

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223332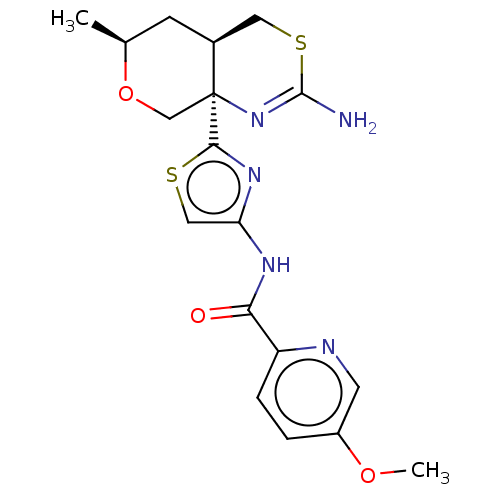 (US9315520, 19 | US9605007, Example 19 | US9744173,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452875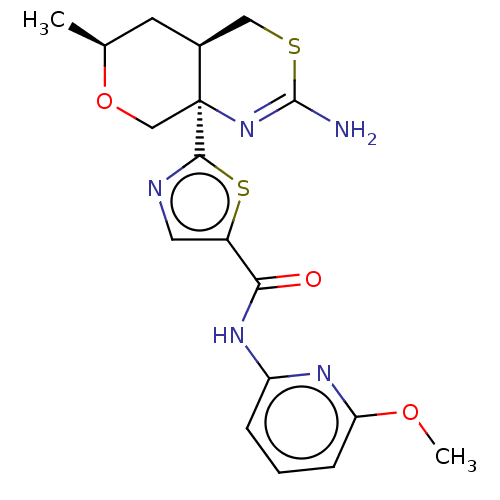 (CHEMBL4212046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452884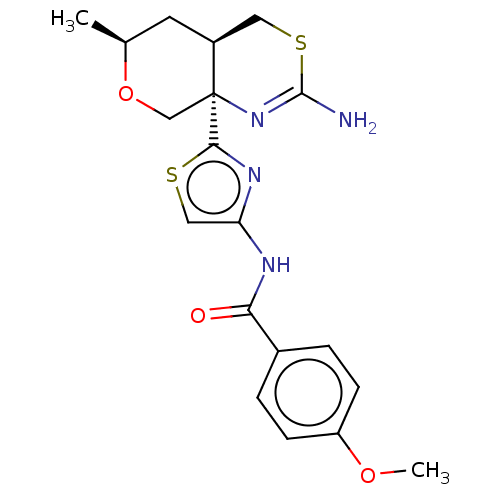 (CHEMBL4217023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452883 (CHEMBL4203860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485444 (CHEMBL2059813) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50458169 (Avagacestat | BMS 708163 | BMS-708163 | BMS-708163...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.225 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353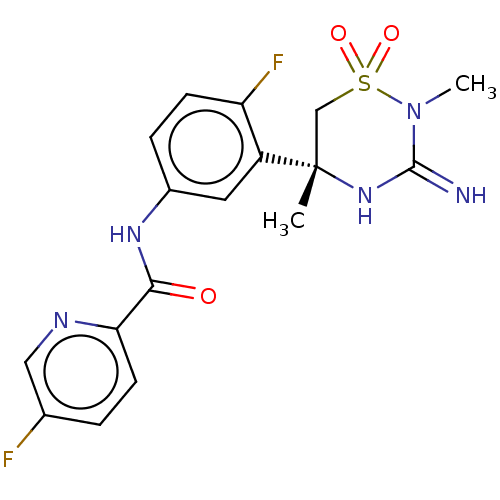 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452874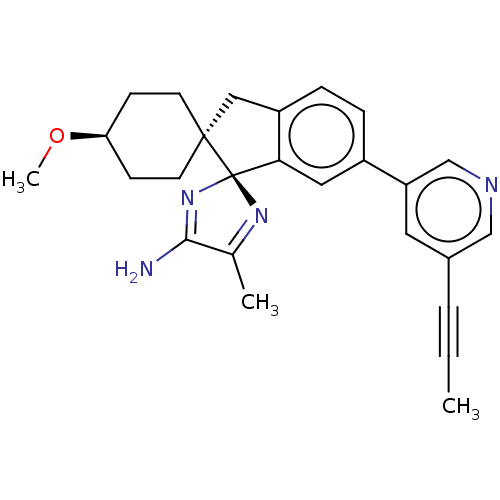 (CHEMBL4217620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50452874 (CHEMBL4217620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485438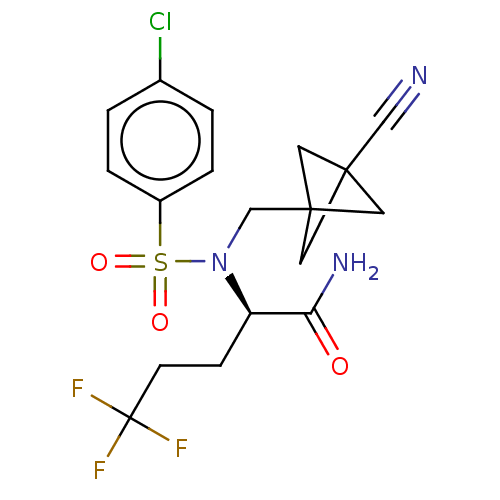 (CHEMBL2059021) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485443 (CHEMBL2059814) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485431 (CHEMBL2059815) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485434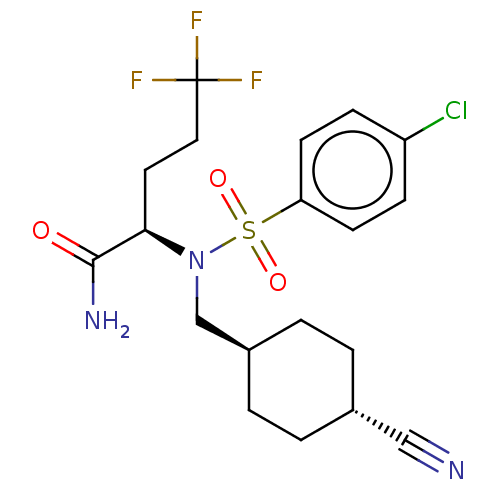 (CHEMBL2059016) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485434 (CHEMBL2059016) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223400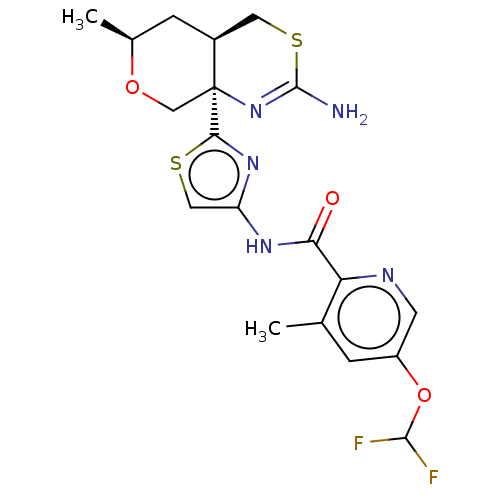 (US9315520, 45 | US9315520, Example 45 | US9605007,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length Myc-DDK-tagged BACE2 expressed in HEK293 cell membranes after 30 mins by parallel ... