 Found 46 hits with Last Name = 'el-hallouty' and Initial = 'sm'
Found 46 hits with Last Name = 'el-hallouty' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50428286

(DABRAFENIB | GSK2118436A)Show SMILES CC(C)(C)c1nc(c(s1)-c1ccnc(N)n1)-c1cccc(NS(=O)(=O)c2c(F)cccc2F)c1F Show InChI InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-kit (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Src (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ABL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM112499
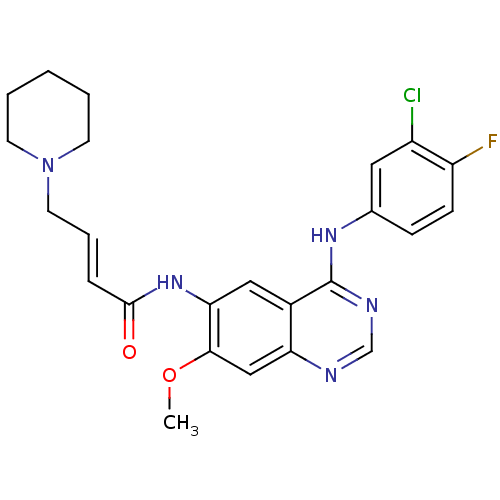
(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50355497
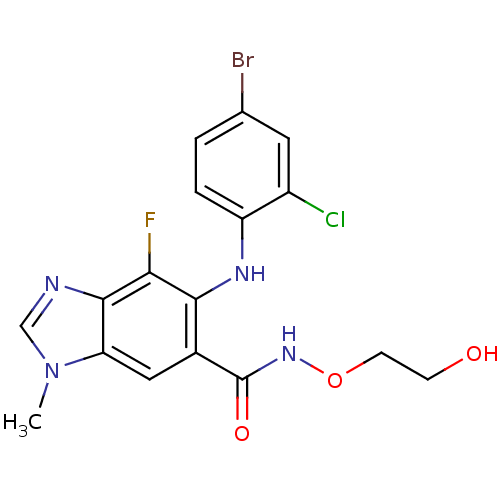
(AZD-6244 | CHEMBL1614701)Show SMILES Cn1cnc2c(F)c(Nc3ccc(Br)cc3Cl)c(cc12)C(=O)NOCCO Show InChI InChI=1S/C17H15BrClFN4O3/c1-24-8-21-16-13(24)7-10(17(26)23-27-5-4-25)15(14(16)20)22-12-3-2-9(18)6-11(12)19/h2-3,6-8,22,25H,4-5H2,1H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MEK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438444
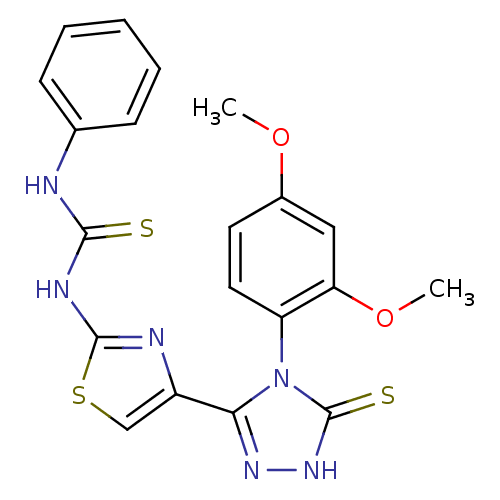
(CHEMBL2414370)Show SMILES COc1ccc(c(OC)c1)-n1c(n[nH]c1=S)-c1csc(NC(=S)Nc2ccccc2)n1 |(37.8,-11.38,;39.13,-12.15,;40.47,-11.38,;41.8,-12.15,;43.14,-11.38,;43.13,-9.83,;41.8,-9.07,;41.79,-7.53,;40.46,-6.76,;40.47,-9.84,;44.46,-9.06,;45.92,-9.55,;46.84,-8.31,;45.94,-7.06,;44.47,-7.53,;43.23,-6.61,;46.37,-11.02,;45.45,-12.25,;46.34,-13.51,;47.81,-13.05,;49.14,-13.82,;50.48,-13.05,;50.48,-11.52,;51.81,-13.83,;53.14,-13.06,;54.47,-13.84,;55.81,-13.08,;55.81,-11.54,;54.47,-10.76,;53.14,-11.53,;47.82,-11.51,)| Show InChI InChI=1S/C20H18N6O2S3/c1-27-13-8-9-15(16(10-13)28-2)26-17(24-25-20(26)30)14-11-31-19(22-14)23-18(29)21-12-6-4-3-5-7-12/h3-11H,1-2H3,(H,25,30)(H2,21,22,23,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561030
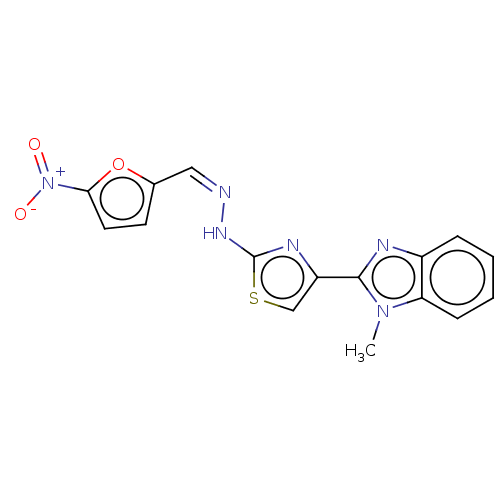
(CHEMBL4750734)Show SMILES Cn1c(nc2ccccc12)-c1csc(N\N=C/c2ccc(o2)[N+]([O-])=O)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561024

