

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145818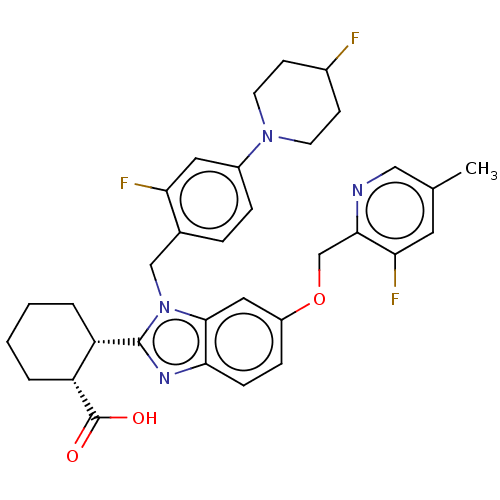 (US8952177, 174 | US9089569, 174 | US9695149, 174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145818 (US8952177, 174 | US9089569, 174 | US9695149, 174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145818 (US8952177, 174 | US9089569, 174 | US9695149, 174) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50371662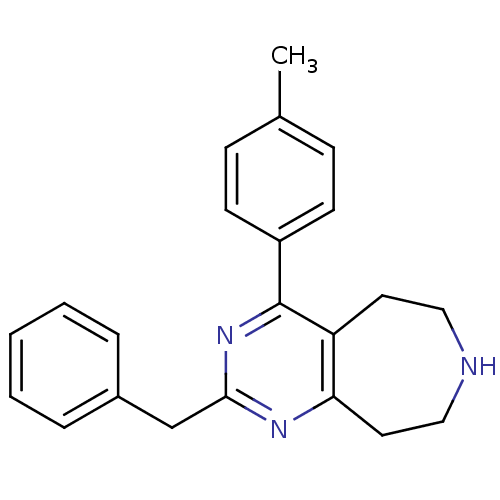 (CHEMBL269974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells | Bioorg Med Chem Lett 18: 2103-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.090 BindingDB Entry DOI: 10.7270/Q2571CW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145820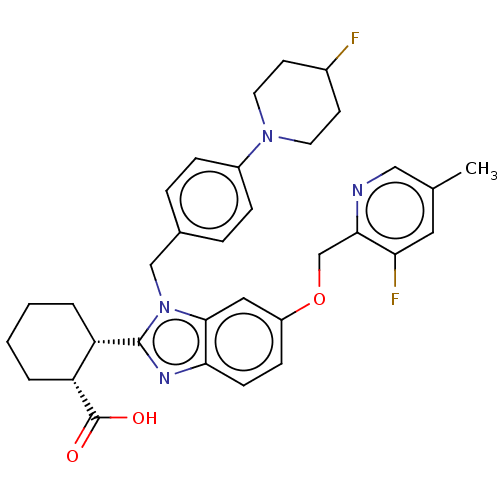 (US8952177, 176 | US9089569, 176 | US9695149, 176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145820 (US8952177, 176 | US9089569, 176 | US9695149, 176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145820 (US8952177, 176 | US9089569, 176 | US9695149, 176) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50410342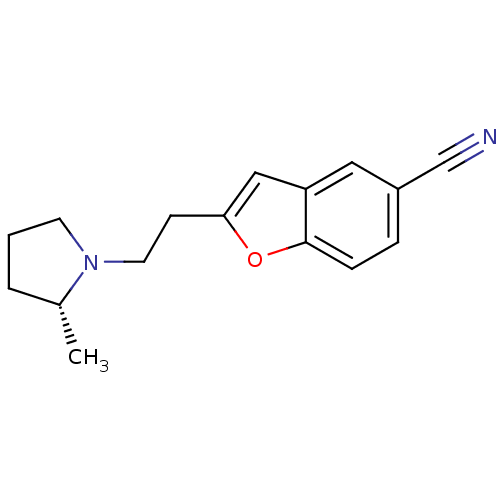 (CHEMBL195408) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Mean binding affinity for human H3 receptor | J Med Chem 48: 2229-38 (2005) Article DOI: 10.1021/jm049212n BindingDB Entry DOI: 10.7270/Q2GB258T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145785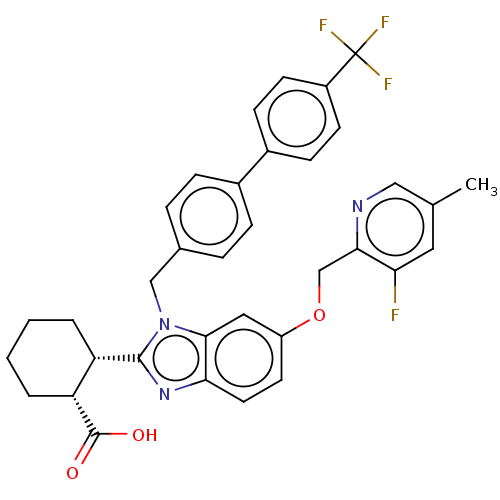 (US8952177, 139 | US9089569, 139 | US9695149, 139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145785 (US8952177, 139 | US9089569, 139 | US9695149, 139) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145785 (US8952177, 139 | US9089569, 139 | US9695149, 139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200636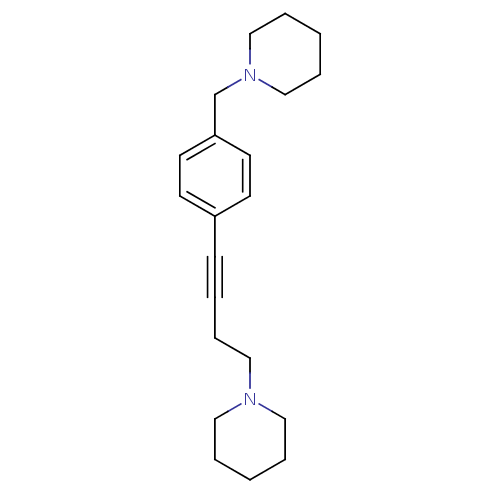 (1-(4-(4-(piperidin-1-ylmethyl)phenyl)but-3-ynyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145770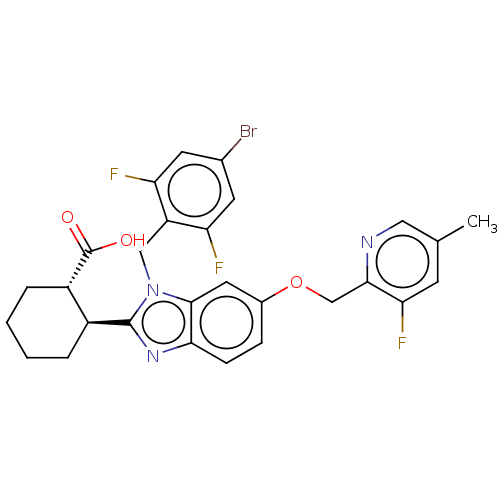 (US8952177, 124 | US9089569, 124 | US9695149, 124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145770 (US8952177, 124 | US9089569, 124 | US9695149, 124) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145770 (US8952177, 124 | US9089569, 124 | US9695149, 124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414797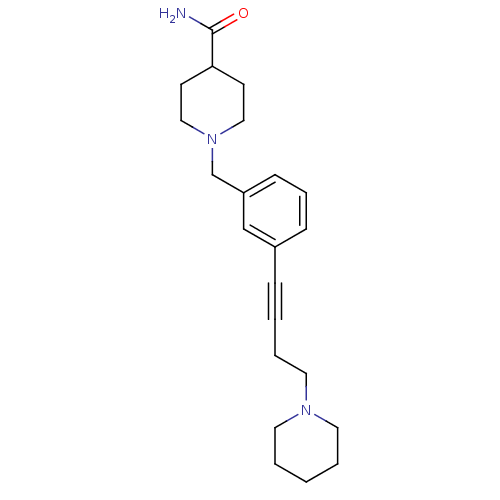 (CHEMBL582977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50371682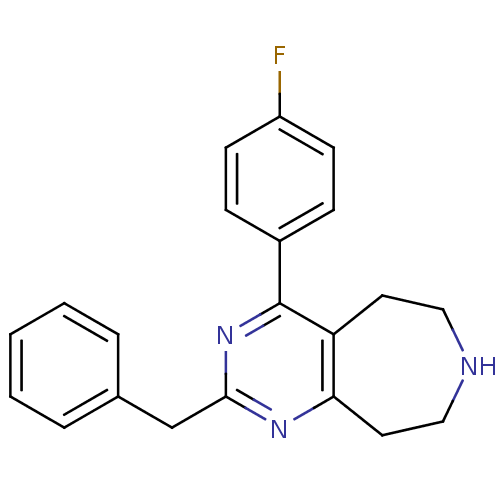 (CHEMBL270188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells | Bioorg Med Chem Lett 18: 