 Found 551 hits with Last Name = 'emayan' and Initial = 'k'
Found 551 hits with Last Name = 'emayan' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81917
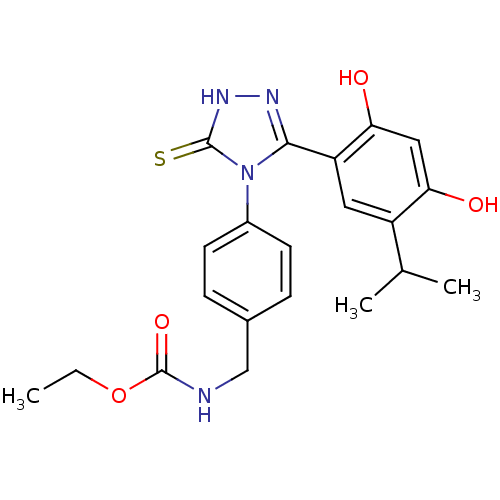
(BX-2819)Show SMILES CCOC(=O)NCc1ccc(cc1)-n1c(n[nH]c1=S)-c1cc(C(C)C)c(O)cc1O Show InChI InChI=1S/C21H24N4O4S/c1-4-29-21(28)22-11-13-5-7-14(8-6-13)25-19(23-24-20(25)30)16-9-15(12(2)3)17(26)10-18(16)27/h5-10,12,26-27H,4,11H2,1-3H3,(H,22,28)(H,24,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81916
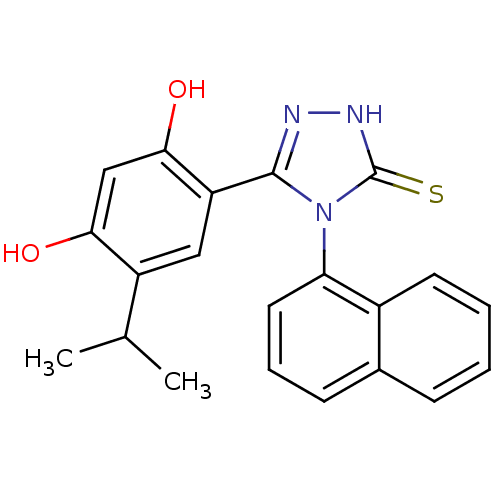
(lspropyl analog, 5)Show SMILES CC(C)c1cc(-c2n[nH]c(=S)n2-c2cccc3ccccc23)c(O)cc1O |(-.66,2.94,;.68,3.71,;.68,5.25,;2.01,2.94,;3.35,3.71,;4.68,2.94,;5.88,3.91,;7.36,3.51,;8.2,4.8,;7.23,6,;7.63,7.48,;5.8,5.45,;4.46,6.22,;3.13,5.44,;1.79,6.21,;1.79,7.76,;3.13,8.53,;3.13,10.07,;4.46,10.84,;5.8,10.07,;5.8,8.53,;4.46,7.76,;4.68,1.4,;6.01,.63,;3.35,.63,;2.01,1.4,;.68,.63,)| Show InChI InChI=1S/C21H19N3O2S/c1-12(2)15-10-16(19(26)11-18(15)25)20-22-23-21(27)24(20)17-9-5-7-13-6-3-4-8-14(13)17/h3-12,25-26H,1-2H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81914
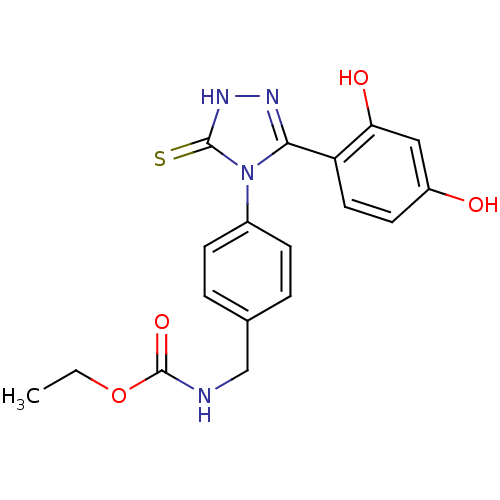
(Ethyl carbamate analog, 3)Show SMILES CCOC(=O)NCc1ccc(cc1)-n1c(n[nH]c1=S)-c1ccc(O)cc1O Show InChI InChI=1S/C18H18N4O4S/c1-2-26-18(25)19-10-11-3-5-12(6-4-11)22-16(20-21-17(22)27)14-8-7-13(23)9-15(14)24/h3-9,23-24H,2,10H2,1H3,(H,19,25)(H,21,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81915
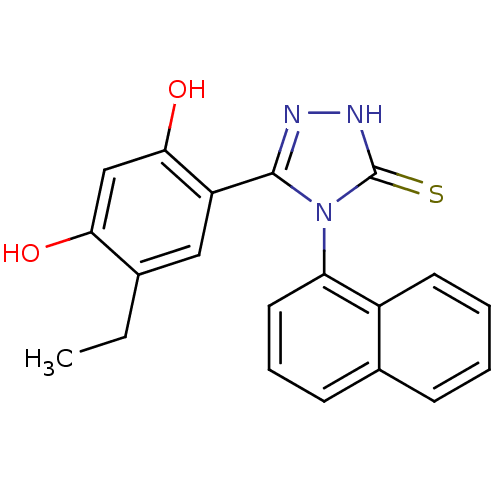
