 Found 679 hits with Last Name = 'durdagi' and Initial = 's'
Found 679 hits with Last Name = 'durdagi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093582
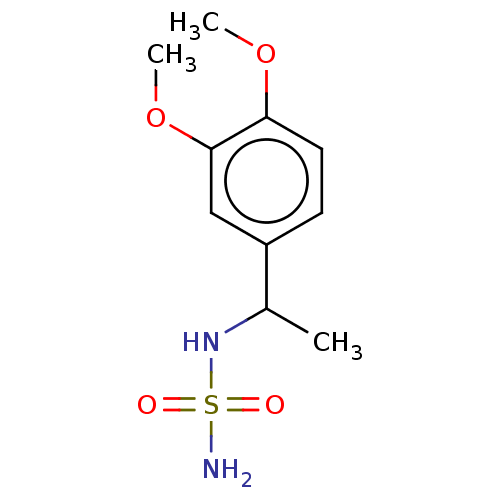
(CHEMBL3585782)Show InChI InChI=1S/C10H16N2O4S/c1-7(12-17(11,13)14)8-4-5-9(15-2)10(6-8)16-3/h4-7,12H,1-3H3,(H2,11,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093587
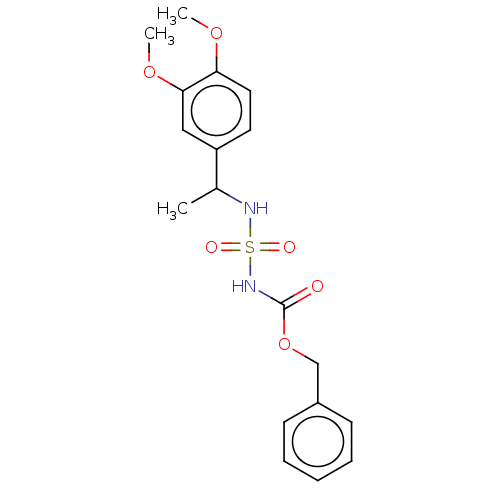
(CHEMBL3585777)Show SMILES COc1ccc(cc1OC)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(15-9-10-16(24-2)17(11-15)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093588
(CHEMBL3585776)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-8-10-16(23-2)11-9-15)18-25(21,22)19-17(20)24-12-14-6-4-3-5-7-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093589
(CHEMBL3585775)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-10-6-7-11-16(15)23-2)18-25(21,22)19-17(20)24-12-14-8-4-3-5-9-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093581
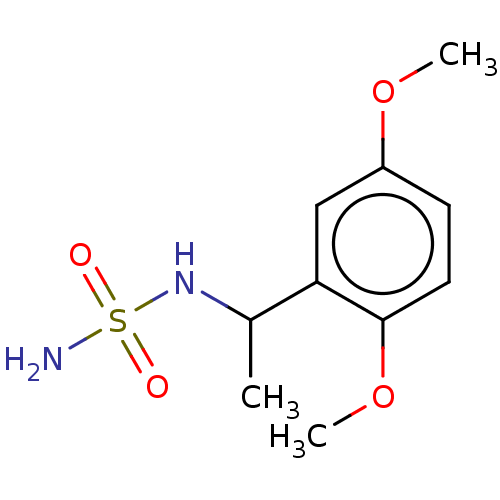
(CHEMBL3585783)Show InChI InChI=1S/C10H16N2O4S/c1-7(12-17(11,13)14)9-6-8(15-2)4-5-10(9)16-3/h4-7,12H,1-3H3,(H2,11,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093583
