N)n3)n2c1 Show InChI InChI=1S/C18H16N10O/c1-9-7-12(29-27-9)11-3-4-14-24-17(23-13-5-6-20-26-13)15(28(14)8-11)16-21-10(2)22-18(19)25-16/h3-8H,1-2H3,(H2,20,23,26)(H2,19,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396316
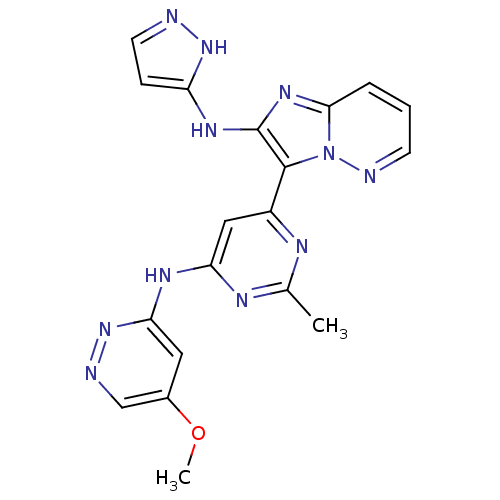
(CHEMBL2172635)Show SMILES COc1cnnc(Nc2cc(nc(C)n2)-c2c(Nc3ccn[nH]3)nc3cccnn23)c1 Show InChI InChI=1S/C19H17N11O/c1-11-23-13(9-15(24-11)25-16-8-12(31-2)10-21-29-16)18-19(26-14-5-7-20-28-14)27-17-4-3-6-22-30(17)18/h3-10H,1-2H3,(H2,20,26,28)(H,23,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396303
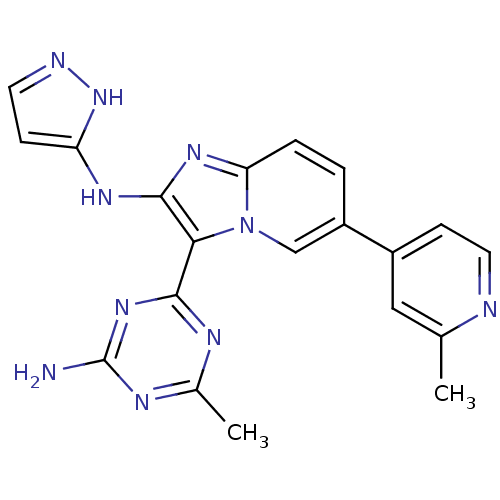
(CHEMBL2172484)Show SMILES Cc1cc(ccn1)-c1ccc2nc(Nc3ccn[nH]3)c(-c3nc(C)nc(N)n3)n2c1 Show InChI InChI=1S/C20H18N10/c1-11-9-13(5-7-22-11)14-3-4-16-27-19(26-15-6-8-23-29-15)17(30(16)10-14)18-24-12(2)25-20(21)28-18/h3-10H,1-2H3,(H2,23,26,29)(H2,21,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396299
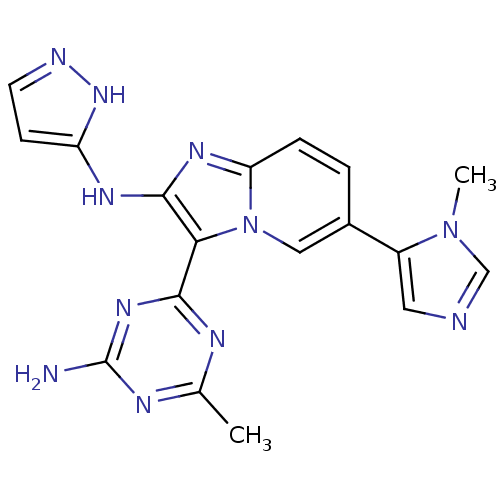
(CHEMBL2172488)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cncn1C Show InChI InChI=1S/C18H17N11/c1-10-22-16(26-18(19)23-10)15-17(24-13-5-6-21-27-13)25-14-4-3-11(8-29(14)15)12-7-20-9-28(12)2/h3-9H,1-2H3,(H2,21,24,27)(H2,19,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human U-87 cells assessed as inhibition of phosphorylation of AKT at S473 |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341035

(1-(4-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-b...)Show SMILES CNC(=O)Nc1ccc(Nc2nc3ccccc3n2-c2nc(C)nc(N)n2)cc1 Show InChI InChI=1S/C19H19N9O/c1-11-22-16(20)27-17(23-11)28-15-6-4-3-5-14(15)26-18(28)24-12-7-9-13(10-8-12)25-19(29)21-2/h3-10H,1-2H3,(H,24,26)(H2,21,25,29)(H2,20,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396309
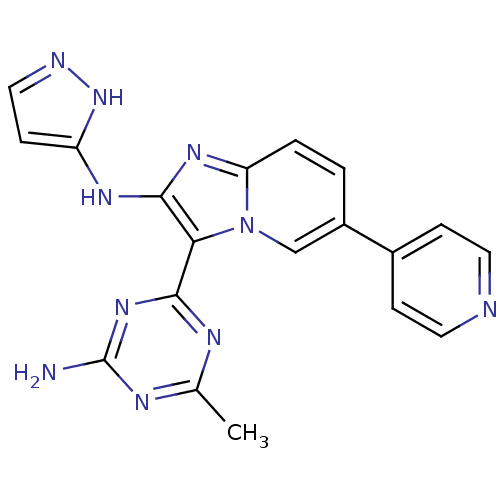