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223396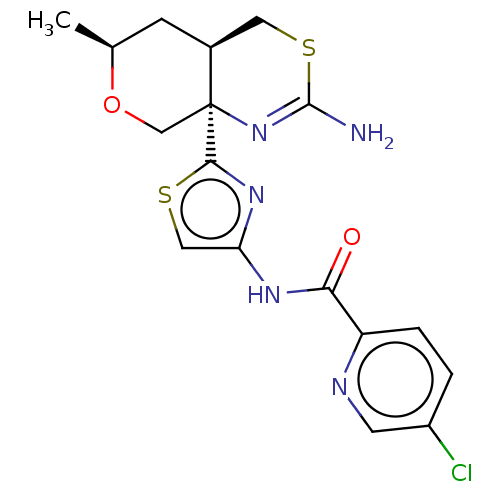 (US9315520, 7 | US9315520, Example 7 | US9605007, E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312940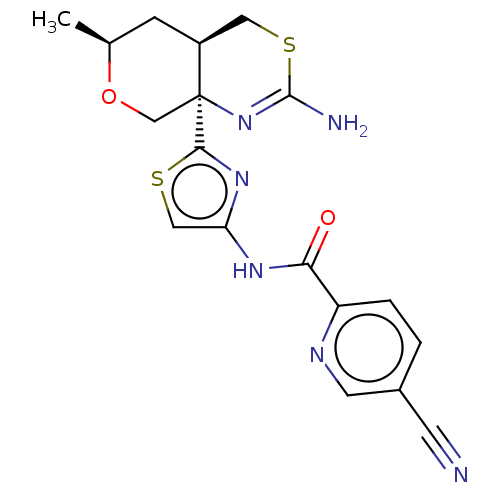 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223330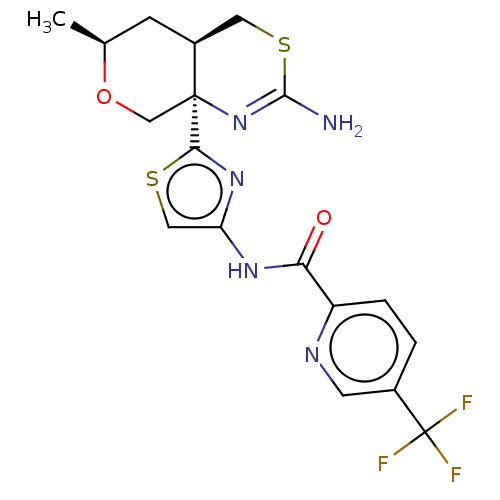 (US9315520, 17 | US9605007, Example 17 | US9744173,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485436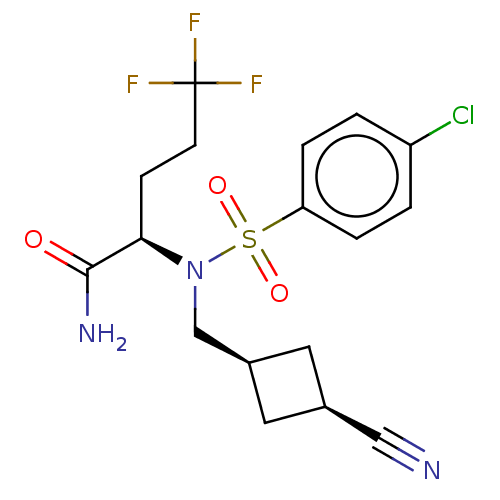 (CHEMBL2059011) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223395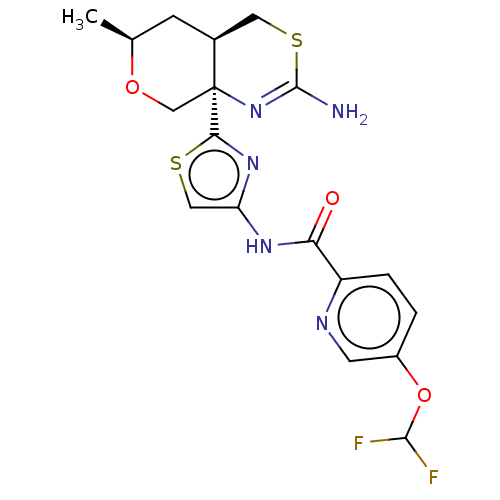 (US9315520, 1 | US9315520, Comparator 1 | US9315520...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484476 (CHEMBL1916325) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma-secretase-mediated cleavage of human amyloid beta protein expressed in human H4 cells assessed as reduction in amyloid beta42 pro... | J Med Chem 54: 7772-83 (2011) Article DOI: 10.1021/jm200893p BindingDB Entry DOI: 10.7270/Q2JW8HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484472 (CHEMBL1916327) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma-secretase-mediated cleavage of human amyloid beta protein expressed in human H4 cells assessed as reduction in amyloid beta42 pro... | J Med Chem 54: 7772-83 (2011) Article DOI: 10.1021/jm200893p BindingDB Entry DOI: 10.7270/Q2JW8HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484455 (CHEMBL1916335) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma-secretase-mediated cleavage of human amyloid beta protein expressed