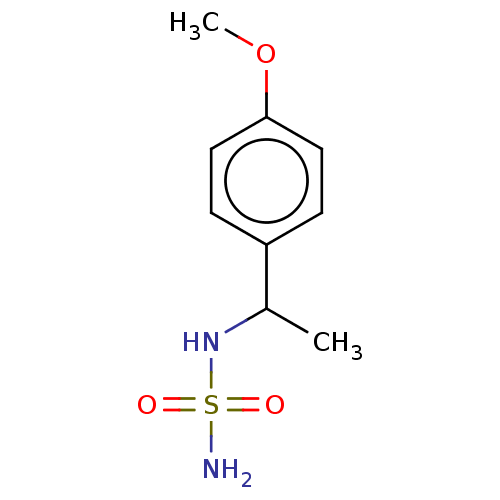
(CHEMBL3585781)Show InChI InChI=1S/C9H14N2O3S/c1-7(11-15(10,12)13)8-3-5-9(14-2)6-4-8/h3-7,11H,1-2H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093597
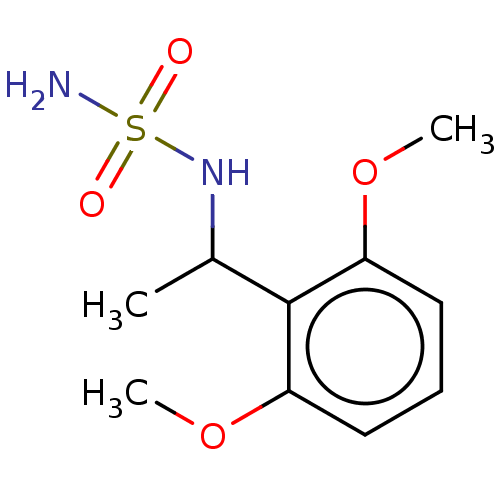
(CHEMBL3585784)Show InChI InChI=1S/C21H33NO2/c1-13(22-24)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4,15-19,23-24H,5-12H2,1-3H3/b22-13-/t15?,16?,17-,18?,19?,20?,21?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093585
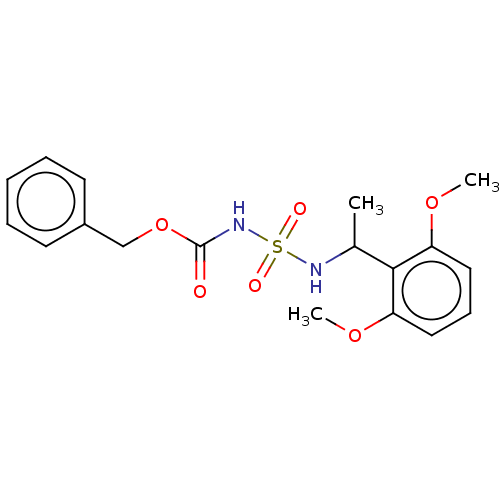
(CHEMBL3585779)Show SMILES COc1cccc(OC)c1C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(17-15(24-2)10-7-11-16(17)25-3)19-27(22,23)20-18(21)26-12-14-8-5-4-6-9-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093586
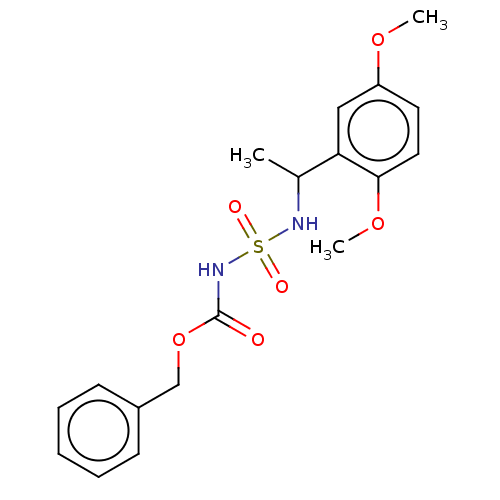
(CHEMBL3585778)Show SMILES COc1ccc(OC)c(c1)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(16-11-15(24-2)9-10-17(16)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093584
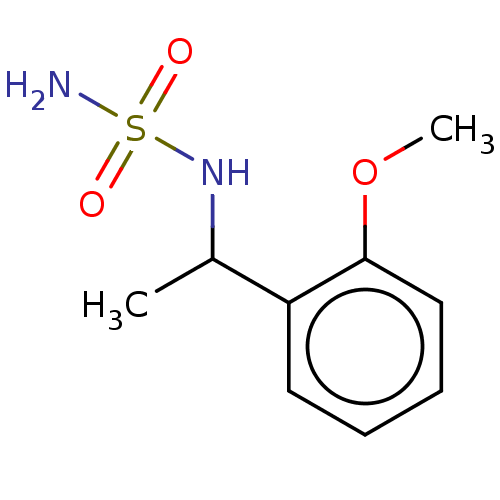
(CHEMBL3585780)Show InChI InChI=1S/C9H14N2O3S/c1-7(11-15(10,12)13)8-5-3-4-6-9(8)14-2/h3-7,11H,1-2H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093585