(CHEMBL4753631)Show SMILES Cn1c(nc2ccccc12)-c1csc(N\N=C/c2ccc(cc2)[N+]([O-])=O)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561025
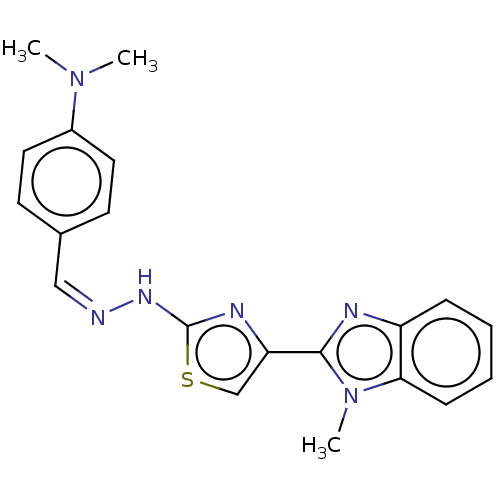
(CHEMBL4786168)Show SMILES CN(C)c1ccc(\C=N/Nc2nc(cs2)-c2nc3ccccc3n2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561017

(CHEMBL4780058)Show SMILES [#6]-n1c(nc2ccccc12)-c1csc(-[#7]\[#7]=[#6]-2\[#6]-[#6]-[#6]-[#6]-2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561020
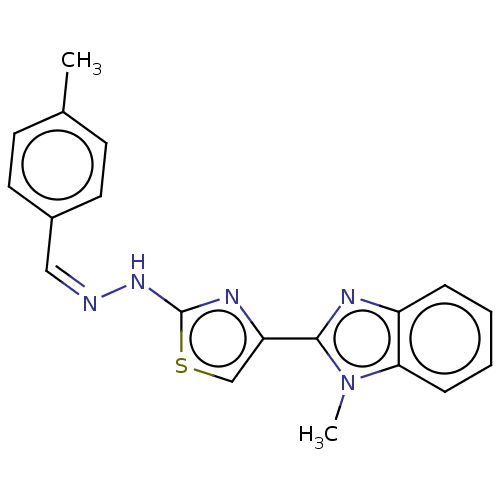
(CHEMBL4783948) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561022

(CHEMBL4740646)Show SMILES COc1ccc(\C=N/Nc2nc(cs2)-c2nc3ccccc3n2C)cc1OC | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561029