(Ethyl analog, 4)Show SMILES CCc1cc(-c2n[nH]c(=S)n2-c2cccc3ccccc23)c(O)cc1O |(-.66,2.94,;.68,3.71,;2.01,2.94,;3.35,3.71,;4.68,2.94,;5.88,3.91,;7.36,3.51,;8.2,4.8,;7.23,6,;7.63,7.48,;5.8,5.45,;4.46,6.22,;3.13,5.44,;1.79,6.21,;1.79,7.76,;3.13,8.53,;3.13,10.07,;4.46,10.84,;5.8,10.07,;5.8,8.53,;4.46,7.76,;4.68,1.4,;6.01,.63,;3.35,.63,;2.01,1.4,;.68,.63,)| Show InChI InChI=1S/C20H17N3O2S/c1-2-12-10-15(18(25)11-17(12)24)19-21-22-20(26)23(19)16-9-5-7-13-6-3-4-8-14(13)16/h3-11,24-25H,2H2,1H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81912
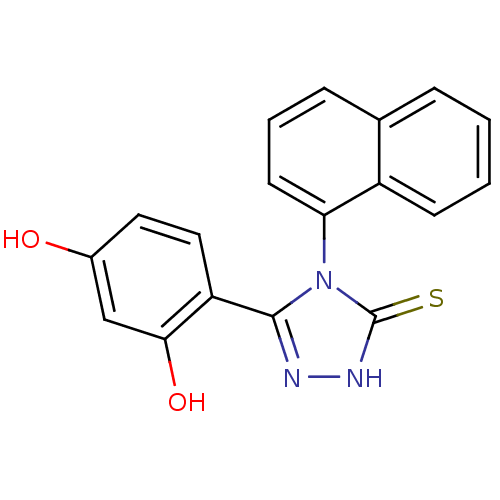
(DC23 | Resorcinol analog, 1)Show SMILES Oc1ccc(-c2n[nH]c(=S)n2-c2cccc3ccccc23)c(O)c1 |(.68,.63,;2.01,1.4,;2.01,2.94,;3.35,3.71,;4.68,2.94,;5.88,3.91,;7.36,3.51,;8.2,4.8,;7.23,6,;7.63,7.48,;5.8,5.45,;4.46,6.22,;3.13,5.44,;1.79,6.21,;1.79,7.76,;3.13,8.53,;3.13,10.06,;4.46,10.84,;5.8,10.07,;5.8,8.52,;4.46,7.76,;4.68,1.4,;6.01,.63,;3.35,.63,)| Show InChI InChI=1S/C18H13N3O2S/c22-12-8-9-14(16(23)10-12)17-19-20-18(24)21(17)15-7-3-5-11-4-1-2-6-13(11)15/h1-10,22-23H,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81913
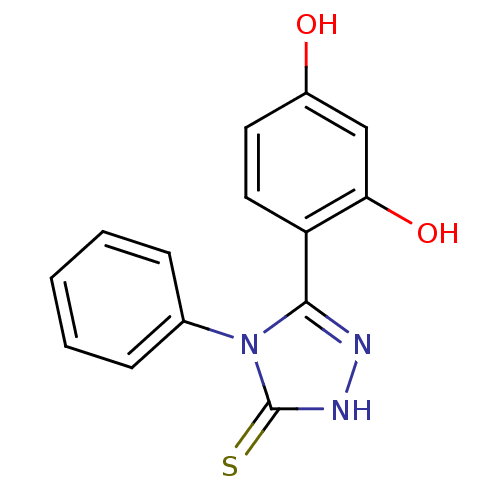
(Unsubstituted phenyl ring analog, 2)Show InChI InChI=1S/C14H11N3O2S/c18-10-6-7-11(12(19)8-10)13-15-16-14(20)17(13)9-4-2-1-3-5-9/h1-8,18-19H,(H,16,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467209
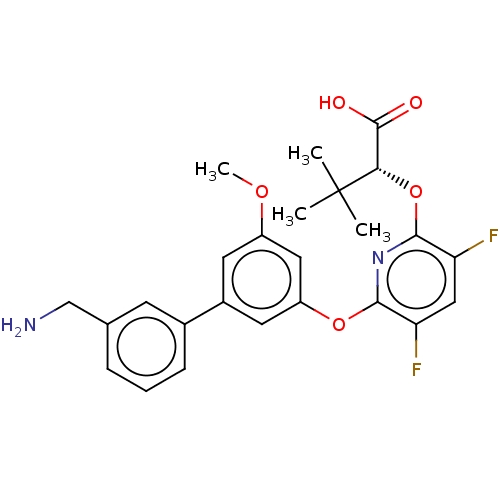
(CHEMBL4291446)Show SMILES COc1cc(Oc2nc(O[C@@H](C(O)=O)C(C)(C)C)c(F)cc2F)cc(c1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C25H26F2N2O5/c1-25(2,3)21(24(30)31)34-23-20(27)12-19(26)22(29-23)33-18-10-16(9-17(11-18)32-4)15-7-5-6-14(8-15)13-28/h5-12,21H,13,28H2,1-4H3,(H,30,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467207