(CHEMBL3585779)Show SMILES COc1cccc(OC)c1C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(17-15(24-2)10-7-11-16(17)25-3)19-27(22,23)20-18(21)26-12-14-8-5-4-6-9-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093587

(CHEMBL3585777)Show SMILES COc1ccc(cc1OC)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(15-9-10-16(24-2)17(11-15)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50093586

(CHEMBL3585778)Show SMILES COc1ccc(OC)c(c1)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(16-11-15(24-2)9-10-17(16)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50093589

(CHEMBL3585775)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-10-6-7-11-16(15)23-2)18-25(21,22)19-17(20)24-12-14-8-4-3-5-9-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50093587

(CHEMBL3585777)Show SMILES COc1ccc(cc1OC)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(15-9-10-16(24-2)17(11-15)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50093585

(CHEMBL3585779)Show SMILES COc1cccc(OC)c1C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(17-15(24-2)10-7-11-16(17)25-3)19-27(22,23)20-18(21)26-12-14-8-5-4-6-9-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093588

(CHEMBL3585776)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-8-10-16(23-2)11-9-15)18-25(21,22)19-17(20)24-12-14-6-4-3-5-7-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093589

(CHEMBL3585775)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-10-6-7-11-16(15)23-2)18-25(21,22)19-17(20)24-12-14-8-4-3-5-9-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50093597

(CHEMBL3585784)Show InChI InChI=1S/C21H33NO2/c1-13(22-24)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4,15-19,23-24H,5-12H2,1-3H3/b22-13-/t15?,16?,17-,18?,19?,20?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093586

(CHEMBL3585778)Show SMILES COc1ccc(OC)c(c1)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(16-11-15(24-2)9-10-17(16)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50093588

(CHEMBL3585776)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-8-10-16(23-2)11-9-15)18-25(21,22)19-17(20)24-12-14-6-4-3-5-7-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50093584

(CHEMBL3585780)Show InChI InChI=1S/C9H14N2O3S/c1-7(11-15(10,12)13)8-5-3-4-6-9(8)14-2/h3-7,11H,1-2H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50067742

(7-((6aR,10aR)-1-Hydroxy-6,6,9-trimethyl-6a,7,10,10...)Show SMILES CC1=CC[C@@H]2[C@@H](C1)c1c(O)cc(cc1OC2(C)C)C(C)(C)CCCCCC#N |t:1| Show InChI InChI=1S/C25H35NO2/c1-17-10-11-20-19(14-17)23-21(27)15-18(16-22(23)28-25(20,4)5)24(2,3)12-8-6-7-9-13-26/h10,15-16,19-20,27H,6-9,11-12,14H2,1-5H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50130624
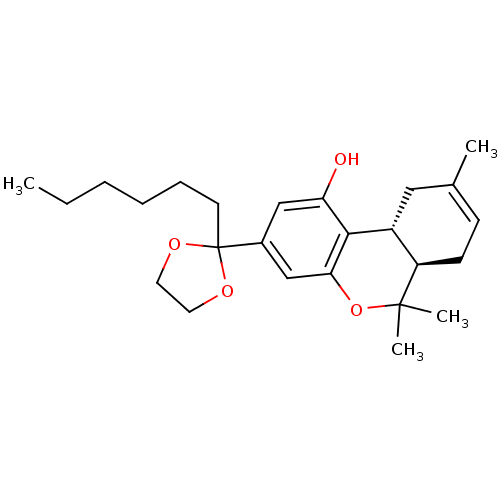
((6aR,10aR)-3-(2-hexyl-1,3-dioxolan-2-yl)-6,6,9-tri...)Show SMILES CCCCCCC1(OCCO1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:20| Show InChI InChI=1S/C25H36O4/c1-5-6-7-8-11-25(27-12-13-28-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)29-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213605
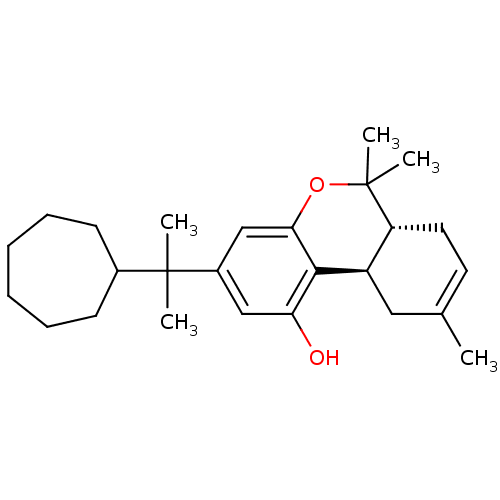
((6aR,10aR)-3-(2-cycloheptylpropan-2-yl)-6,6,9-trim...)Show SMILES CC1=CC[C@@H]2[C@@H](C1)c1c(O)cc(cc1OC2(C)C)C(C)(C)C1CCCCCC1 |t:1| Show InChI InChI=1S/C26H38O2/c1-17-12-13-21-20(14-17)24-22(27)15-19(16-23(24)28-26(21,4)5)25(2,3)18-10-8-6-7-9-11-18/h12,15-16,18,20-21,27H,6-11,13-14H2,1-5H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093584