(CHEMBL4746023) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561026
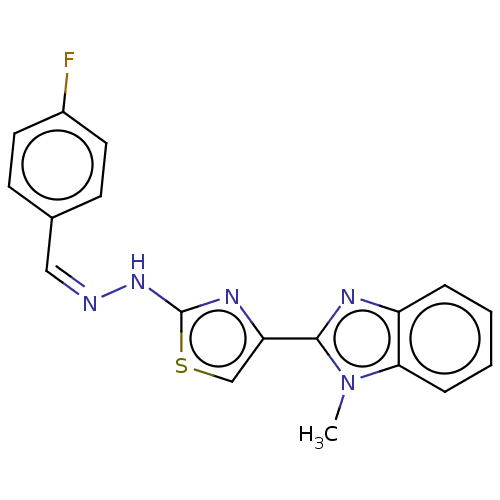
(CHEMBL4752843)Show SMILES Cn1c(nc2ccccc12)-c1csc(N\N=C/c2ccc(F)cc2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561018
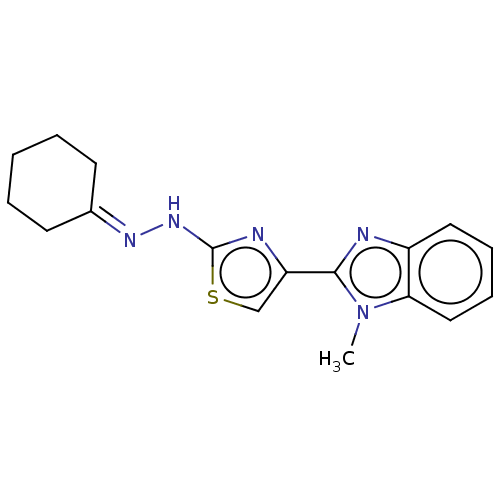
(CHEMBL4747759)Show SMILES [#6]-n1c(nc2ccccc12)-c1csc(-[#7]\[#7]=[#6]-2\[#6]-[#6]-[#6]-[#6]-[#6]-2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561021

(CHEMBL4794131) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561032

(CHEMBL4749518) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561027

(CHEMBL4746321)Show SMILES Cn1c(nc2ccccc12)-c1csc(N\N=C/c2ccc(Br)cc2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561028

(CHEMBL4762604)Show SMILES Cn1c(nc2ccccc12)-c1csc(N\N=C/c2ccc(Cl)cc2Cl)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 514 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561033

(CHEMBL4796408) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 569 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561023
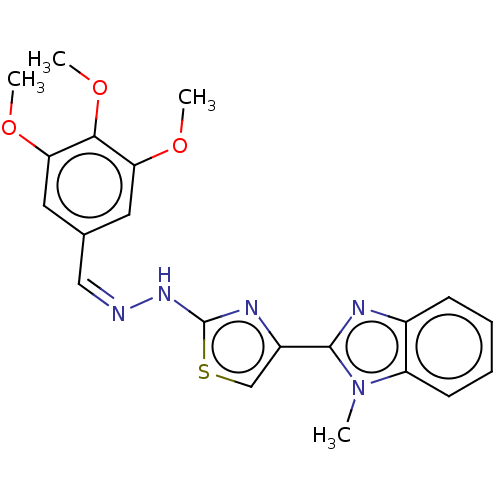
(CHEMBL4758204)Show SMILES COc1cc(\C=N/Nc2nc(cs2)-c2nc3ccccc3n2C)cc(OC)c1OC | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 623 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561031