in human H4 cells assessed as reduction in amyloid beta42 pro... | J Med Chem 54: 7772-83 (2011) Article DOI: 10.1021/jm200893p BindingDB Entry DOI: 10.7270/Q2JW8HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485447 (CHEMBL2059819) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312934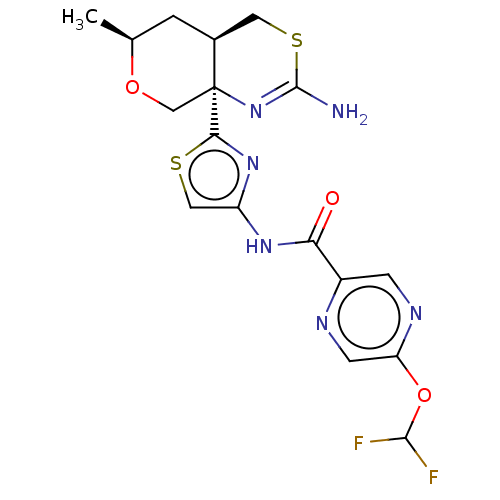 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223395 (US9315520, 1 | US9315520, Comparator 1 | US9315520...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484467 (CHEMBL1916326) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma-secretase-mediated cleavage of human amyloid beta protein expressed in human H4 cells assessed as reduction in amyloid beta42 pro... | J Med Chem 54: 7772-83 (2011) Article DOI: 10.1021/jm200893p BindingDB Entry DOI: 10.7270/Q2JW8HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485435 (CHEMBL2059014) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484465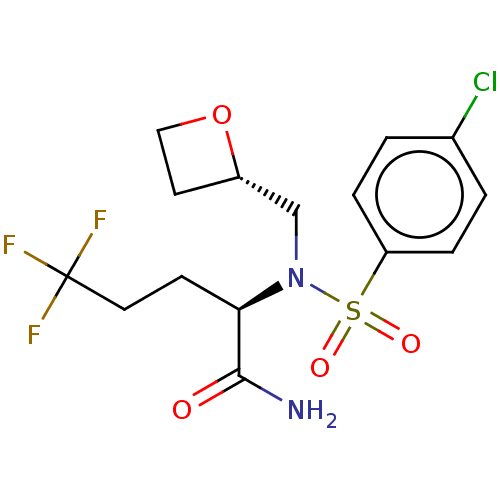 (CHEMBL1916331) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma-secretase-mediated cleavage of human amyloid beta protein expressed in human H4 cells assessed as reduction in amyloid beta42 pro... | J Med Chem 54: 7772-83 (2011) Article DOI: 10.1021/jm200893p BindingDB Entry DOI: 10.7270/Q2JW8HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484460 (CHEMBL1916320) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma-secretase-mediated cleavage of human amyloid beta protein expressed in human H4 cells assessed as reduction in amyloid beta42 pro... | J Med Chem 54: 7772-83 (2011) Article DOI: 10.1021/jm200893p BindingDB Entry DOI: 