(CHEMBL3585780)Show InChI InChI=1S/C9H14N2O3S/c1-7(11-15(10,12)13)8-5-3-4-6-9(8)14-2/h3-7,11H,1-2H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50121425
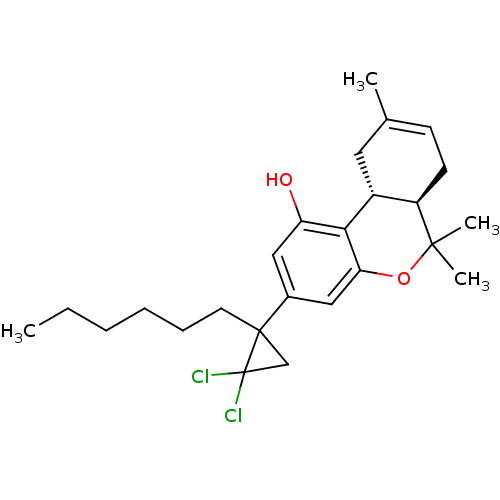
((6aR,10aR)-3-(2,2-Dichloro-1-hexyl-cyclopropyl)-6,...)Show SMILES CCCCCCC1(CC1(Cl)Cl)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:20| Show InChI InChI=1S/C25H34Cl2O2/c1-5-6-7-8-11-24(15-25(24,26)27)17-13-20(28)22-18-12-16(2)9-10-19(18)23(3,4)29-21(22)14-17/h9,13-14,18-19,28H,5-8,10-12,15H2,1-4H3/t18-,19-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50063885
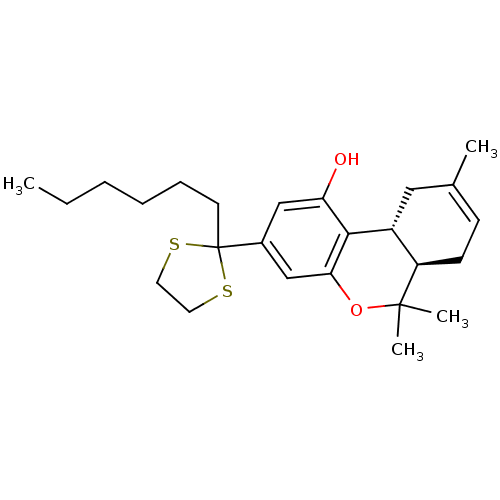
((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...)Show SMILES CCCCCCC1(SCCS1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:20| Show InChI InChI=1S/C25H36O2S2/c1-5-6-7-8-11-25(28-12-13-29-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)27-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50063885

((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...)Show SMILES CCCCCCC1(SCCS1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:20| Show InChI InChI=1S/C25H36O2S2/c1-5-6-7-8-11-25(28-12-13-29-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)27-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor (unknown origin) |
Bioorg Med Chem 16: 7377-87 (2008)
Article DOI: 10.1016/j.bmc.2008.06.019
BindingDB Entry DOI: 10.7270/Q2TX3F6K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50063885