(CHEMBL4763960) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 773 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50561019
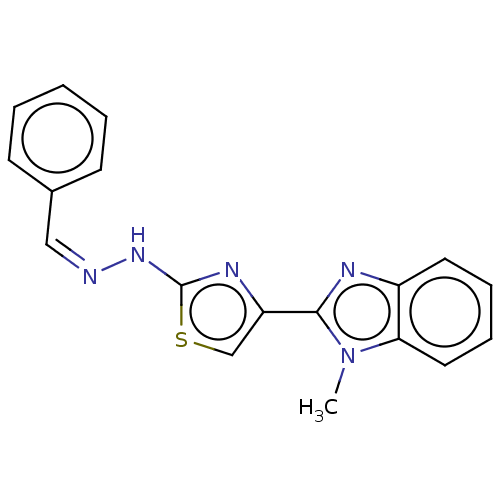
(CHEMBL4749342) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) using ATF-2 as substrate after 1 hr by radiometric based ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438443
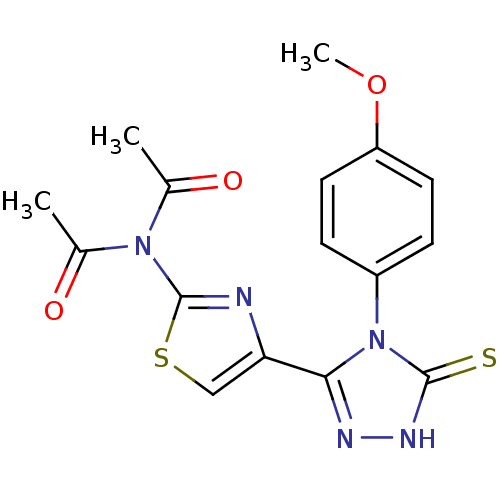
(CHEMBL2414364)Show SMILES COc1ccc(cc1)-n1c(n[nH]c1=S)-c1csc(n1)N(C(C)=O)C(C)=O Show InChI InChI=1S/C16H15N5O3S2/c1-9(22)20(10(2)23)16-17-13(8-26-16)14-18-19-15(25)21(14)11-4-6-12(24-3)7-5-11/h4-8H,1-3H3,(H,19,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438442
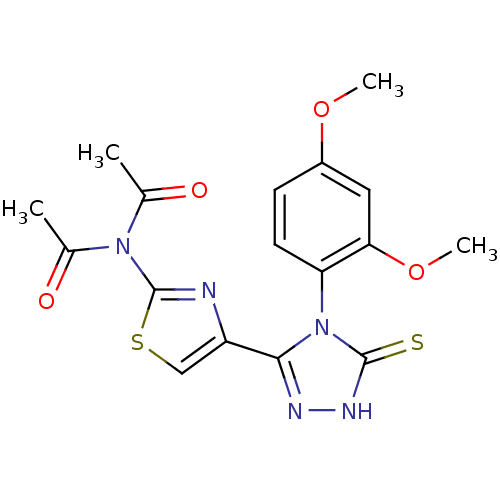
(CHEMBL2414365)Show SMILES COc1ccc(c(OC)c1)-n1c(n[nH]c1=S)-c1csc(n1)N(C(C)=O)C(C)=O |(3.25,-7.69,;4.59,-8.46,;5.92,-7.69,;7.26,-8.46,;8.59,-7.69,;8.59,-6.14,;7.25,-5.37,;7.25,-3.83,;5.91,-3.07,;5.92,-6.14,;9.92,-5.37,;11.38,-5.86,;12.3,-4.62,;11.4,-3.37,;9.93,-3.83,;8.69,-2.92,;11.83,-7.33,;10.91,-8.56,;11.8,-9.82,;13.27,-9.36,;13.28,-7.81,;14.51,-10.27,;14.33,-11.8,;15.57,-12.72,;12.92,-12.42,;15.92,-9.66,;17.16,-10.57,;16.09,-8.13,)| Show InChI InChI=1S/C17H17N5O4S2/c1-9(23)21(10(2)24)17-18-12(8-28-17)15-19-20-16(27)22(15)13-6-5-11(25-3)7-14(13)26-4/h5-8H,1-4H3,(H,20,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438440
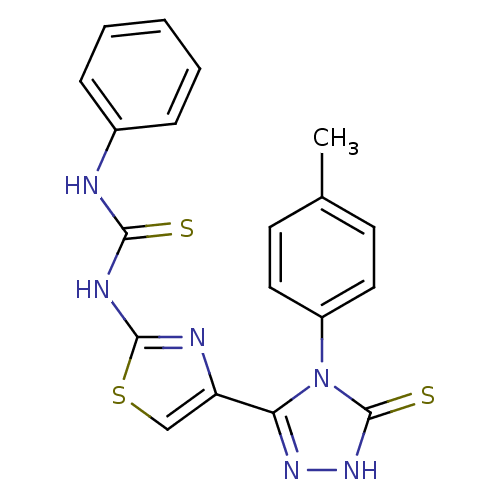
(CHEMBL2414371)Show SMILES Cc1ccc(cc1)-n1c(n[nH]c1=S)-c1csc(NC(=S)Nc2ccccc2)n1 Show InChI InChI=1S/C19H16N6S3/c1-12-7-9-14(10-8-12)25-16(23-24-19(25)27)15-11-28-18(21-15)22-17(26)20-13-5-3-2-4-6-13/h2-11H,1H3,(H,24,27)(H2,20,21,22,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438441
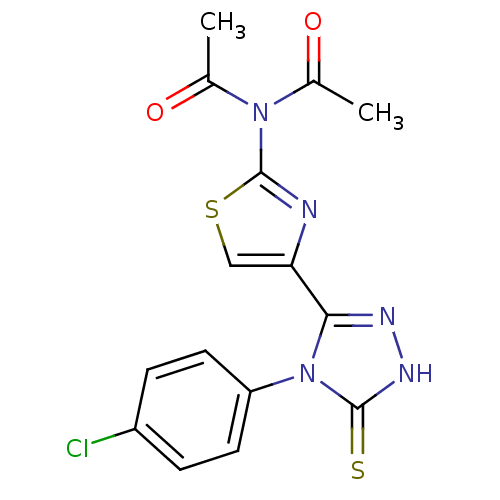
(CHEMBL2414362)Show SMILES CC(=O)N(C(C)=O)c1nc(cs1)-c1n[nH]c(=S)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C15H12ClN5O2S2/c1-8(22)20(9(2)23)15-17-12(7-25-15)13-18-19-14(24)21(13)11-5-3-10(16)4-6-11/h3-7H,1-2H3,(H,19,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438439
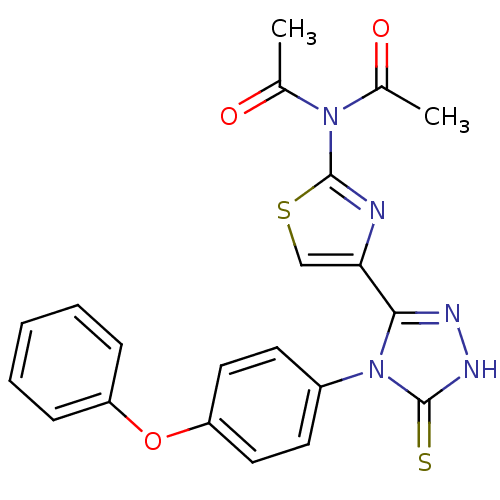
(CHEMBL2414366)Show SMILES CC(=O)N(C(C)=O)c1nc(cs1)-c1n[nH]c(=S)n1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C21H17N5O3S2/c1-13(27)25(14(2)28)21-22-18(12-31-21)19-23-24-20(30)26(19)15-8-10-17(11-9-15)29-16-6-4-3-5-7-16/h3-12H,1-2H3,(H,24,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438438
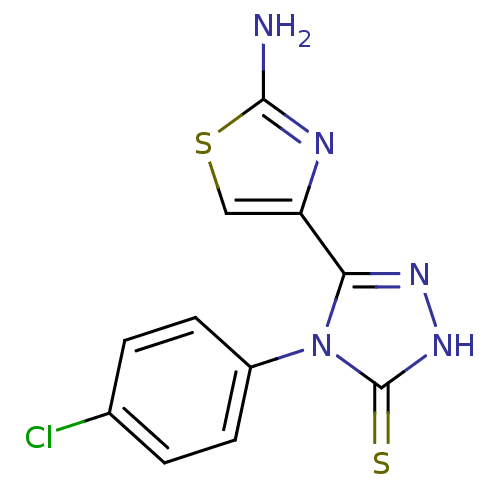
(CHEMBL2414374)Show InChI InChI=1S/C11H8ClN5S2/c12-6-1-3-7(4-2-6)17-9(15-16-11(17)18)8-5-19-10(13)14-8/h1-5H,(H2,13,14)(H,16,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438437