(CHEMBL4288828)Show SMILES CC(C)(C)[C@@H](Oc1nc(Oc2cc(cc(c2)-c2cccc(CN)c2)C#N)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C25H23F2N3O4/c1-25(2,3)21(24(31)32)34-23-20(27)11-19(26)22(30-23)33-18-9-15(13-29)8-17(10-18)16-6-4-5-14(7-16)12-28/h4-11,21H,12,28H2,1-3H3,(H,31,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467211

(CHEMBL4281179)Show SMILES Cc1cc(Oc2nc(O[C@@H](C(O)=O)C(C)(C)C)c(F)cc2F)cc(c1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C25H26F2N2O4/c1-14-8-17(16-7-5-6-15(10-16)13-28)11-18(9-14)32-22-19(26)12-20(27)23(29-22)33-21(24(30)31)25(2,3)4/h5-12,21H,13,28H2,1-4H3,(H,30,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467212

(CHEMBL4285028)Show SMILES CC[C@@H](Oc1nc(Oc2cc(C)cc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C23H22F2N2O4/c1-3-20(23(28)29)31-22-19(25)11-18(24)21(27-22)30-17-8-13(2)7-16(10-17)15-6-4-5-14(9-15)12-26/h4-11,20H,3,12,26H2,1-2H3,(H,28,29)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467210

(CHEMBL4281574)Show SMILES CC(C)(C)[C@@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C24H24F2N2O4/c1-24(2,3)20(23(29)30)32-22-19(26)12-18(25)21(28-22)31-17-9-5-8-16(11-17)15-7-4-6-14(10-15)13-27/h4-12,20H,13,27H2,1-3H3,(H,29,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467208
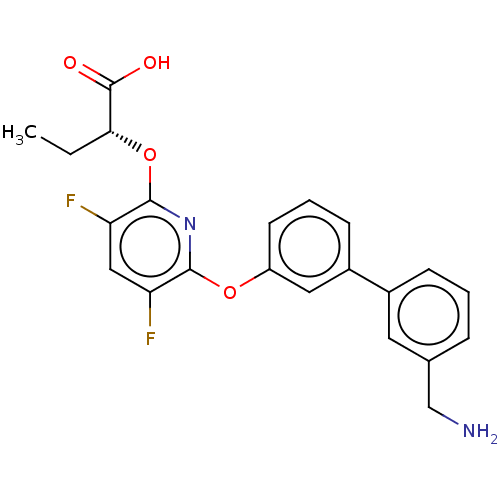
(CHEMBL4277939)Show SMILES CC[C@@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C22H20F2N2O4/c1-2-19(22(27)28)30-21-18(24)11-17(23)20(26-21)29-16-8-4-7-15(10-16)14-6-3-5-13(9-14)12-25/h3-11,19H,2,12,25H2,1H3,(H,27,28)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467214

