

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016658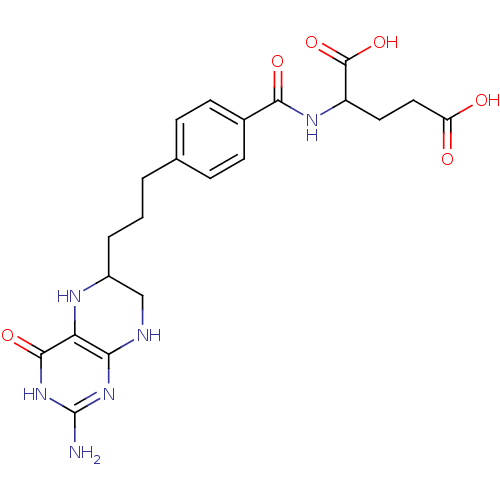 (2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016659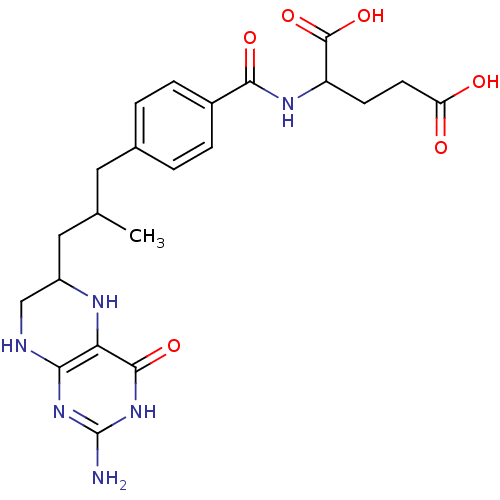 (2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016660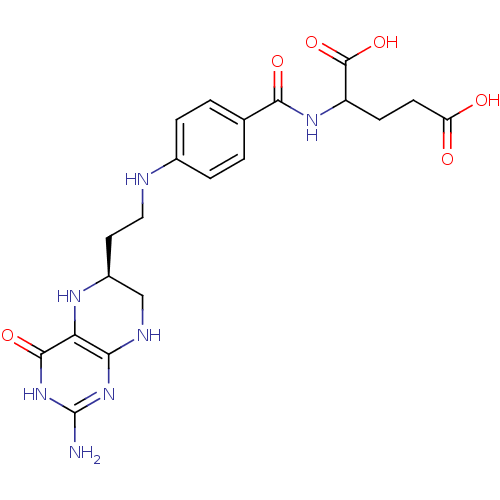 (2-{4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016662 (2-{4-[3-(2-Amino-4-oxo-3,4,7,8-tetrahydro-pteridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016658 (2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in MOLT-4 human leukemia cells | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50016658 (2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in L1210 | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50016660 (2-{4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in L1210 | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CXCR4-mediated chemotaxis in SDF1-stimulated human U937 cells treated 15 mins before SDF1 challenge measured after 2 hrs by luminescenc... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331659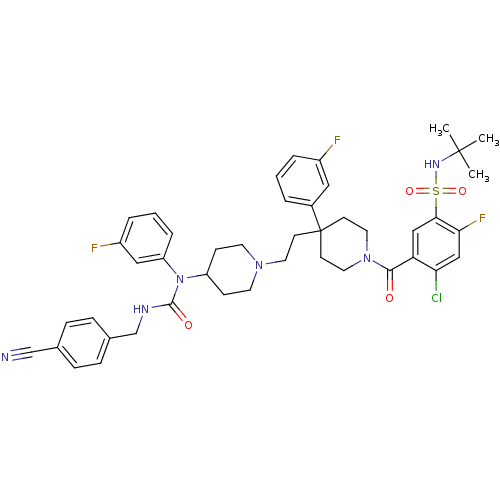 (CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7751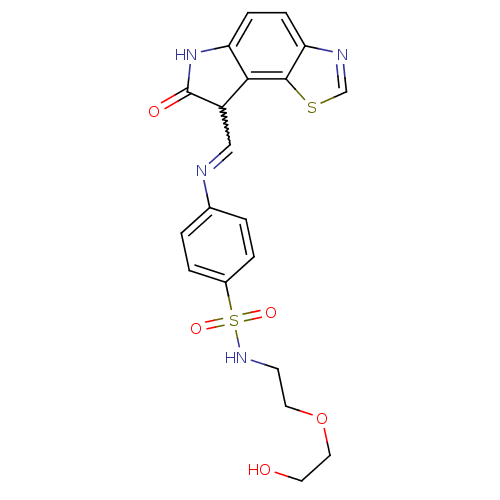 ((12Z)-12-{[(4-{[2-(2-hydroxyethoxy)ethyl]sulfamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated 30 mins before agonist challenge... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7753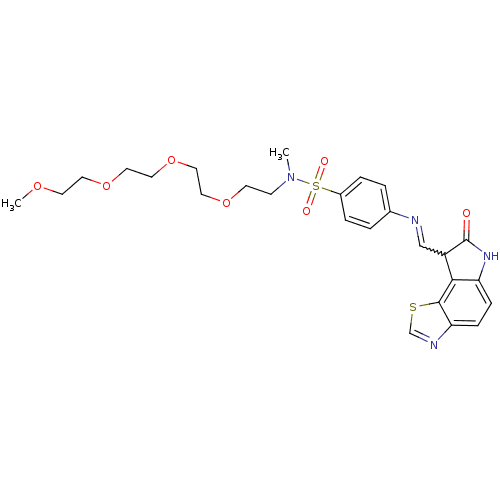 (N-methyl-4-({[(12Z)-11-oxo-3-thia-5,10-diazatricyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated before agonist challenge measure... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7692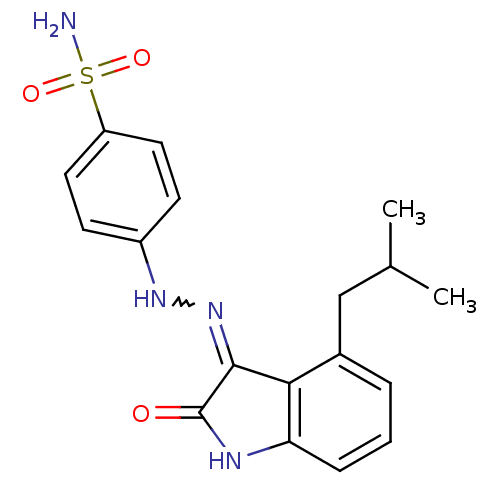 (4-[N -(4-Isobutyl-2-oxo-1,2-dihydro-indol-3-yliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7746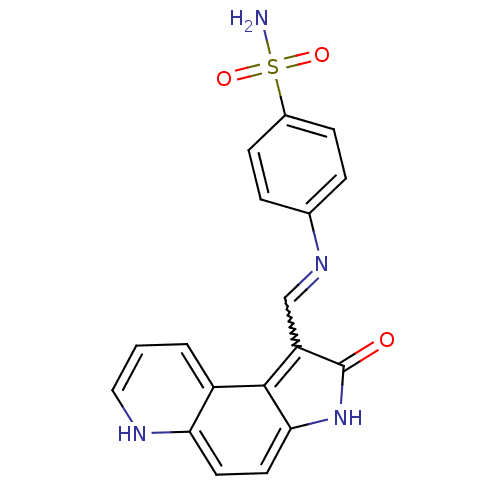 (4-({[(3Z)-4-oxo-5,10-diazatricyclo[7.4.0.0^{2,6}]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7693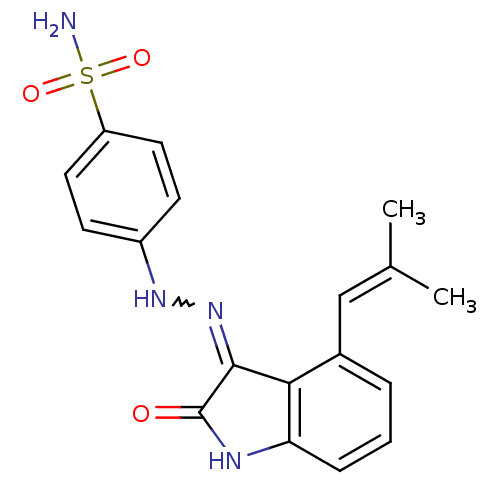 (4-{2-[(3Z)-4-(2-methylprop-1-en-1-yl)-2-oxo-2,3-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7745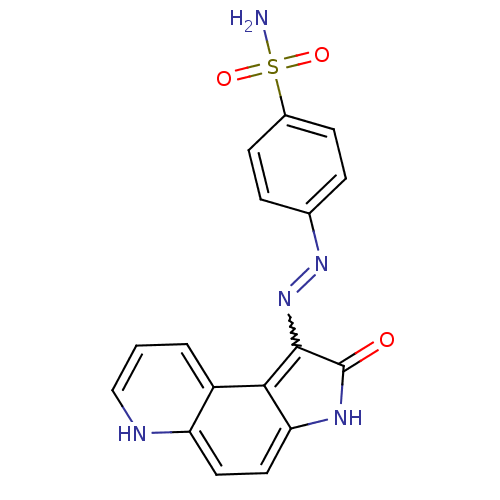 (4-[N -2-Oxo-2,3-dihydropyrrolo[3,2-f]quinolin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331659 (CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7727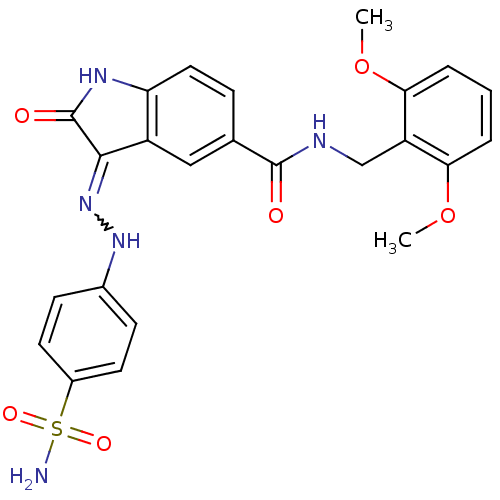 ((3Z)-N-[(2,6-dimethoxyphenyl)methyl]-2-oxo-3-[2-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 45 mg/ml human ... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7719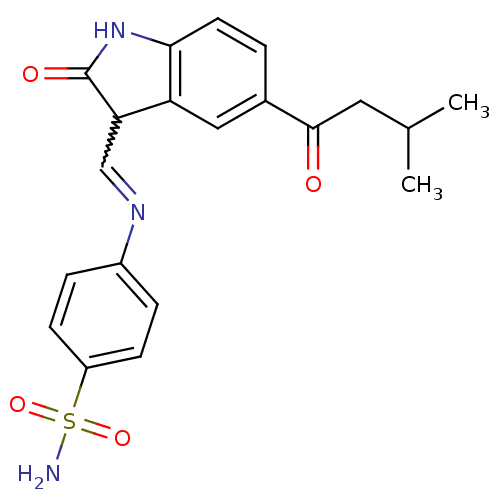 (4-({[(3Z)-5-(3-methylbutanoyl)-2-oxo-2,3-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of alpha-acid glyc... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 1 mg/ml alpha-a... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7688 (4-({1-[(3Z)-5-(1,3-oxazol-5-yl)-2-oxo-2,3-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7725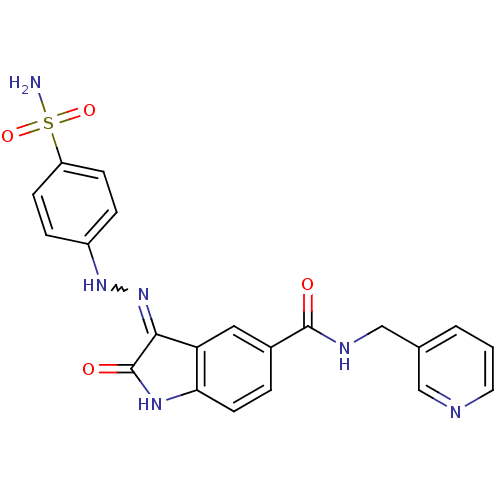 ((3Z)-2-oxo-N-(pyridin-3-ylmethyl)-3-[2-(4-sulfamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7717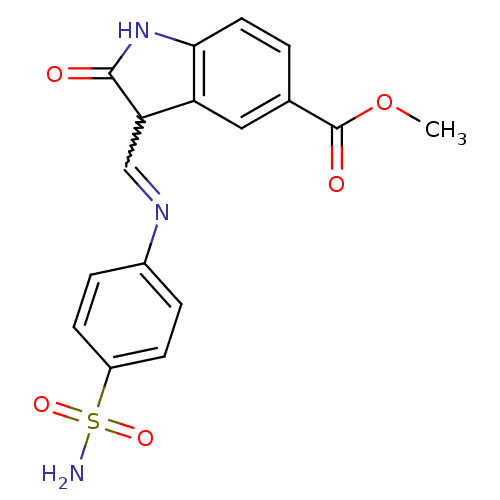 (Oxindole-Based Inhibitor 53 | methyl (3Z)-2-oxo-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-X-C chemokine receptor type 4 (Mus musculus) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR4 expressed in human U2OS cells assessed as inhibition of SDF1-induced increase in intracellular calcium level by FL... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7741 (4-[N -(1-Chloro-7-oxo-6,7-dihydro-3H-pyrrolo[3,2-e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7686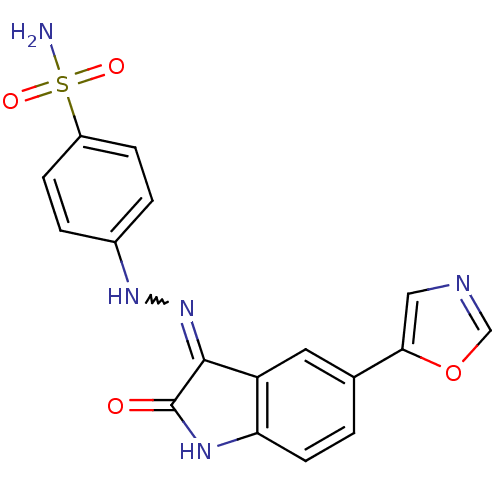 (4-[1-(5-Oxazol-5-yl-2-oxo-1,2-dihydro-indol-3-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.41 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced increase in intracellular calcium level treated 1... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7691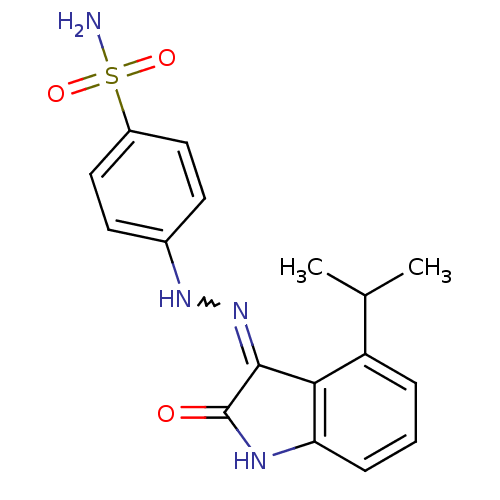 (4-[N¢-(4-Isopropyl-2-oxo-1,2-dihydro-indol-3-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7687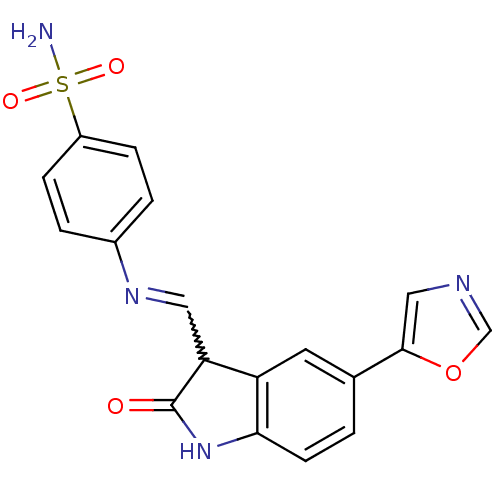 (4-({[(3Z)-5-(1,3-oxazol-5-yl)-2-oxo-2,3-dihydro-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331658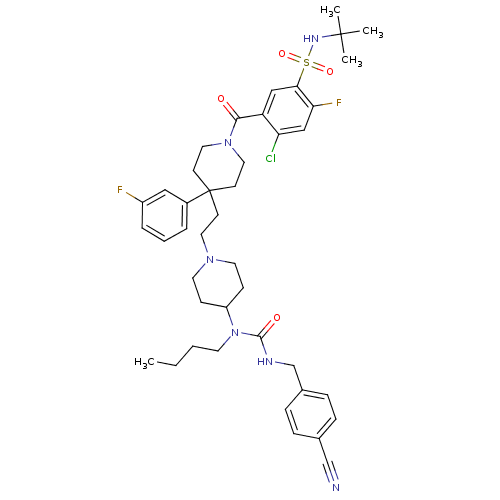 (CHEMBL1288924 | N-tert-butyl-5-(4-(2-(4-(1-butyl-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM7720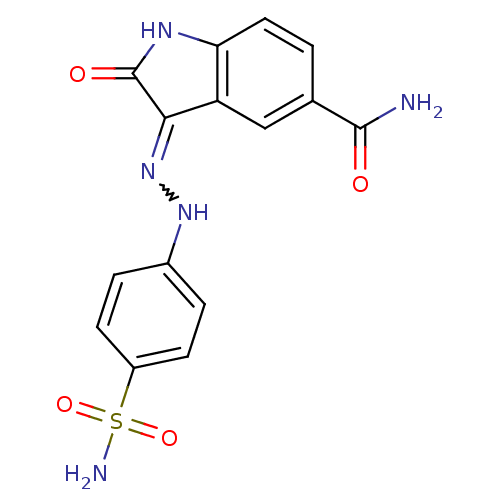 ((3Z)-2-oxo-3-[2-(4-sulfamoylphenyl)hydrazin-1-ylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7744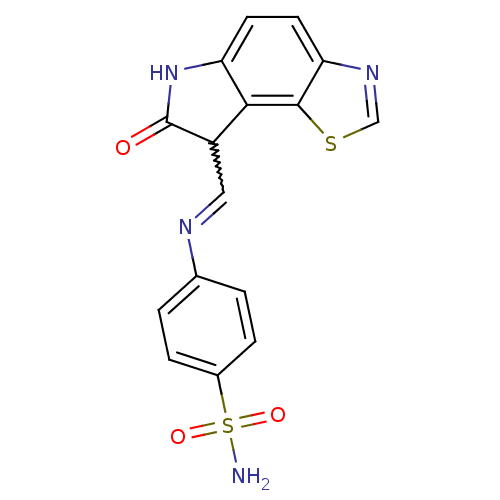 (4-({[(12Z)-11-oxo-3-thia-5,10-diazatricyclo[7.3.0....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7718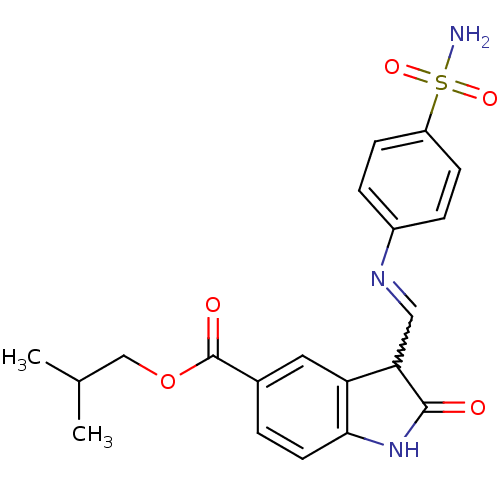 (2-methylpropyl (3Z)-2-oxo-3-{[(4-sulfamoylphenyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7760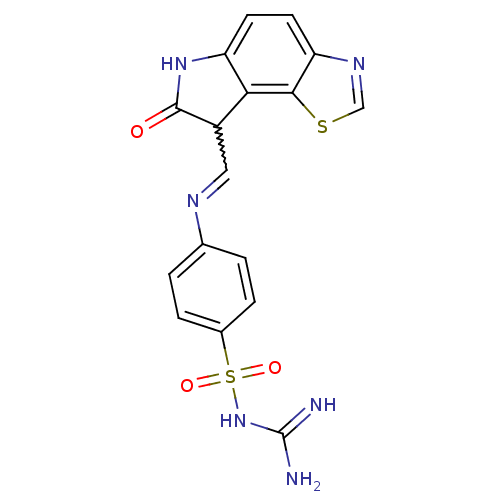 (3-{[4-({[(12Z)-11-oxo-3-thia-5,10-diazatricyclo[7....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331651 (CHEMBL1288917 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated before agonist challenge measure... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7695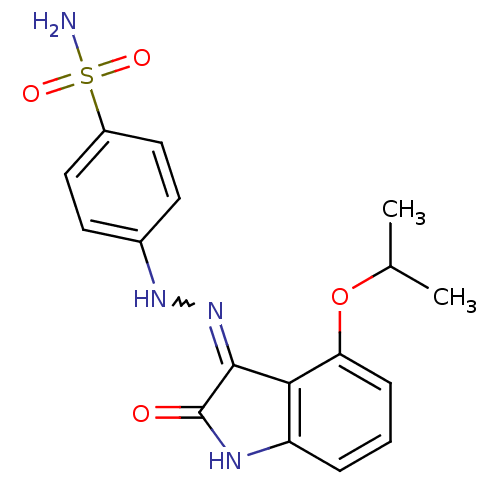 (4-{2-[(3Z)-2-oxo-4-(propan-2-yloxy)-2,3-dihydro-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7750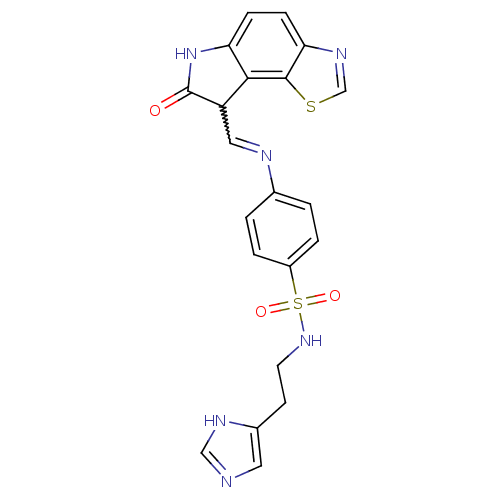 (N-[2-(1H-Imidazol-5-yl)ethyl]-4-{[(7-oxo-6,7-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331646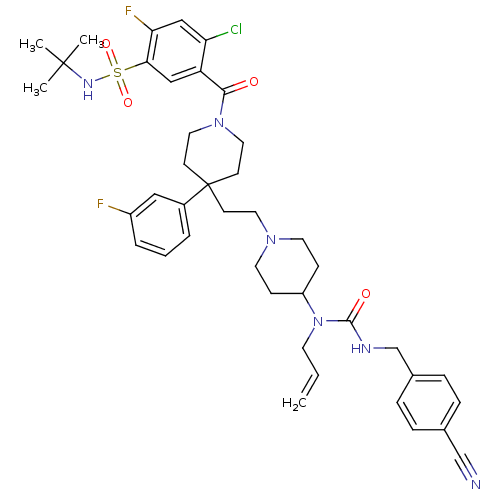 (5-(4-(2-(4-(1-allyl-3-(4-cyanobenzyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331646 (5-(4-(2-(4-(1-allyl-3-(4-cyanobenzyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7754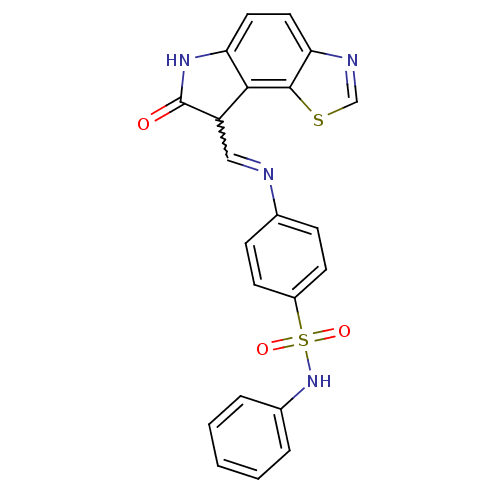 (4-({[(12Z)-11-oxo-3-thia-5,10-diazatricyclo[7.3.0....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-X-C chemokine receptor type 4 (Rattus norvegicus (Rat)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rat CXCR4 expressed in human U2OS cells assessed as inhibition of SDF1-induced increase in intracellular calcium level by FLIP... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7720 ((3Z)-2-oxo-3-[2-(4-sulfamoylphenyl)hydrazin-1-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7752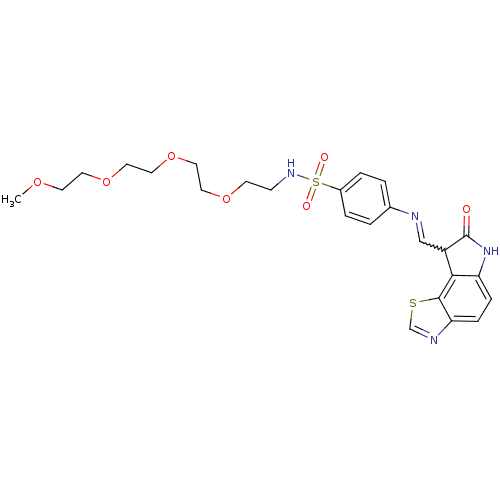 (4-({[(12Z)-11-oxo-3-thia-5,10-diazatricyclo[7.3.0....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331638 (4-(methylsulfonyl)benzyl allyl(1-(2-(1-(5-(N-tert-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331639 (4-cyanobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 269 total ) | Next | Last >> |