(CHEMBL2414376)Show InChI InChI=1S/C12H11N5OS2/c1-18-8-4-2-7(3-5-8)17-10(15-16-12(17)19)9-6-20-11(13)14-9/h2-6H,1H3,(H2,13,14)(H,16,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438435
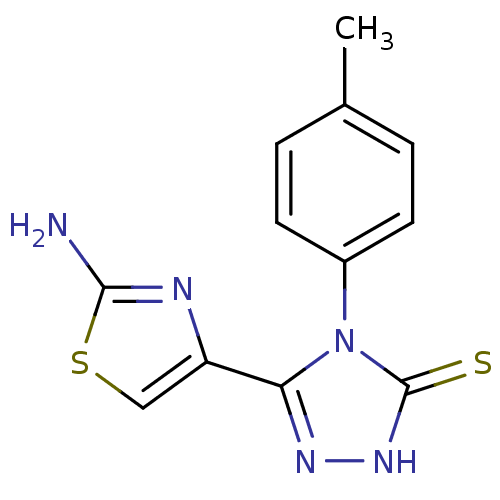
(CHEMBL2414377)Show InChI InChI=1S/C12H11N5S2/c1-7-2-4-8(5-3-7)17-10(15-16-12(17)18)9-6-19-11(13)14-9/h2-6H,1H3,(H2,13,14)(H,16,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438436
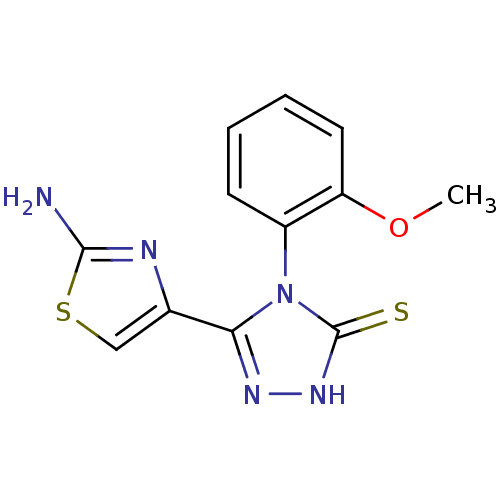
(CHEMBL2414375)Show SMILES COc1ccccc1-n1c(n[nH]c1=S)-c1csc(N)n1 |(26.2,-4.75,;27.54,-5.52,;27.54,-7.06,;26.21,-7.83,;26.21,-9.37,;27.55,-10.14,;28.88,-9.37,;28.88,-7.82,;30.21,-7.05,;31.67,-7.55,;32.58,-6.31,;31.69,-5.05,;30.22,-5.52,;28.98,-4.6,;32.12,-9.01,;31.19,-10.25,;32.09,-11.5,;33.56,-11.04,;34.79,-11.96,;33.57,-9.5,)| Show InChI InChI=1S/C12H11N5OS2/c1-18-9-5-3-2-4-8(9)17-10(15-16-12(17)19)7-6-20-11(13)14-7/h2-6H,1H3,(H2,13,14)(H,16,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50561016