(CHEMBL4277252)Show SMILES C[C@@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C21H18F2N2O4/c1-12(21(26)27)28-19-17(22)10-18(23)20(25-19)29-16-7-3-6-15(9-16)14-5-2-4-13(8-14)11-24/h2-10,12H,11,24H2,1H3,(H,26,27)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50467212

(CHEMBL4285028)Show SMILES CC[C@@H](Oc1nc(Oc2cc(C)cc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C23H22F2N2O4/c1-3-20(23(28)29)31-22-19(25)11-18(24)21(27-22)30-17-8-13(2)7-16(10-17)15-6-4-5-14(9-15)12-26/h4-11,20H,3,12,26H2,1-2H3,(H,28,29)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467211

(CHEMBL4281179)Show SMILES Cc1cc(Oc2nc(O[C@@H](C(O)=O)C(C)(C)C)c(F)cc2F)cc(c1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C25H26F2N2O4/c1-14-8-17(16-7-5-6-15(10-16)13-28)11-18(9-14)32-22-19(26)12-20(27)23(29-22)33-21(24(30)31)25(2,3)4/h5-12,21H,13,28H2,1-4H3,(H,30,31)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human tPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Mus musculus) | BDBM50467212

(CHEMBL4285028)Show SMILES CC[C@@H](Oc1nc(Oc2cc(C)cc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C23H22F2N2O4/c1-3-20(23(28)29)31-22-19(25)11-18(24)21(27-22)30-17-8-13(2)7-16(10-17)15-6-4-5-14(9-15)12-26/h4-11,20H,3,12,26H2,1-2H3,(H,28,29)/t20-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse tPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50467212

(CHEMBL4285028)Show SMILES CC[C@@H](Oc1nc(Oc2cc(C)cc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C23H22F2N2O4/c1-3-20(23(28)29)31-22-19(25)11-18(24)21(27-22)30-17-8-13(2)7-16(10-17)15-6-4-5-14(9-15)12-26/h4-11,20H,3,12,26H2,1-2H3,(H,28,29)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467210

(CHEMBL4281574)Show SMILES CC(C)(C)[C@@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C24H24F2N2O4/c1-24(2,3)20(23(29)30)32-22-19(26)12-18(25)21(28-22)31-17-9-5-8-16(11-17)15-7-4-6-14(10-15)13-27/h4-12,20H,13,27H2,1-3H3,(H,29,30)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human tPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467212

(CHEMBL4285028)Show SMILES CC[C@@H](Oc1nc(Oc2cc(C)cc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C23H22F2N2O4/c1-3-20(23(28)29)31-22-19(25)11-18(24)21(27-22)30-17-8-13(2)7-16(10-17)15-6-4-5-14(9-15)12-26/h4-11,20H,3,12,26H2,1-2H3,(H,28,29)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human tPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Mus musculus) | BDBM50467212

(CHEMBL4285028)Show SMILES CC[C@@H](Oc1nc(Oc2cc(C)cc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C23H22F2N2O4/c1-3-20(23(28)29)31-22-19(25)11-18(24)21(27-22)30-17-8-13(2)7-16(10-17)15-6-4-5-14(9-15)12-26/h4-11,20H,3,12,26H2,1-2H3,(H,28,29)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse plasmin |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467208

(CHEMBL4277939)Show SMILES CC[C@@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C22H20F2N2O4/c1-2-19(22(27)28)30-21-18(24)11-17(23)20(26-21)29-16-8-4-7-15(10-16)14-6-3-5-13(9-14)12-25/h3-11,19H,2,12,25H2,1H3,(H,27,28)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human tPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467209

(CHEMBL4291446)Show SMILES COc1cc(Oc2nc(O[C@@H](C(O)=O)C(C)(C)C)c(F)cc2F)cc(c1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C25H26F2N2O5/c1-25(2,3)21(24(30)31)34-23-20(27)12-19(26)22(29-23)33-18-10-16(9-17(11-18)32-4)15-7-5-6-14(8-15)13-28/h5-12,21H,13,28H2,1-4H3,(H,30,31)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human tPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50467208

(CHEMBL4277939)Show SMILES CC[C@@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C22H20F2N2O4/c1-2-19(22(27)28)30-21-18(24)11-17(23)20(26-21)29-16-8-4-7-15(10-16)14-6-3-5-13(9-14)12-25/h3-11,19H,2,12,25H2,1H3,(H,27,28)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50467211