((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...)Show SMILES CCCCCCC1(SCCS1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:20| Show InChI InChI=1S/C25H36O2S2/c1-5-6-7-8-11-25(28-12-13-29-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)27-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity at CB1 receptor |
Bioorg Med Chem Lett 17: 6754-63 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.044
BindingDB Entry DOI: 10.7270/Q2QV3Q9M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213607
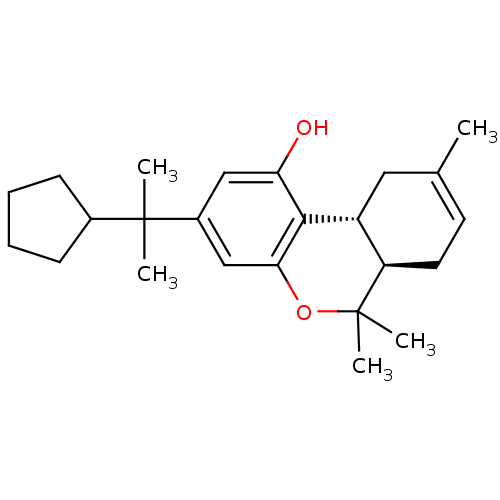
((6aR,10aR)-3-(2-cyclopentylpropan-2-yl)-6,6,9-trim...)Show SMILES CC1=CC[C@@H]2[C@@H](C1)c1c(O)cc(cc1OC2(C)C)C(C)(C)C1CCCC1 |t:1| Show InChI InChI=1S/C24H34O2/c1-15-10-11-19-18(12-15)22-20(25)13-17(14-21(22)26-24(19,4)5)23(2,3)16-8-6-7-9-16/h10,13-14,16,18-19,25H,6-9,11-12H2,1-5H3/t18-,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50093581

(CHEMBL3585783)Show InChI InChI=1S/C10H16N2O4S/c1-7(12-17(11,13)14)9-6-8(15-2)4-5-10(9)16-3/h4-7,12H,1-3H3,(H2,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213607

((6aR,10aR)-3-(2-cyclopentylpropan-2-yl)-6,6,9-trim...)Show SMILES CC1=CC[C@@H]2[C@@H](C1)c1c(O)cc(cc1OC2(C)C)C(C)(C)C1CCCC1 |t:1| Show InChI InChI=1S/C24H34O2/c1-15-10-11-19-18(12-15)22-20(25)13-17(14-21(22)26-24(19,4)5)23(2,3)16-8-6-7-9-16/h10,13-14,16,18-19,25H,6-9,11-12H2,1-5H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213599
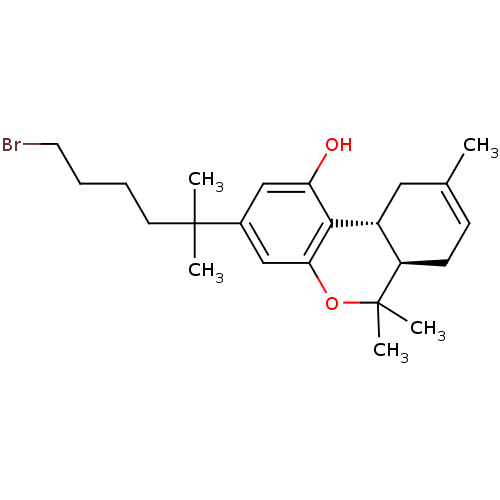
((6aR,10aR)-3-(6-bromo-2-methylhexan-2-yl)-6,6,9-tr...)Show SMILES CC1=CC[C@@H]2[C@@H](C1)c1c(O)cc(cc1OC2(C)C)C(C)(C)CCCCBr |t:1| Show InChI InChI=1S/C23H33BrO2/c1-15-8-9-18-17(12-15)21-19(25)13-16(14-20(21)26-23(18,4)5)22(2,3)10-6-7-11-24/h8,13-14,17-18,25H,6-7,9-12H2,1-5H3/t17-,18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50063884
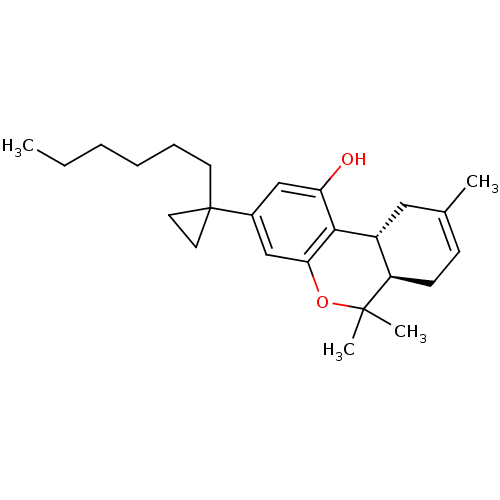
((6aR,10aR)-3-(1-Hexyl-cyclopropyl)-6,6,9-trimethyl...)Show SMILES CCCCCCC1(CC1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H36O2/c1-5-6-7-8-11-25(12-13-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)27-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity at CB1 receptor |
Bioorg Med Chem Lett 17: 6754-63 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.044
BindingDB Entry DOI: 10.7270/Q2QV3Q9M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50063884