(CHEMBL2172640)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1ccncc1 Show InChI InChI=1S/C19H16N10/c1-11-23-17(27-19(20)24-11)16-18(25-14-6-9-22-28-14)26-15-3-2-13(10-29(15)16)12-4-7-21-8-5-12/h2-10H,1H3,(H2,22,25,28)(H2,20,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396321
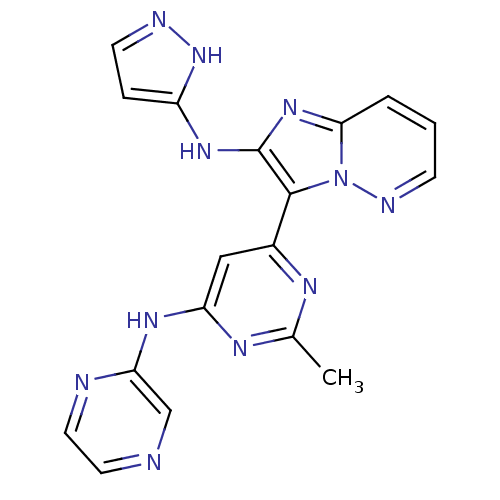
(CHEMBL2172630)Show SMILES Cc1nc(Nc2cnccn2)cc(n1)-c1c(Nc2ccn[nH]2)nc2cccnn12 Show InChI InChI=1S/C18H15N11/c1-11-23-12(9-14(24-11)25-15-10-19-7-8-20-15)17-18(26-13-4-6-21-28-13)27-16-3-2-5-22-29(16)17/h2-10H,1H3,(H2,21,26,28)(H,20,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341063
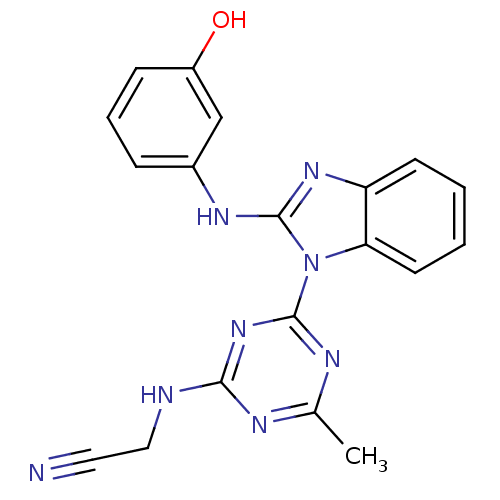
(2-(4-(2-(3-hydroxyphenylamino)-1H-benzo[d]imidazol...)Show SMILES Cc1nc(NCC#N)nc(n1)-n1c(Nc2cccc(O)c2)nc2ccccc12 Show InChI InChI=1S/C19H16N8O/c1-12-22-17(21-10-9-20)26-18(23-12)27-16-8-3-2-7-15(16)25-19(27)24-13-5-4-6-14(28)11-13/h2-8,11,28H,10H2,1H3,(H,24,25)(H,21,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human U-87 cells assessed as inhibition of phosphorylation of AKT at S473 |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
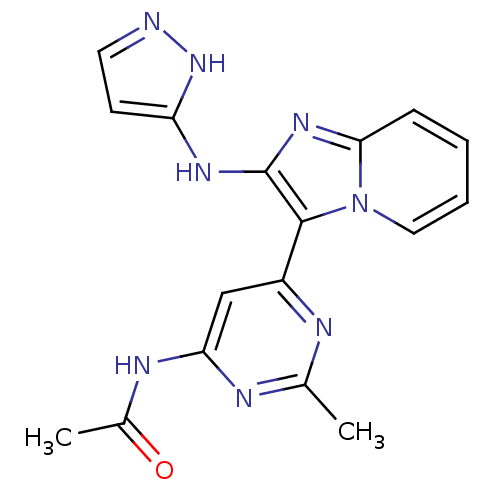
(Homo sapiens (Human)) | BDBM50396314
(CHEMBL2172475)Show SMILES CC(=O)Nc1cc(nc(C)n1)-c1c(Nc2ccn[nH]2)nc2ccccn12 Show InChI InChI=1S/C17H16N8O/c1-10-19-12(9-14(20-10)21-11(2)26)16-17(22-13-6-7-18-24-13)23-15-5-3-4-8-25(15)16/h3-9H,1-2H3,(H2,18,22,24)(H,19,20,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
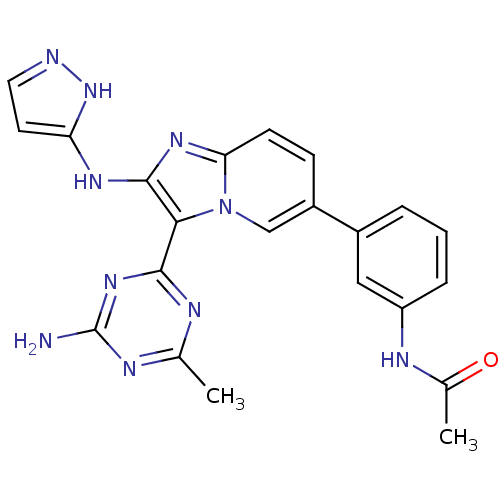
(Homo sapiens (Human)) | BDBM50396300
(CHEMBL2172487)Show SMILES CC(=O)Nc1cccc(c1)-c1ccc2nc(Nc3ccn[nH]3)c(-c3nc(C)nc(N)n3)n2c1 Show InChI InChI=1S/C22H20N10O/c1-12-25-20(30-22(23)26-12)19-21(28-17-8-9-24-31-17)29-18-7-6-15(11-32(18)19)14-4-3-5-16(10-14)27-13(2)33/h3-11H,1-2H3,(H,27,33)(H2,24,28,31)(H2,23,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data