

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RET | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50362985 (CHEMBL1945502) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of KDR | J Med Chem 55: 197-208 (2012) Article DOI: 10.1021/jm2011172 BindingDB Entry DOI: 10.7270/Q21C1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Abl1 | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RET | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Discoidin domain-containing receptor 2 (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of DDR2 | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339615 ((2S)-7-({2-[(Cyclopropylcarbonyl)amino]pyridin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type B-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339613 ((2S)-N-[3-(aminomethyl)-5-(trifluoromethyl)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339616 (7-({2-[(Cyclopropylcarbonyl)amino]pyridin-4-yl}oxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50339609 (3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type B-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM50362985 (CHEMBL1945502) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of PLK3 | J Med Chem 55: 197-208 (2012) Article DOI: 10.1021/jm2011172 BindingDB Entry DOI: 10.7270/Q21C1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM50362988 (CHEMBL1945500) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of PLK3 | J Med Chem 55: 197-208 (2012) Article DOI: 10.1021/jm2011172 BindingDB Entry DOI: 10.7270/Q21C1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339611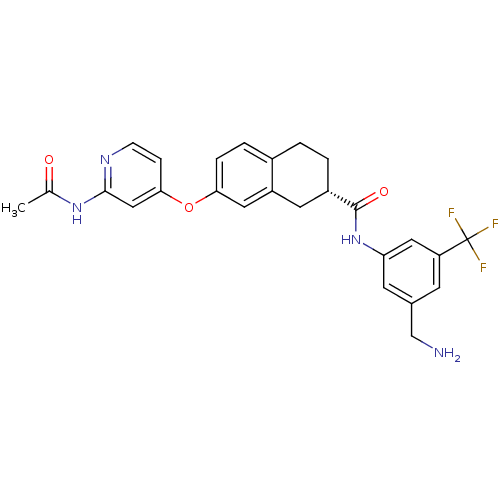 ((2S)-7-{[2-(acetylamino)pyridin-4-yl]oxy}-N-[3-(am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339614 ((2R)-N-[3-(aminomethyl)-5-(trifluoromethyl)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of KDR | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339612 (CHEMBL1688868 | N-[3-(Aminomethyl)-5-(trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Discoidin domain-containing receptor 2 (Homo sapiens (Human)) | BDBM50339608 (3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of DDR2 | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM50362986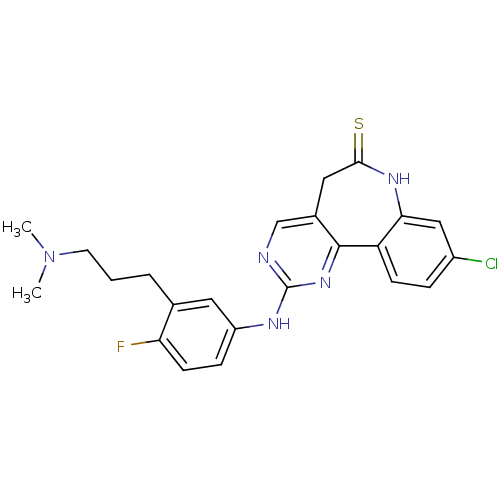 (CHEMBL1945501) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of PLK3 | J Med Chem 55: 197-208 (2012) Article DOI: 10.1021/jm2011172 BindingDB Entry DOI: 10.7270/Q21C1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50339623 (4-[3-(3-{[4-Chloro-3-(trifluoromethyl)phenyl]amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay | J Med Chem 54: 1836-46 (2011) Article DOI: 10.1021/jm101479y BindingDB Entry DOI: 10.7270/Q2DB824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM50362984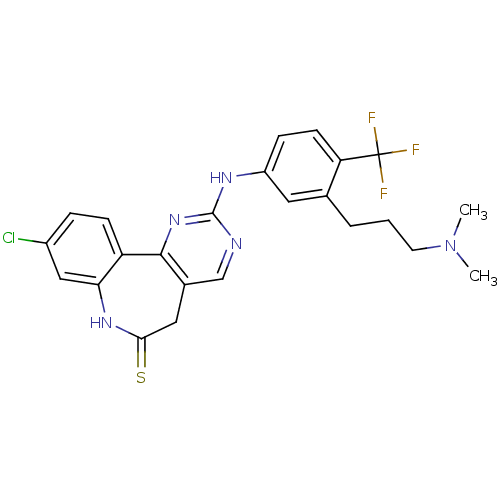 (CHEMBL1945801) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of PLK3 | J Med Chem 55: 197-208 (2012) Article DOI: 10.1021/jm2011172 BindingDB Entry DOI: 10.7270/Q21C1XBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462804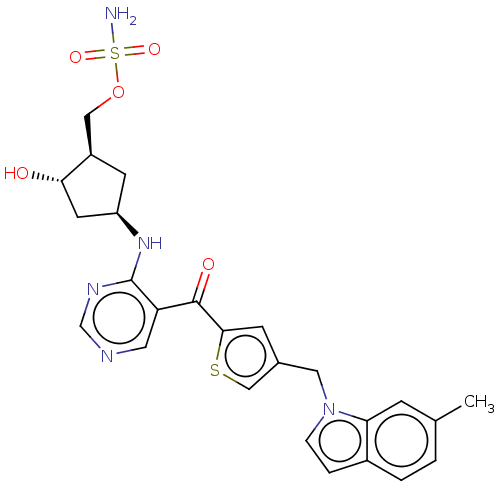 (US10780090, Compound I-132 | [(1R,2S,4R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462805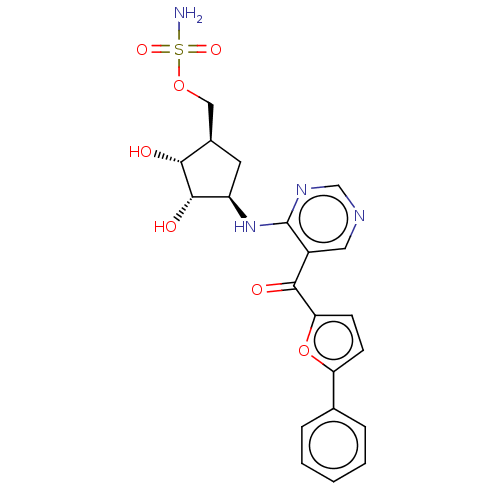 (US10780090, Compound I-134 | [(1R,2R,3S,4R)-2,3-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462806 (US10780090, Compound I-135 | US10780090, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462809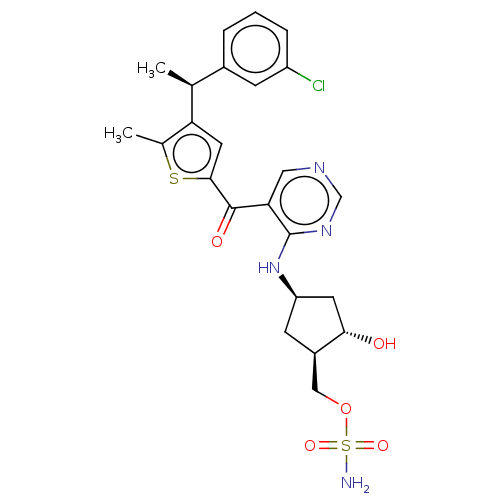 (US10780090, Compound I-136 | [(1R,2S,4R)-4-{[5-({5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462810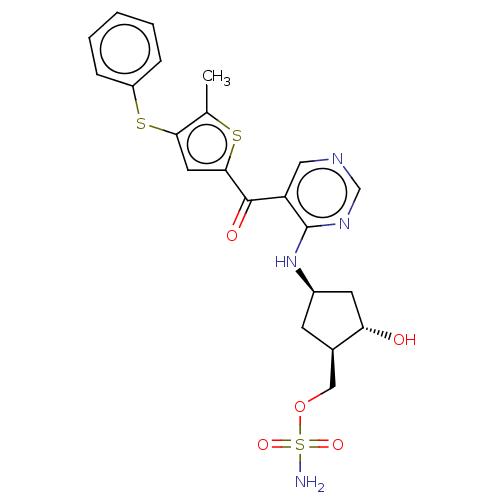 (US10780090, Compound I-137 | {(1R,2S,4R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462811 (US10780090, Compound I-138 | [(1R,2S,4R)-4-({5-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462812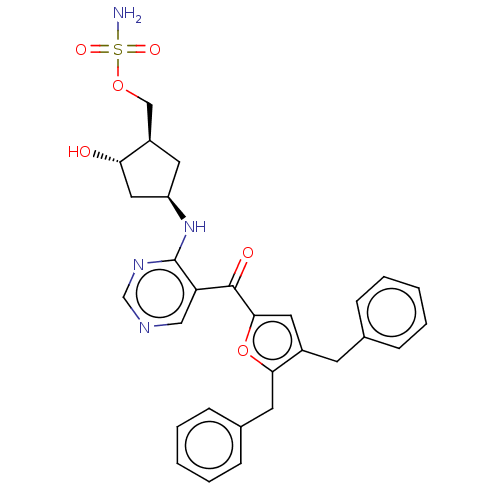 (US10780090, Compound I-139 | [(1R,2S,4R)-4-{[5-(4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462813 (US10780090, Compound I-140 | {(1R,2S,4R)-4-[(5-{[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462814 (US10780090, Compound I-141 | [(1R,2S,4R)-4-{[5-({5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462815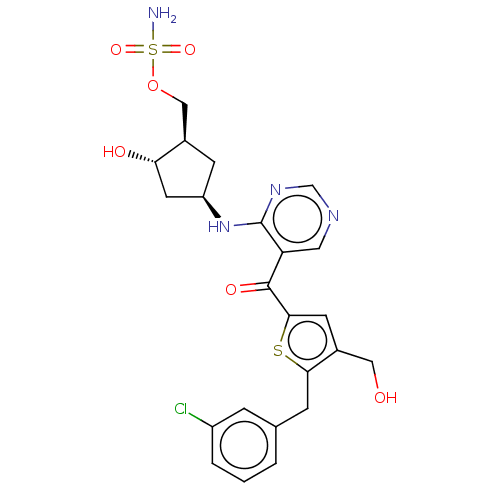 (US10780090, Compound I-142 | {(1R,2S,4R)-4-[(5-{[5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462816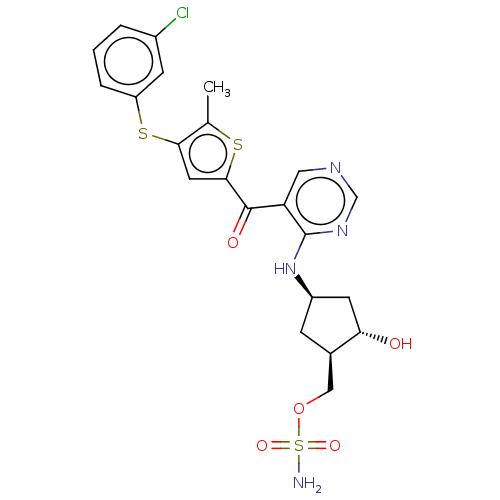 (US10780090, Compound I-143 | [(1R,2S,4R)-4-{[5-({4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462817 (US10780090, Compound I-144 | [(1R,2S,4R)-4-({5-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462818 (US10780090, Compound I-145 | [(1R,2S,4R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462819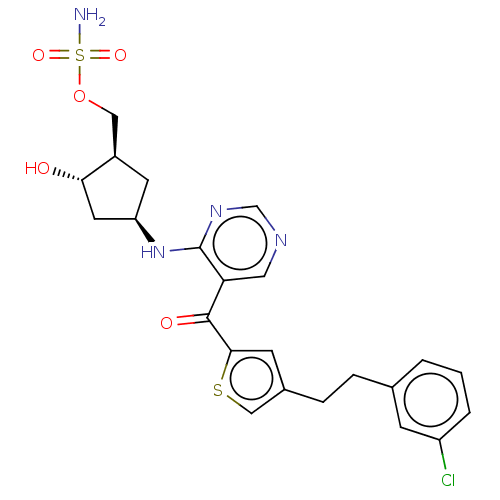 (US10780090, Compound I-146 | [(1R,2S,4R)-4-{[5-({4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462820 (US10780090, Compound I-147 | [(1R,2S,4R)-4-({5-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462821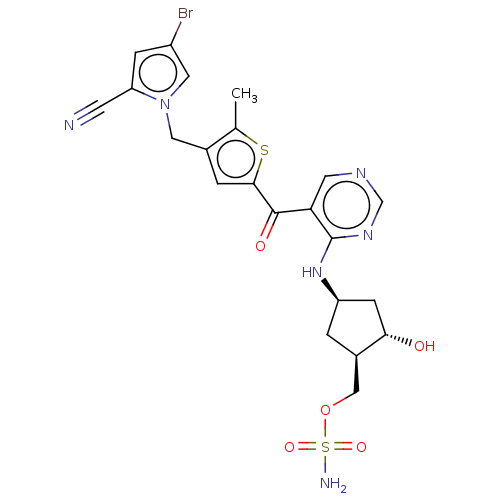 (US10780090, Compound I-148 | [(1R,2S,4R)-4-{[5-({4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462822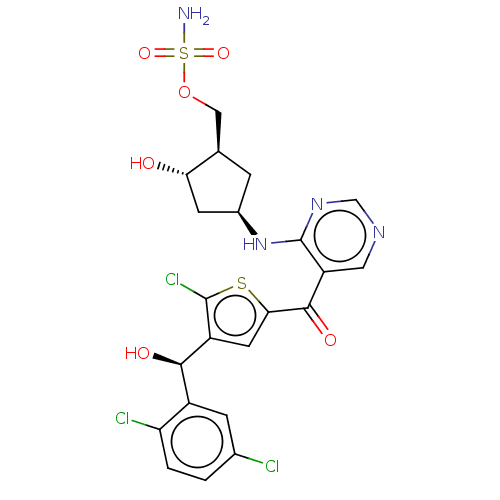 (US10780090, Compound I-149 | [(1R,2S,4R)-4-{[5-({5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462823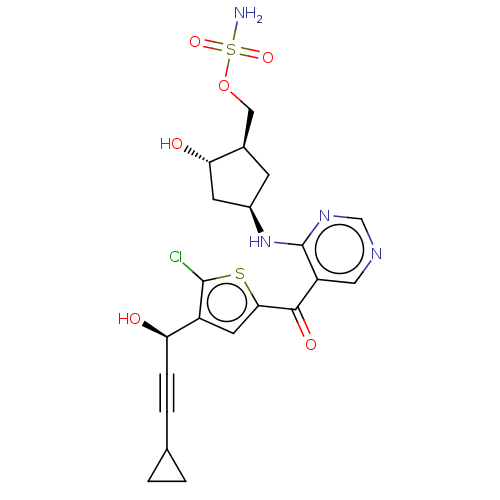 (US10780090, Compound I-150 | [(1R,2S,4R)-4-{[5-({5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462826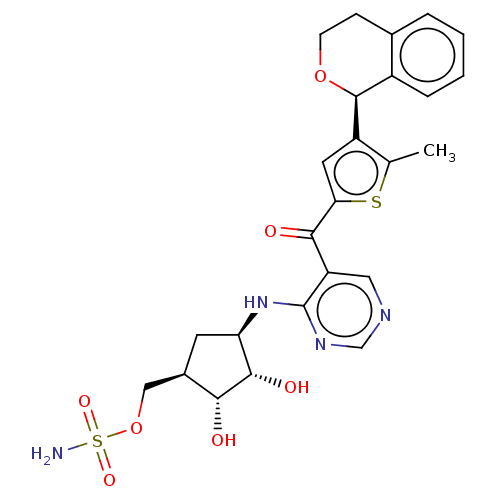 (US10780090, Compound I-151b | US10780090, Compound...