 Found 219 hits with Last Name = 'elkamhawy' and Initial = 'a'
Found 219 hits with Last Name = 'elkamhawy' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2b (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Homeodomain-interacting protein kinase 1
(Homo sapiens (Human)) | BDBM50400734
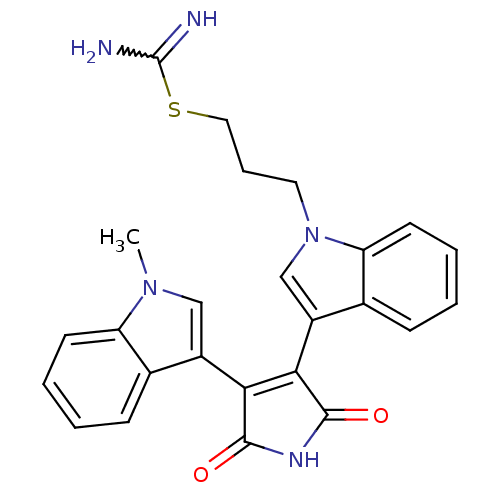
(CHEMBL1591531)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCSC(N)=N)c3ccccc23)c2ccccc12 |w:18.19,t:4| Show InChI InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.367 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of HIPK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of MINK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.473 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LYN (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Phosphorylase b kinase gamma catalytic chain, liver/testis isoform
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PHKg2 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin A (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50358430
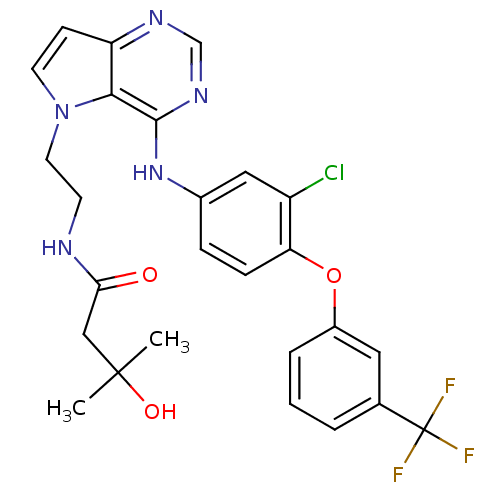
(CHEMBL1614725)Show SMILES CC(C)(O)CC(=O)NCCn1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c12 Show InChI InChI=1S/C26H25ClF3N5O3/c1-25(2,37)14-22(36)31-9-11-35-10-8-20-23(35)24(33-15-32-20)34-17-6-7-21(19(27)13-17)38-18-5-3-4-16(12-18)26(28,29)30/h3-8,10,12-13,15,37H,9,11,14H2,1-2H3,(H,31,36)(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-diprenorphine binding to kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) after 120 mins in presence of 33P-ATP |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/cyclin B (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of RET (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of c-SRC (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G1/S-specific cyclin- 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/cyclin E (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PAK2 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jouf University
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora A using [H-LRRASLG] as substrate in presence of [gamma-33P]-ATP incubated for 2 hrs by hotspot kinase assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115525
BindingDB Entry DOI: 10.7270/Q2ZP49PD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132489

(CHEMBL3633929)Show SMILES CC(=O)Nc1ccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H18ClN5O2/c1-14(29)27-15-5-7-20-18(10-15)22(26-13-25-20)28-16-6-8-21(19(23)11-16)30-12-17-4-2-3-9-24-17/h2-11,13H,12H2,1H3,(H,27,29)(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50358430

(CHEMBL1614725)Show SMILES CC(C)(O)CC(=O)NCCn1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c12 Show InChI InChI=1S/C26H25ClF3N5O3/c1-25(2,37)14-22(36)31-9-11-35-10-8-20-23(35)24(33-15-32-20)34-17-6-7-21(19(27)13-17)38-18-5-3-4-16(12-18)26(28,29)30/h3-8,10,12-13,15,37H,9,11,14H2,1-2H3,(H,31,36)(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-diprenorphine binding to kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132492

(CHEMBL3633774)Show SMILES NC(=O)Nc1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H15ClF3N5O2/c23-17-10-14(5-7-19(17)33-15-3-1-2-12(8-15)22(24,25)26)30-20-16-9-13(31-21(27)32)4-6-18(16)28-11-29-20/h1-11H,(H3,27,31,32)(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Thrombin was determined |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50289118