2103-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.090 BindingDB Entry DOI: 10.7270/Q2571CW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145769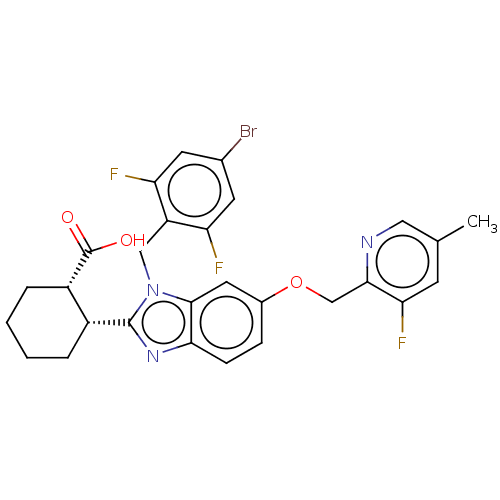 (US8952177, 123 | US9089569, 123 | US9695149, 123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145769 (US8952177, 123 | US9089569, 123 | US9695149, 123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145769 (US8952177, 123 | US9089569, 123 | US9695149, 123) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414804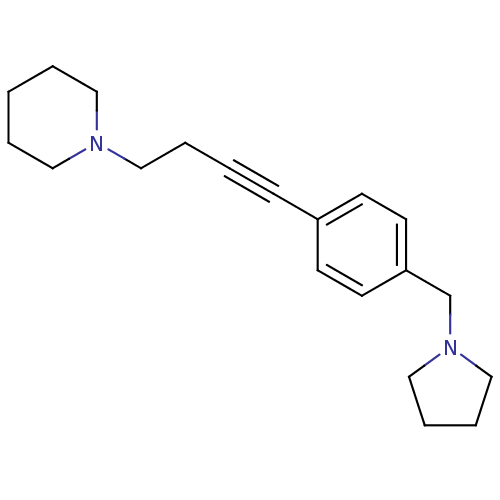 (CHEMBL574712) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50410347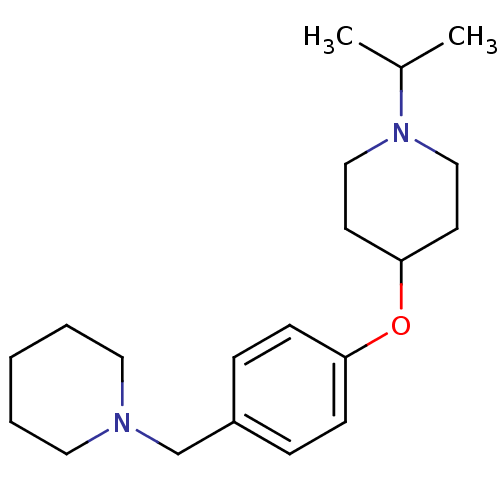 (CHEMBL194441) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Mean binding affinity for human H3 receptor | J Med Chem 48: 2229-38 (2005) Article DOI: 10.1021/jm049212n BindingDB Entry DOI: 10.7270/Q2GB258T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145782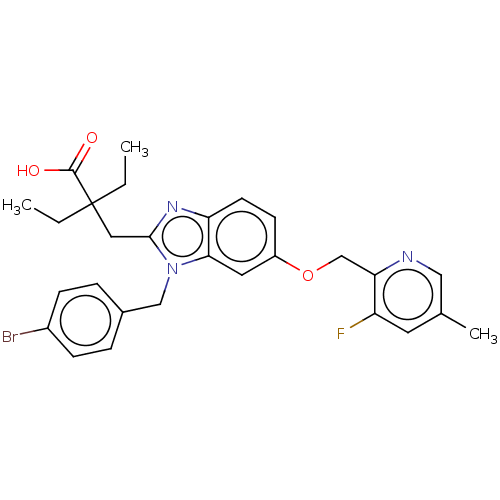 (US8952177, 136 | US9089569, 136 | US9695149, 136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145782 (US8952177, 136 | US9089569, 136 | US9695149, 136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145782 (US8952177, 136 | US9089569, 136 | US9695149, 136) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145783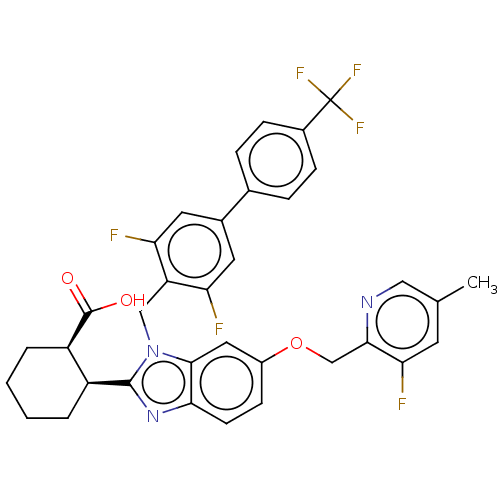 (US8952177, 137 | US9089569, 137 | US9695149, 137) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145783 (US8952177, 137 | US9089569, 137 | US9695149, 137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145783 (US8952177, 137 | US9089569, 137 | US9695149, 137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414811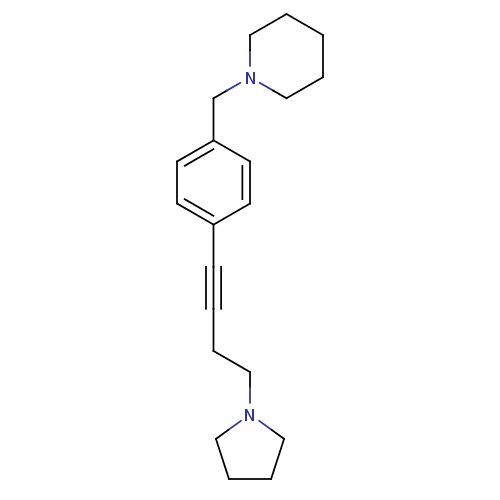 (CHEMBL574721) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to the human histamine H3 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50371669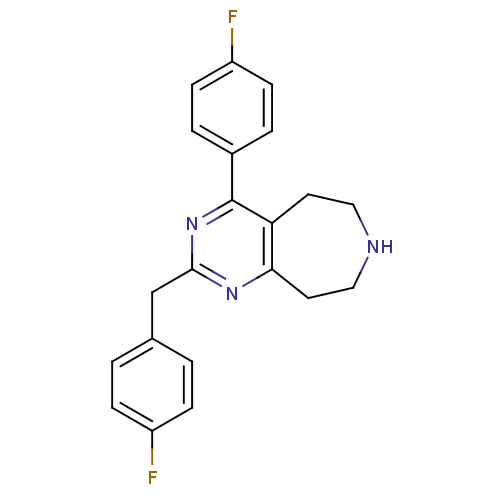 (CHEMBL402164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells | Bioorg Med Chem Lett 18: 2103-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.090 BindingDB Entry DOI: 10.7270/Q2571CW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414798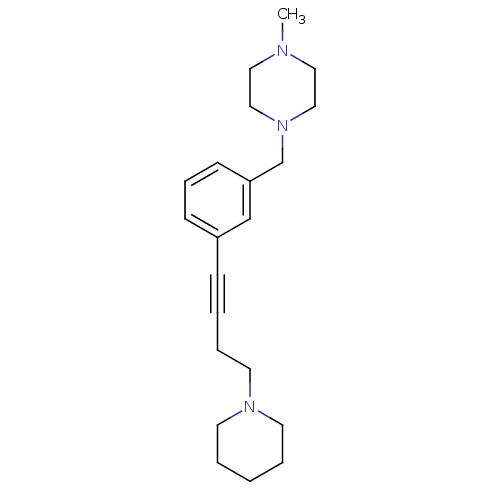 (CHEMBL575172) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145657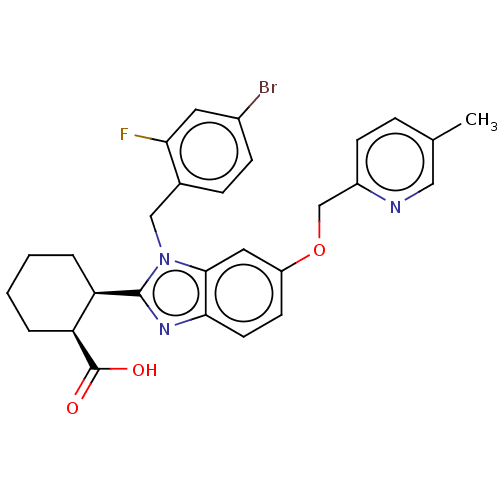 (US8952177, 11 | US9089569, 11 | US9695149, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145657 (US8952177, 11 | US9089569, 11 | US9695149, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145657 (US8952177, 11 | US9089569, 