

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50089779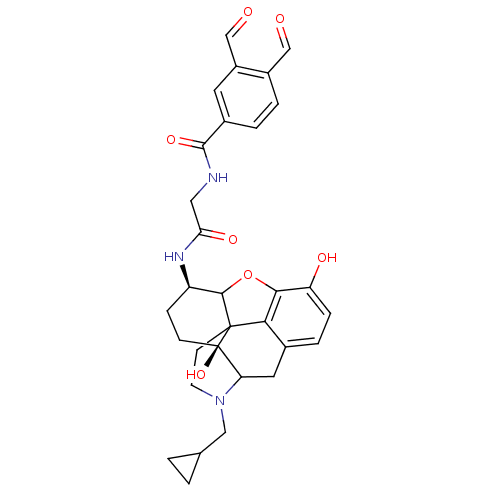 (4-cyclopropylmethyl-14-(3,4-diformylphenylcarboxam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]- diprenorphine binding to Opioid receptor mu 1 (83 fmol/mg protein) stably expressed in membranes from CHO cells | J Med Chem 43: 2489-92 (2000) BindingDB Entry DOI: 10.7270/Q2XW4KH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50089779 (4-cyclopropylmethyl-14-(3,4-diformylphenylcarboxam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to Opioid receptor kappa 1 | J Med Chem 43: 2489-92 (2000) BindingDB Entry DOI: 10.7270/Q2XW4KH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50115095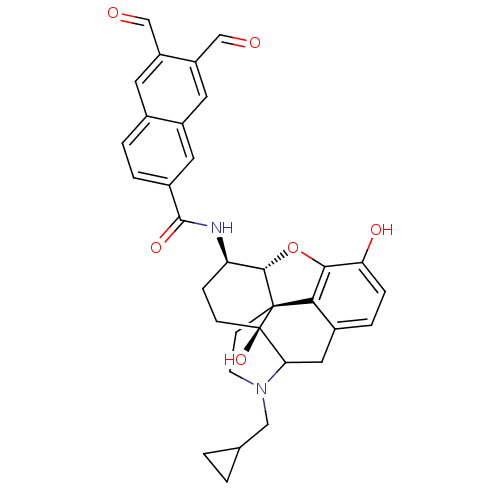 (4-cyclopropylmethyl-14-(6,7-diformyl-2-naphthylcar...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.744 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]- diprenorphine binding on Opioid receptor mu 1 expressed in human embryonic kidney (HEK) cells | J Med Chem 45: 2887-90 (2002) BindingDB Entry DOI: 10.7270/Q23N243V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50098209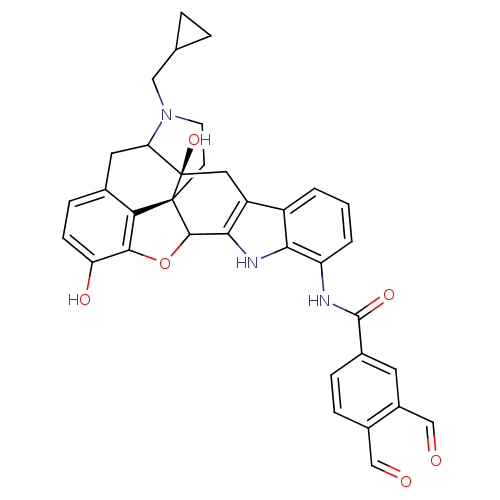 (22-cyclopropylmethyl-9-(3,4-diformylphenylcarboxam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards delta-opioid receptor from