((6aR,10aR)-3-(1-Hexyl-cyclopropyl)-6,6,9-trimethyl...)Show SMILES CCCCCCC1(CC1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H36O2/c1-5-6-7-8-11-25(12-13-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)27-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50130623
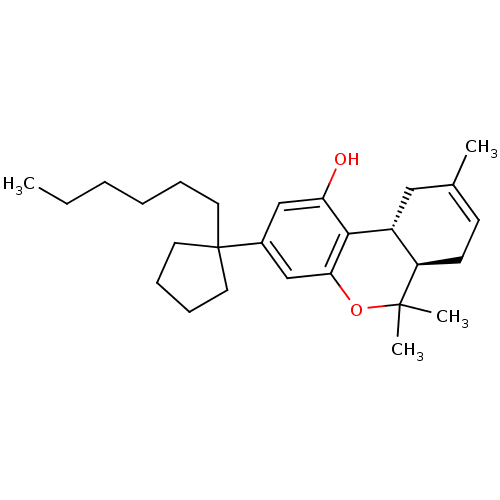
((6aR,10aR)-3-(1-hexylcyclopentyl)-6,6,9-trimethyl-...)Show SMILES CCCCCCC1(CCCC1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:20| Show InChI InChI=1S/C27H40O2/c1-5-6-7-8-13-27(14-9-10-15-27)20-17-23(28)25-21-16-19(2)11-12-22(21)26(3,4)29-24(25)18-20/h11,17-18,21-22,28H,5-10,12-16H2,1-4H3/t21-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity at CB1 receptor |
Bioorg Med Chem Lett 17: 6754-63 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.044
BindingDB Entry DOI: 10.7270/Q2QV3Q9M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161477
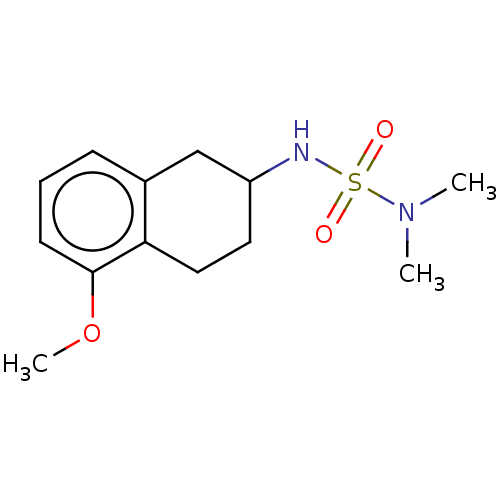
(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50130623

((6aR,10aR)-3-(1-hexylcyclopentyl)-6,6,9-trimethyl-...)Show SMILES CCCCCCC1(CCCC1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:20| Show InChI InChI=1S/C27H40O2/c1-5-6-7-8-13-27(14-9-10-15-27)20-17-23(28)25-21-16-19(2)11-12-22(21)26(3,4)29-24(25)18-20/h11,17-18,21-22,28H,5-10,12-16H2,1-4H3/t21-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50093582

(CHEMBL3585782)Show InChI InChI=1S/C10H16N2O4S/c1-7(12-17(11,13)14)8-4-5-9(15-2)10(6-8)16-3/h4-7,12H,1-3H3,(H2,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.454 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093583