11 | US9695149, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50410346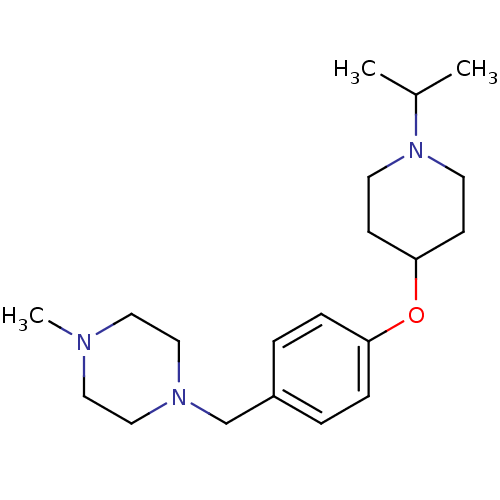 (CHEMBL372471) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Mean binding affinity for human H3 receptor | J Med Chem 48: 2229-38 (2005) Article DOI: 10.1021/jm049212n BindingDB Entry DOI: 10.7270/Q2GB258T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414800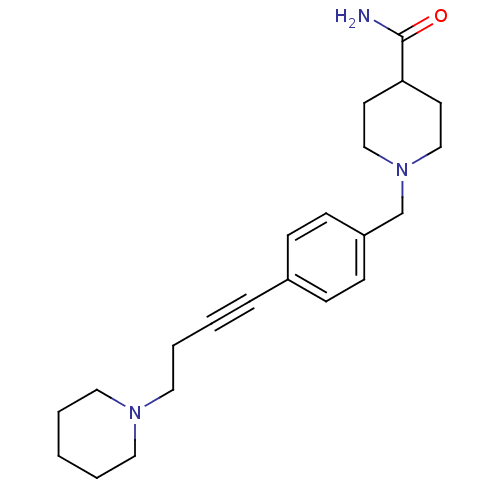 (CHEMBL583182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414792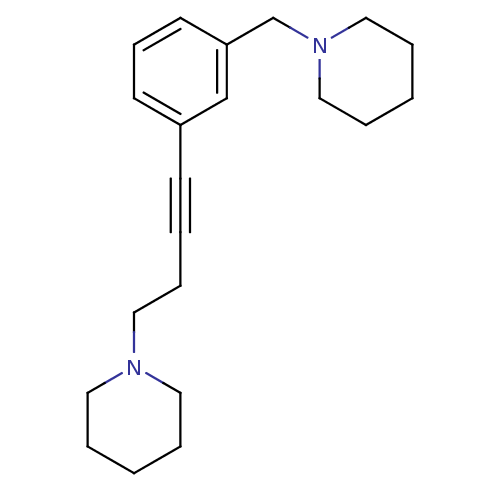 (CHEMBL573817) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50371681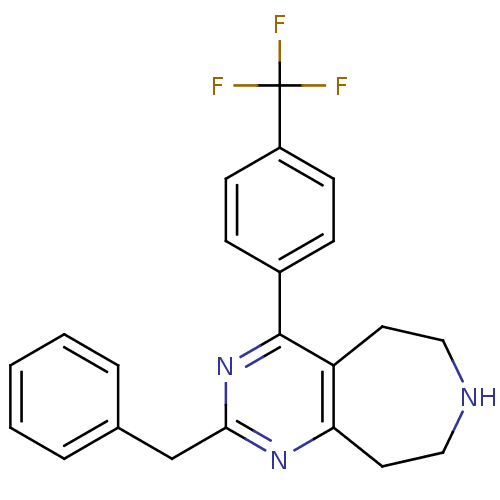 (CHEMBL271418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells | Bioorg Med Chem Lett 18: 2103-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.090 BindingDB Entry DOI: 10.7270/Q2571CW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145817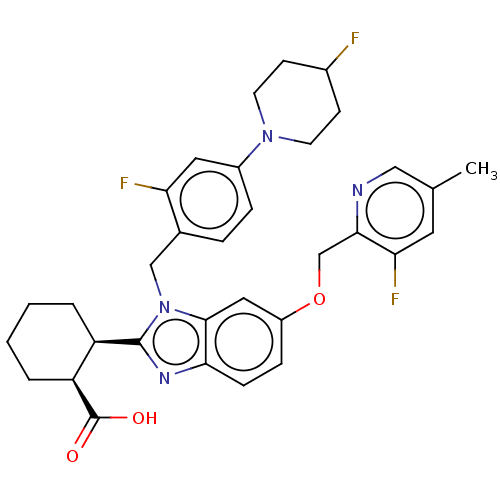 (US8952177, 173 | US8952177, 175 | US9089569, 175 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145817 (US8952177, 173 | US8952177, 175 | US9089569, 175 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145817 (US8952177, 173 | US8952177, 175 | US9089569, 175 