(CHEMBL34922)Show SMILES Cl.Cl.Cl.CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1ccc2nc([nH]c2c1)-c1ccc(O)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Topoisomerase 2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438434
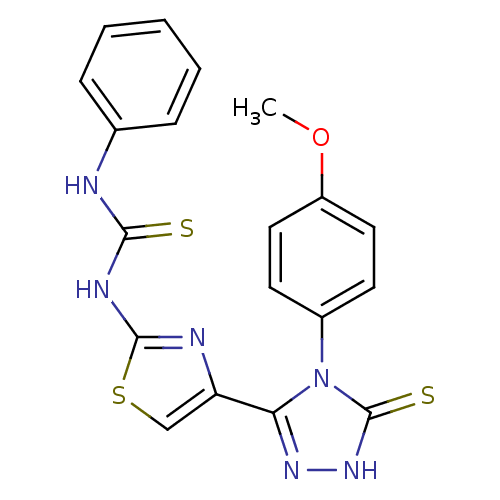
(CHEMBL2414369)Show SMILES COc1ccc(cc1)-n1c(n[nH]c1=S)-c1csc(NC(=S)Nc2ccccc2)n1 Show InChI InChI=1S/C19H16N6OS3/c1-26-14-9-7-13(8-10-14)25-16(23-24-19(25)28)15-11-29-18(21-15)22-17(27)20-12-5-3-2-4-6-12/h2-11H,1H3,(H,24,28)(H2,20,21,22,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50561016