CHO cells | J Med Chem 44: 1017-20 (2001) BindingDB Entry DOI: 10.7270/Q2QF8TK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50089779 (4-cyclopropylmethyl-14-(3,4-diformylphenylcarboxam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]- diprenorphine binding to Opioid receptor delta 1 | J Med Chem 43: 2489-92 (2000) BindingDB Entry DOI: 10.7270/Q2XW4KH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20458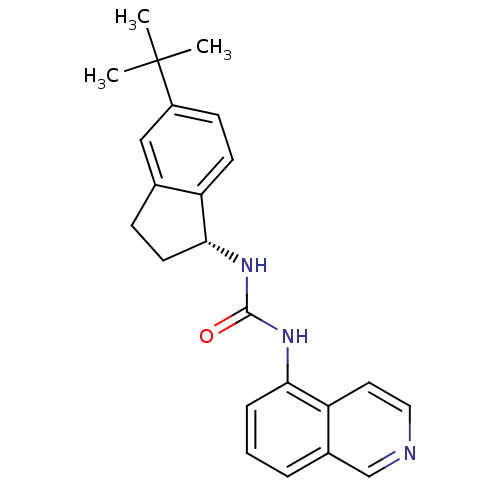 (1-[(1R)-5-tert-butyl-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -46.5 | n/a | n/a | 5 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50098209 (22-cyclopropylmethyl-9-(3,4-diformylphenylcarboxam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor from CHO cells | J Med Chem 44: 1017-20 (2001) BindingDB Entry DOI: 10.7270/Q2QF8TK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50098209 (22-cyclopropylmethyl-9-(3,4-diformylphenylcarboxam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards opioid receptor kappa 1 from CHO cells | J Med Chem 44: 1017-20 (2001) BindingDB Entry DOI: 10.7270/Q2QF8TK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20464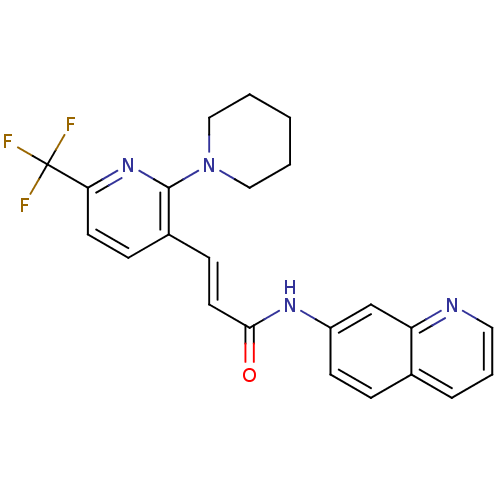 ((2E)-3-[2-(piperidin-1-yl)-6-(trifluoromethyl)pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | -43.2 | n/a | n/a | 34 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20334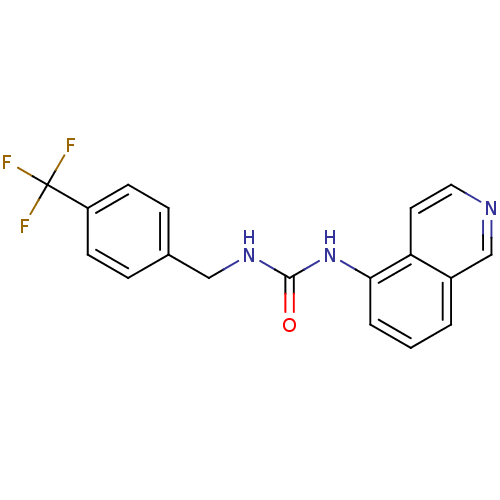 (1-Isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-ur...