(CHEMBL4281179)Show SMILES Cc1cc(Oc2nc(O[C@@H](C(O)=O)C(C)(C)C)c(F)cc2F)cc(c1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C25H26F2N2O4/c1-14-8-17(16-7-5-6-15(10-16)13-28)11-18(9-14)32-22-19(26)12-20(27)23(29-22)33-21(24(30)31)25(2,3)4/h5-12,21H,13,28H2,1-4H3,(H,30,31)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467213
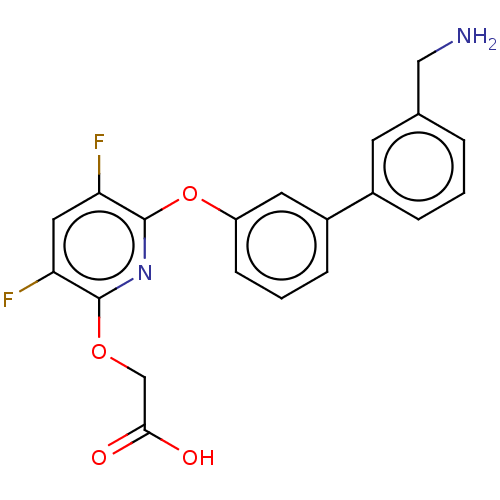
(CHEMBL4278454)Show SMILES NCc1cccc(c1)-c1cccc(Oc2nc(OCC(O)=O)c(F)cc2F)c1 Show InChI InChI=1S/C20H16F2N2O4/c21-16-9-17(22)20(24-19(16)27-11-18(25)26)28-15-6-2-5-14(8-15)13-4-1-3-12(7-13)10-23/h1-9H,10-11,23H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50467209

(CHEMBL4291446)Show SMILES COc1cc(Oc2nc(O[C@@H](C(O)=O)C(C)(C)C)c(F)cc2F)cc(c1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C25H26F2N2O5/c1-25(2,3)21(24(30)31)34-23-20(27)12-19(26)22(29-23)33-18-10-16(9-17(11-18)32-4)15-7-5-6-14(8-15)13-28/h5-12,21H,13,28H2,1-4H3,(H,30,31)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50467214

(CHEMBL4277252)Show SMILES C[C@@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C21H18F2N2O4/c1-12(21(26)27)28-19-17(22)10-18(23)20(25-19)29-16-7-3-6-15(9-16)14-5-2-4-13(8-14)11-24/h2-10,12H,11,24H2,1H3,(H,26,27)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50467207

(CHEMBL4288828)Show SMILES CC(C)(C)[C@@H](Oc1nc(Oc2cc(cc(c2)-c2cccc(CN)c2)C#N)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C25H23F2N3O4/c1-25(2,3)21(24(31)32)34-23-20(27)11-19(26)22(30-23)33-18-9-15(13-29)8-17(10-18)16-6-4-5-14(7-16)12-28/h4-11,21H,12,28H2,1-3H3,(H,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50467210

(CHEMBL4281574)Show SMILES CC(C)(C)[C@@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C24H24F2N2O4/c1-24(2,3)20(23(29)30)32-22-19(26)12-18(25)21(28-22)31-17-9-5-8-16(11-17)15-7-4-6-14(10-15)13-27/h4-12,20H,13,27H2,1-3H3,(H,29,30)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467207

(CHEMBL4288828)Show SMILES CC(C)(C)[C@@H](Oc1nc(Oc2cc(cc(c2)-c2cccc(CN)c2)C#N)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C25H23F2N3O4/c1-25(2,3)21(24(31)32)34-23-20(27)11-19(26)22(30-23)33-18-9-15(13-29)8-17(10-18)16-6-4-5-14(7-16)12-28/h4-11,21H,12,28H2,1-3H3,(H,31,32)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human tPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467215

(CHEMBL4290603)Show SMILES CC[C@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C22H20F2N2O4/c1-2-19(22(27)28)30-21-18(24)11-17(23)20(26-21)29-16-8-4-7-15(10-16)14-6-3-5-13(9-14)12-25/h3-11,19H,2,12,25H2,1H3,(H,27,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50467215

(CHEMBL4290603)Show SMILES CC[C@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C22H20F2N2O4/c1-2-19(22(27)28)30-21-18(24)11-17(23)20(26-21)29-16-8-4-7-15(10-16)14-6-3-5-13(9-14)12-25/h3-11,19H,2,12,25H2,1H3,(H,27,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467215