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462827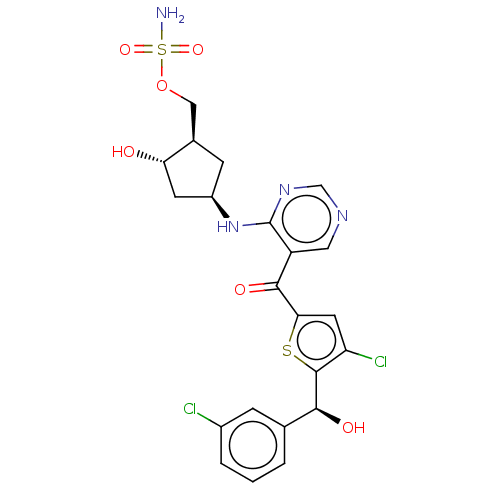 (US10780090, Compound I-152 | [(1R,2S,4R)-4-{[5-({5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462828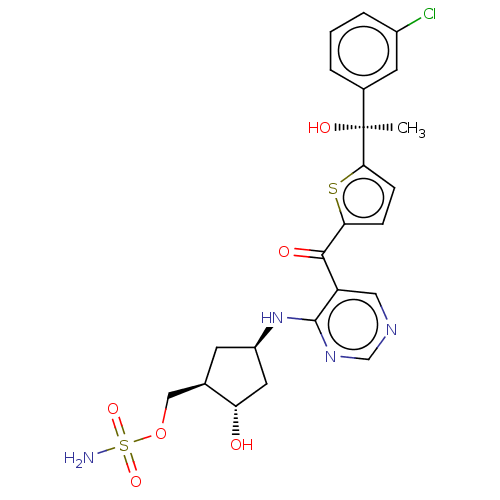 (US10780090, Compound I-153 | US10780090, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462831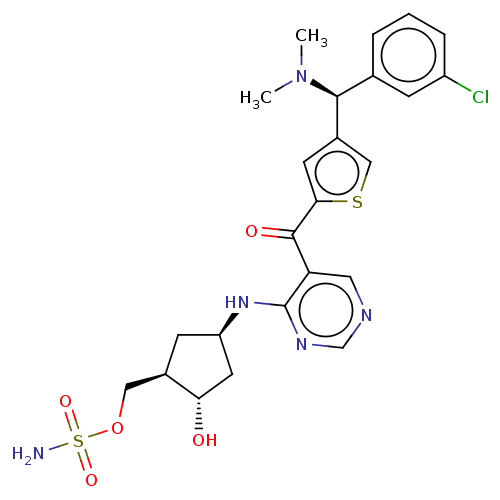 (US10780090, Compound I-154 | [(1R,2S,4R)-4-{[5-({4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462832 (US10780090, Compound I-155 | {(1R,2S,4R)-4-[(5-{[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462833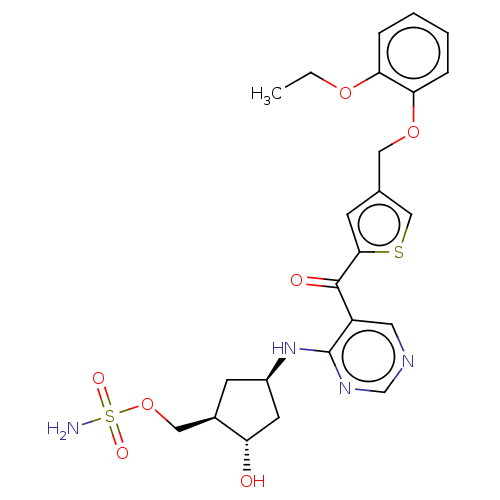 (US10780090, Compound I-156 | [(1R,2S,4R)-4-{[5-({4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462834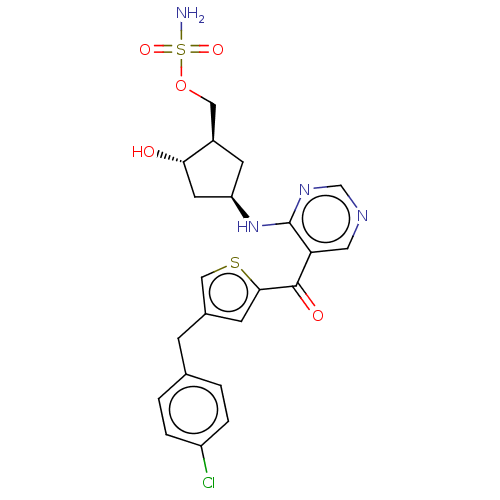 (US10780090, Compound I-157 | {(1R,2S,4R)-4-[(5-{[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462835 (US10780090, Compound I-158 | [(1R,2R,3S,4R)-4-({5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SUMO-activating enzyme subunit 1 (Homo sapiens (Human)) | BDBM462836 (US10780090, Compound I-159 | [(1R,2S,4R)-4-{[5-({4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description The SAE enzymatic reaction totals 50 μl and contains 50 mM HEPES Hemisodium (pH 7.5), 0.05% BSA, 5 mM MgCl2, 0.5 μM ATP, 250 μM GSH, 0... | US Patent US10780090 (2020) BindingDB Entry DOI: 10.7270/Q29Z980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 689 total ) | Next | Last >> |