(CHEMBL3585781)Show InChI InChI=1S/C9H14N2O3S/c1-7(11-15(10,12)13)8-3-5-9(14-2)6-4-8/h3-7,11H,1-2H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50067735
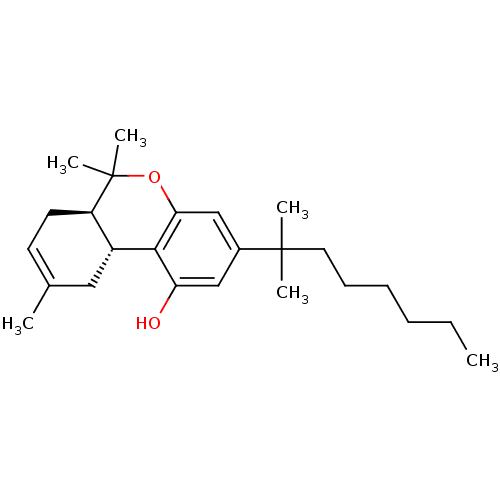
((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6,9-trimethyl...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:17| Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h11,15-16,19-20,26H,7-10,12-14H2,1-6H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50063885

((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...)Show SMILES CCCCCCC1(SCCS1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:20| Show InChI InChI=1S/C25H36O2S2/c1-5-6-7-8-11-25(28-12-13-29-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)27-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50063885

((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...)Show SMILES CCCCCCC1(SCCS1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:20| Show InChI InChI=1S/C25H36O2S2/c1-5-6-7-8-11-25(28-12-13-29-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)27-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Bioorg Med Chem 16: 7377-87 (2008)
Article DOI: 10.1016/j.bmc.2008.06.019
BindingDB Entry DOI: 10.7270/Q2TX3F6K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50130624

((6aR,10aR)-3-(2-hexyl-1,3-dioxolan-2-yl)-6,6,9-tri...)Show SMILES CCCCCCC1(OCCO1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:20| Show InChI InChI=1S/C25H36O4/c1-5-6-7-8-11-25(27-12-13-28-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)29-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50130624

((6aR,10aR)-3-(2-hexyl-1,3-dioxolan-2-yl)-6,6,9-tri...)Show SMILES CCCCCCC1(OCCO1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:20| Show InChI InChI=1S/C25H36O4/c1-5-6-7-8-11-25(27-12-13-28-25)18-15-21(26)23-19-14-17(2)9-10-20(19)24(3,4)29-22(23)16-18/h9,15-16,19-20,26H,5-8,10-14H2,1-4H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity at CB1 receptor |
Bioorg Med Chem Lett 17: 6754-63 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.044
BindingDB Entry DOI: 10.7270/Q2QV3Q9M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093581

(CHEMBL3585783)Show InChI InChI=1S/C10H16N2O4S/c1-7(12-17(11,13)14)9-6-8(15-2)4-5-10(9)16-3/h4-7,12H,1-3H3,(H2,11,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50133544

((6aR,10aR)-3-(1-Cyclohexyl-1-methyl-ethyl)-6,6,9-t...)Show SMILES CC1=CC[C@@H]2[C@@H](C1)c1c(O)cc(cc1OC2(C)C)C(C)(C)C1CCCCC1 |t:1| Show InChI InChI=1S/C25H36O2/c1-16-11-12-20-19(13-16)23-21(26)14-18(15-22(23)27-25(20,4)5)24(2,3)17-9-7-6-8-10-17/h11,14-15,17,19-20,26H,6-10,12-13H2,1-5H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50133544

((6aR,10aR)-3-(1-Cyclohexyl-1-methyl-ethyl)-6,6,9-t...)Show SMILES CC1=CC[C@@H]2[C@@H](C1)c1c(O)cc(cc1OC2(C)C)C(C)(C)C1CCCCC1 |t:1| Show InChI InChI=1S/C25H36O2/c1-16-11-12-20-19(13-16)23-21(26)14-18(15-22(23)27-25(20,4)5)24(2,3)17-9-7-6-8-10-17/h11,14-15,17,19-20,26H,6-10,12-13H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor |
J Med Chem 50: 2875-85 (2007)
Article DOI: 10.1021/jm0610705
BindingDB Entry DOI: 10.7270/Q2ST7PJ4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50093582

(CHEMBL3585782)Show InChI InChI=1S/C10H16N2O4S/c1-7(12-17(11,13)14)8-4-5-9(15-2)10(6-8)16-3/h4-7,12H,1-3H3,(H2,11,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.657 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data