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | -41.8 | n/a | n/a | 11 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20465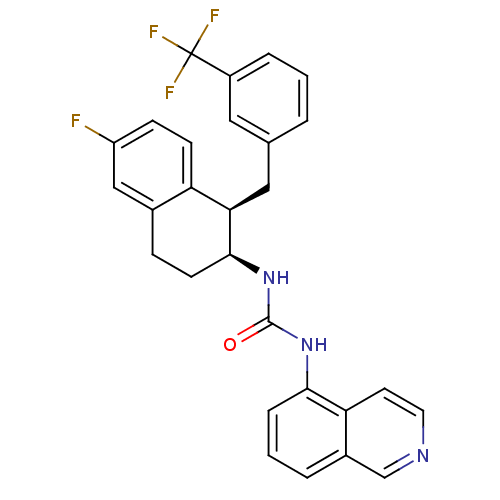 (1-[(1R,2S)-6-fluoro-1-{[3-(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 58 | -41.3 | n/a | n/a | 46 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20285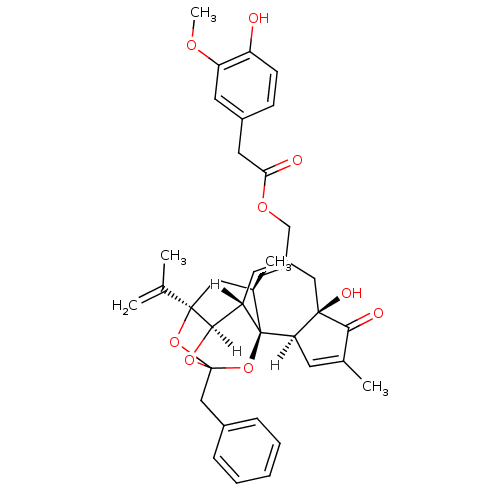 (Resiniferatoxin | [(1R,2R,6R,10S,11R,15R,17R)-13-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | -41.0 | n/a | n/a | 24 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20466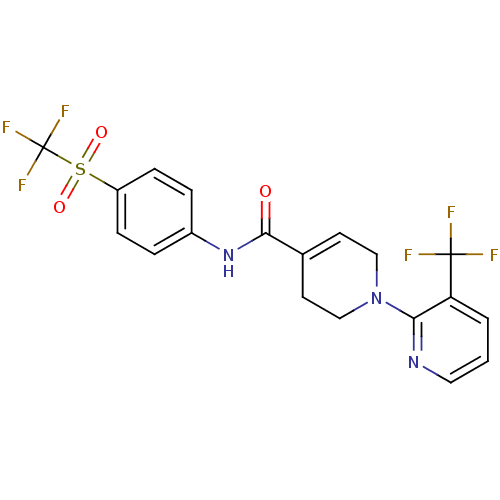 (A-784168 | CHEMBL482834 | N-[4-(trifluoromethane)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | -40.8 | n/a | n/a | 74 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20467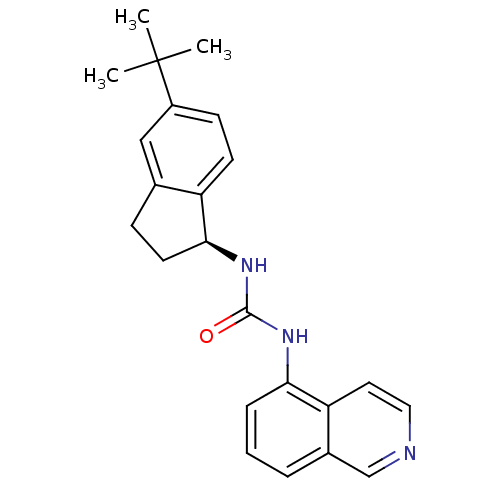 (3-[(1S)-5-tert-butyl-2,3-dihydro-1H-inden-1-yl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 112 | -39.7 | n/a | n/a | 34 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20459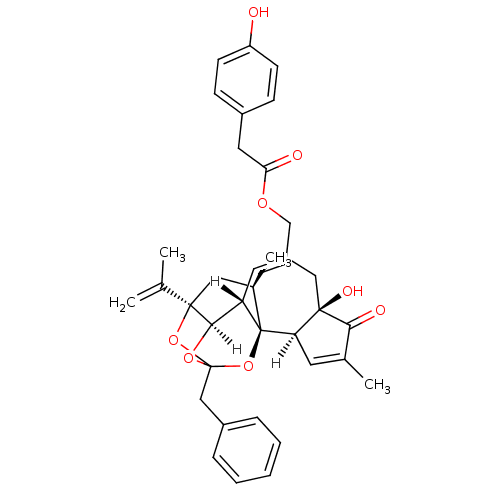 (Tinyatoxin | [(1R,2R,6R,10S,11R,15R,17R)-13-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 589 | -35.6 | n/a | n/a | 129 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20468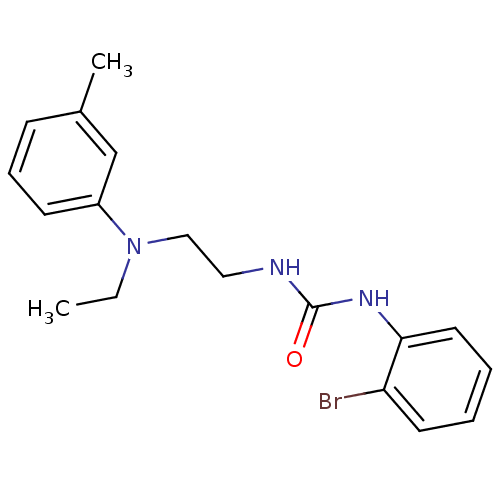 (3-(2-bromophenyl)-1-{2-[ethyl(3-methylphenyl)amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 603 | -35.5 | n/a | n/a | 95 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20284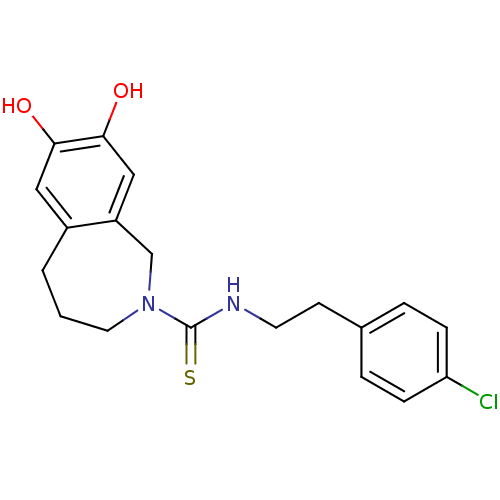 (CHEMBL391997 | CPZ | Capsazepine | N-[2-(4-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.29E+3 | -33.6 | n/a | n/a | 282 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20460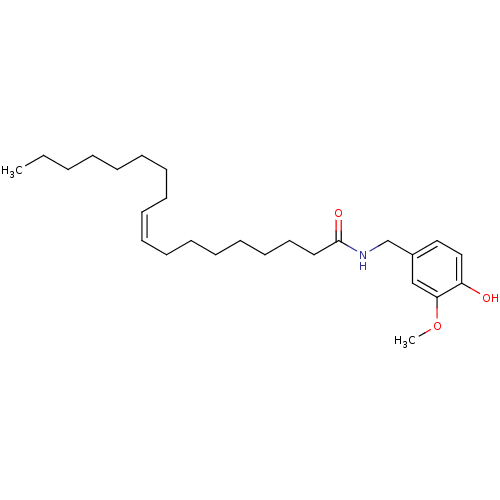 ((9Z)-N-[(4-hydroxy-3-methoxyphenyl)methyl]octadec-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.59E+3 | -33.1 | n/a | n/a | 132 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20462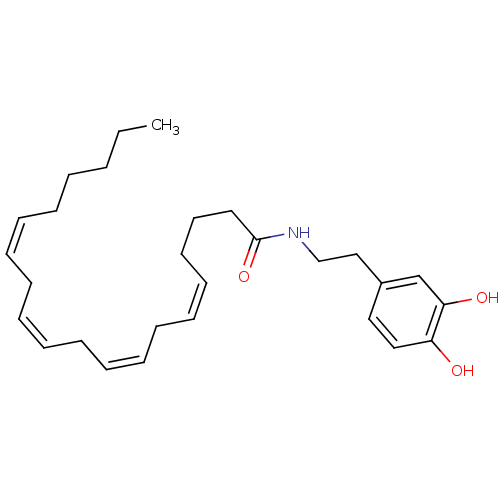 ((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >6.31E+3 | >-29.7 | n/a | n/a | 1.48E+3 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20461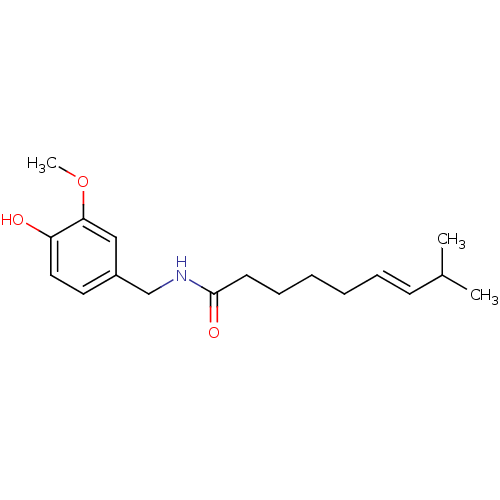 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.00E+4 | -26.8 | n/a | n/a | 29 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... | J Pharmacol Exp Ther 323: 285-93 (2007) Article DOI: 10.1124/jpet.107.124305 BindingDB Entry DOI: 10.7270/Q28K77B0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||