10.7270/Q2JW8HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223396 (US9315520, 7 | US9315520, Example 7 | US9605007, E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484474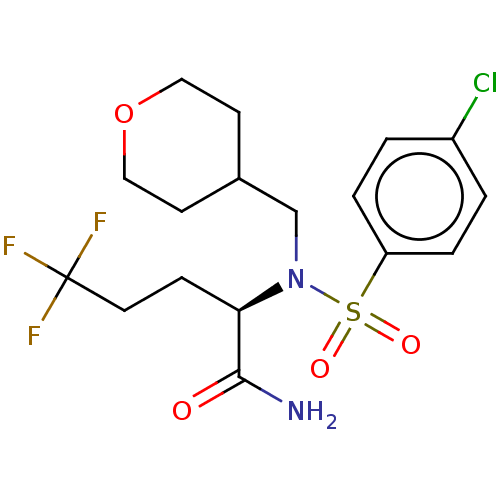 (CHEMBL1916324) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma-secretase-mediated cleavage of human amyloid beta protein expressed in human H4 cells assessed as reduction in amyloid beta42 pro... | J Med Chem 54: 7772-83 (2011) Article DOI: 10.1021/jm200893p BindingDB Entry DOI: 10.7270/Q2JW8HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223400 (US9315520, 45 | US9315520, Example 45 | US9605007,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50485446 (CHEMBL2059015) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant | J Med Chem 55: 3414-24 (2012) Article DOI: 10.1021/jm300094u BindingDB Entry DOI: 10.7270/Q23F4SHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50452877 (CHEMBL4205912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182352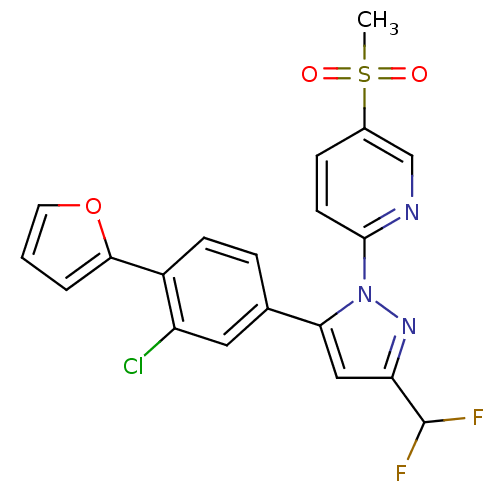 (2-(5-(3-chloro-4-(furan-2-yl)phenyl)-3-(difluorome...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182338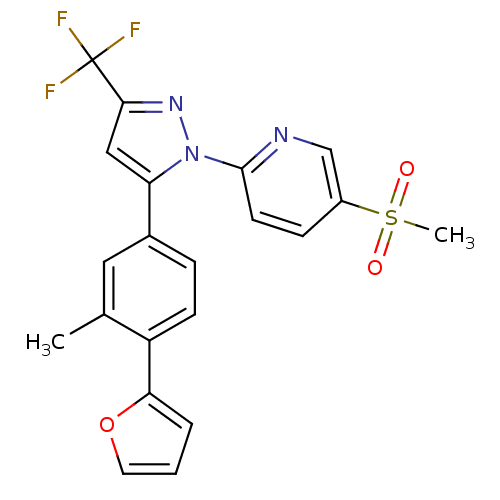 (2-(5-(4-(furan-2-yl)-3-methylphenyl)-3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484454 (CHEMBL1916334) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of gamma-secretase-mediated cleavage of human amyloid beta protein expressed in human H4 cells assessed as reduction in amyloid beta42 pro... | J Med Chem 54: 7772-83 (2011) Article DOI: 10.1021/jm200893p BindingDB Entry DOI: 10.7270/Q2JW8HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 348 total ) | Next | Last >> |