(CHEMBL4165412)Show SMILES COc1ccc(Oc2nc(Nc3ccc(cc3)N3CCOCC3)ncc2\N=C\c2cc(OC)cc(OC)c2)cc1 Show InChI InChI=1S/C30H31N5O5/c1-36-24-8-10-25(11-9-24)40-29-28(31-19-21-16-26(37-2)18-27(17-21)38-3)20-32-30(34-29)33-22-4-6-23(7-5-22)35-12-14-39-15-13-35/h4-11,16-20H,12-15H2,1-3H3,(H,32,33,34)/b31-19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of FMS (unknown origin) after 120 mins in presence of 33P-ATP |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of STK3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CHK2 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004231
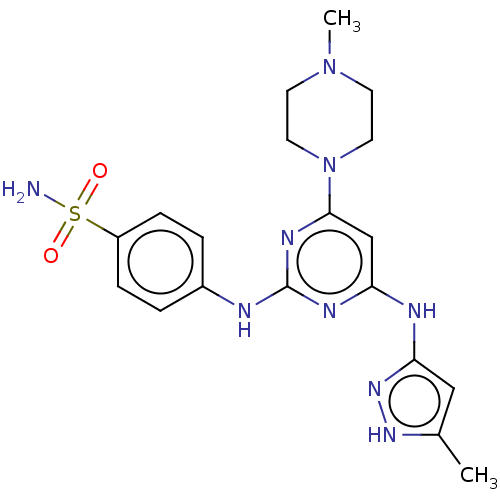
(CHEMBL3237167)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Nc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C19H25N9O2S/c1-13-11-17(26-25-13)22-16-12-18(28-9-7-27(2)8-10-28)24-19(23-16)21-14-3-5-15(6-4-14)31(20,29)30/h3-6,11-12H,7-10H2,1-2H3,(H2,20,29,30)(H3,21,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jouf University
Curated by ChEMBL
| Assay Description
Inhibition of AURKA (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115525
BindingDB Entry DOI: 10.7270/Q2ZP49PD |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Jouf University
Curated by ChEMBL
| Assay Description
Inhibition of human Aurora B using [H-LRRASLG] as substrate in presence of [gamma-33P]-ATP incubated for 2 hrs by hotspot kinase assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115525
BindingDB Entry DOI: 10.7270/Q2ZP49PD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50289144

(CHEMBL4164968)Show SMILES COc1cc(C)ccc1Oc1nc(Nc2ccc(cc2)N2CCOCC2)ncc1\N=C\c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C31H27F6N5O3/c1-19-3-8-26(27(13-19)43-2)45-28-25(38-17-20-14-21(30(32,33)34)16-22(15-20)31(35,36)37)18-39-29(41-28)40-23-4-6-24(7-5-23)42-9-11-44-12-10-42/h3-8,13-18H,9-12H2,1-2H3,(H,39,40,41)/b38-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) after 120 mins in presence of 33P-ATP |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50289129

(CHEMBL4172630)Show SMILES COc1cc(C)ccc1Oc1nc(Nc2ccc(cc2)N2CCOCC2)ncc1\N=C\c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C29H27Cl2N5O3/c1-19-3-8-26(27(13-19)37-2)39-28-25(32-17-20-14-21(30)16-22(31)15-20)18-33-29(35-28)34-23-4-6-24(7-5-23)36-9-11-38-12-10-36/h3-8,13-18H,9-12H2,1-2H3,(H,33,34,35)/b32-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) after 120 mins in presence of 33P-ATP |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 15
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of MAPK15 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50288937

(CHEMBL4172190)Show SMILES COc1cccc(Oc2nc(Nc3ccc(cc3)N3CCOCC3)ncc2\N=C\c2cc(OC)cc(OC)c2)c1 Show InChI InChI=1S/C30H31N5O5/c1-36-24-5-4-6-25(17-24)40-29-28(31-19-21-15-26(37-2)18-27(16-21)38-3)20-32-30(34-29)33-22-7-9-23(10-8-22)35-11-13-39-14-12-35/h4-10,15-20H,11-14H2,1-3H3,(H,32,33,34)/b31-19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of FMS (unknown origin) after 120 mins in presence of 33P-ATP |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory constant against Adenosine A2A receptor using [3H]-SCH-58,261 as radio ligand |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50132495
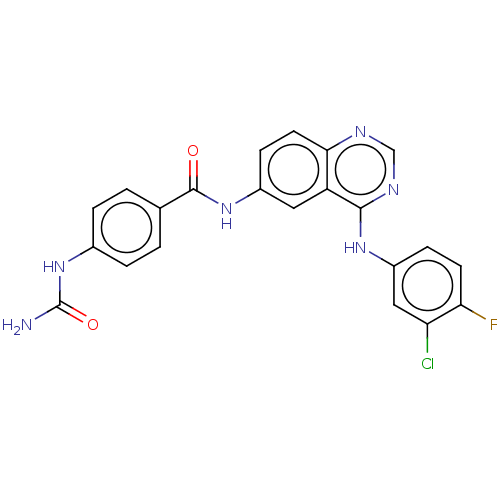
(CHEMBL3633940)Show SMILES NC(=O)Nc1ccc(cc1)C(=O)Nc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H16ClFN6O2/c23-17-10-15(5-7-18(17)24)28-20-16-9-14(6-8-19(16)26-11-27-20)29-21(31)12-1-3-13(4-2-12)30-22(25)32/h1-11H,(H,29,31)(H3,25,30,32)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibitory activity against metabotropic glutamate receptor 5 (mGluR5) |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM22032
(1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...)Show InChI InChI=1S/C21H21ClN2O/c1-4-14(2)24(3)21(25)19-13-15-9-5-6-10-16(15)20(23-19)17-11-7-8-12-18(17)22/h5-14H,4H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO receptor in Sprague-Dawley rat cerebral cortex membrane by radiometric competitive assay |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data