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145816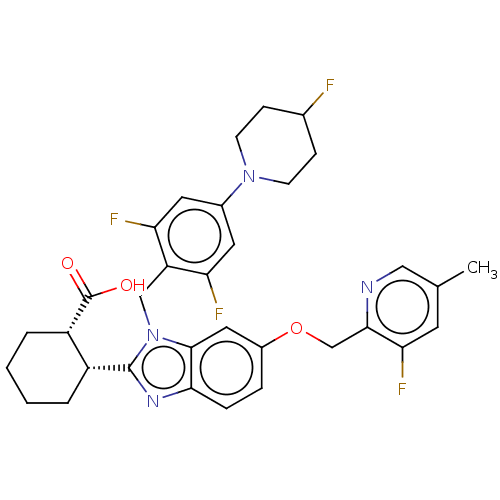 (US8952177, 172 | US9089569, 172 | US9695149, 172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145816 (US8952177, 172 | US9089569, 172 | US9695149, 172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145816 (US8952177, 172 | US9089569, 172 | US9695149, 172) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414813 (CHEMBL573328) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146838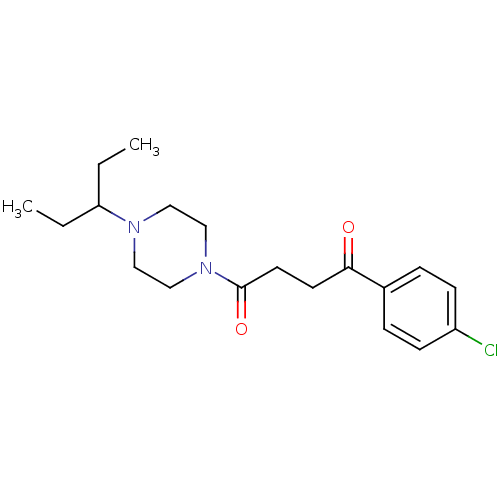 (1-(4-Chloro-phenyl)-4-[4-(1-ethyl-propyl)-piperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Mean binding affinity for human H3 receptor | J Med Chem 48: 2229-38 (2005) Article DOI: 10.1021/jm049212n BindingDB Entry DOI: 10.7270/Q2GB258T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414799 (CHEMBL573815) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50414806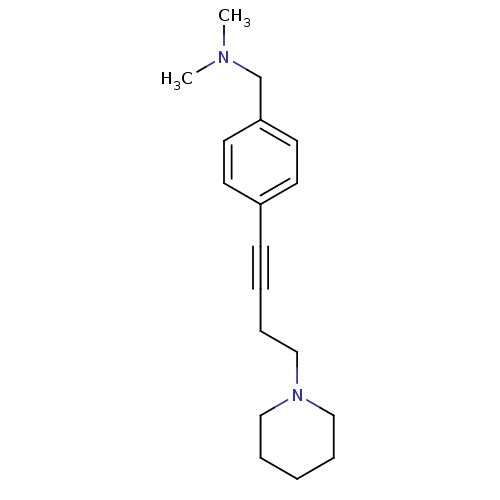 (CHEMBL572845) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4098-106 (2009) Article DOI: 10.1016/j.ejmech.2009.04.049 BindingDB Entry DOI: 10.7270/Q2VH5Q3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM102301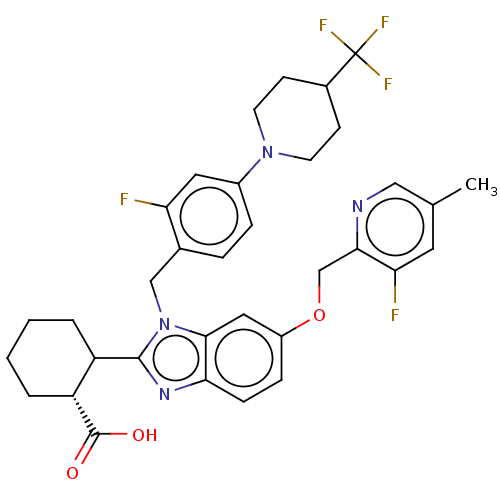 (US9695149, 192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11896 total ) | Next | Last >> |