(CHEMBL34922)Show SMILES Cl.Cl.Cl.CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1ccc2nc([nH]c2c1)-c1ccc(O)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Topoisomerase 1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115657
BindingDB Entry DOI: 10.7270/Q21J9FHZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438433
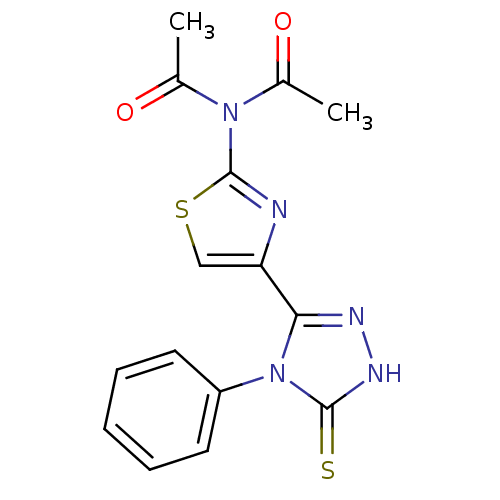
(CHEMBL2414361)Show SMILES CC(=O)N(C(C)=O)c1nc(cs1)-c1n[nH]c(=S)n1-c1ccccc1 Show InChI InChI=1S/C15H13N5O2S2/c1-9(21)19(10(2)22)15-16-12(8-24-15)13-17-18-14(23)20(13)11-6-4-3-5-7-11/h3-8H,1-2H3,(H,18,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438432
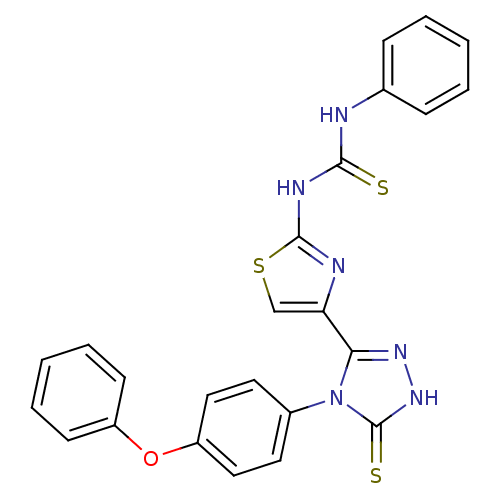
(CHEMBL2414372)Show SMILES S=C(Nc1nc(cs1)-c1n[nH]c(=S)n1-c1ccc(Oc2ccccc2)cc1)Nc1ccccc1 Show InChI InChI=1S/C24H18N6OS3/c32-22(25-16-7-3-1-4-8-16)27-23-26-20(15-34-23)21-28-29-24(33)30(21)17-11-13-19(14-12-17)31-18-9-5-2-6-10-18/h1-15H,(H,29,33)(H2,25,26,27,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Bos taurus (Cattle)) | BDBM50438431
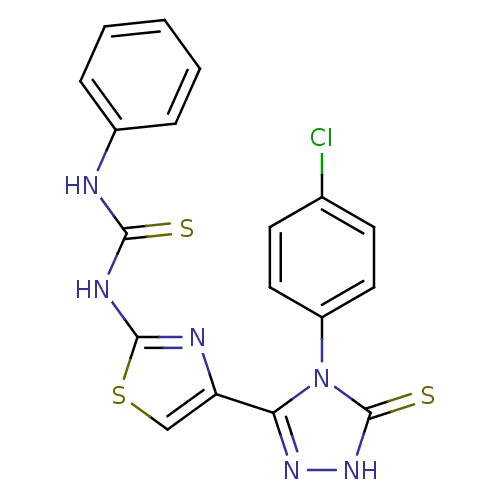
(CHEMBL2414367)Show SMILES Clc1ccc(cc1)-n1c(n[nH]c1=S)-c1csc(NC(=S)Nc2ccccc2)n1 Show InChI InChI=1S/C18H13ClN6S3/c19-11-6-8-13(9-7-11)25-15(23-24-18(25)27)14-10-28-17(21-14)22-16(26)20-12-4-2-1-3-5-12/h1-10H,(H,24,27)(H2,20,21,22,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver DHFR measured every 1 min for 10 mins |
Eur J Med Chem 66: 135-45 (2013)
Article DOI: 10.1016/j.ejmech.2013.05.039
BindingDB Entry DOI: 10.7270/Q21C1Z9Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data