(CHEMBL4290603)Show SMILES CC[C@H](Oc1nc(Oc2cccc(c2)-c2cccc(CN)c2)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C22H20F2N2O4/c1-2-19(22(27)28)30-21-18(24)11-17(23)20(26-21)29-16-8-4-7-15(10-16)14-6-3-5-13(9-14)12-25/h3-11,19H,2,12,25H2,1H3,(H,27,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human tPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168437
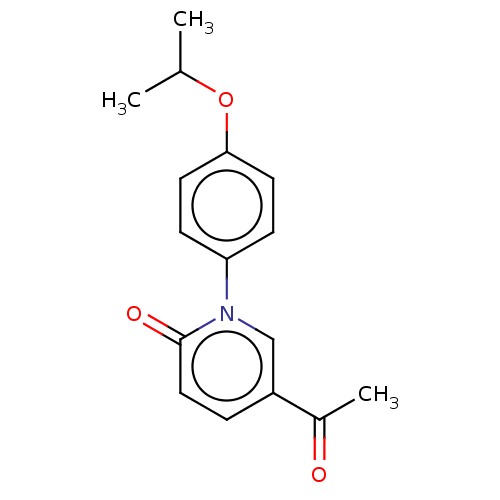
(US9675593, 10)Show InChI InChI=1S/C16H17NO3/c1-11(2)20-15-7-5-14(6-8-15)17-10-13(12(3)18)4-9-16(17)19/h4-11H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168438
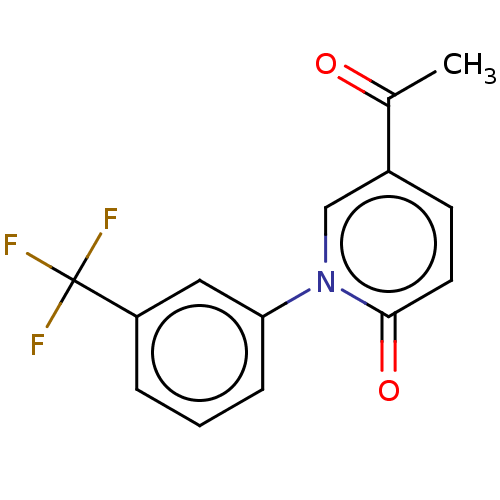
(US9675593, 11)Show InChI InChI=1S/C14H10F3NO2/c1-9(19)10-5-6-13(20)18(8-10)12-4-2-3-11(7-12)14(15,16)17/h2-8H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168439
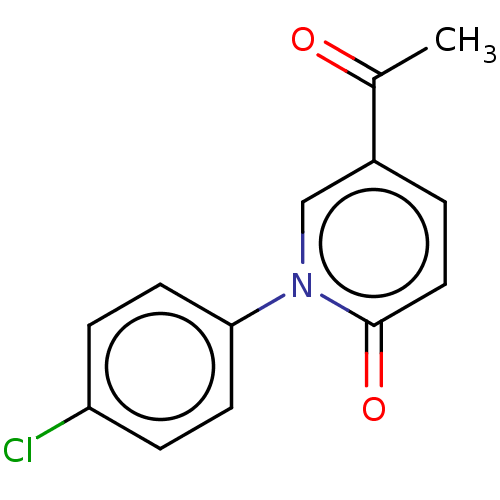
(US9675593, 12)Show InChI InChI=1S/C13H10ClNO2/c1-9(16)10-2-7-13(17)15(8-10)12-5-3-11(14)4-6-12/h2-8H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168440
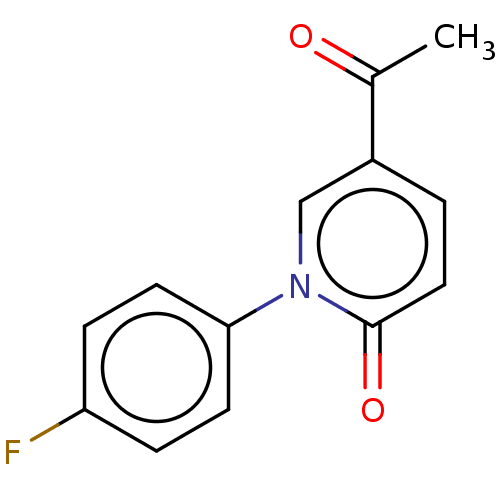
(US9675593, 13)Show InChI InChI=1S/C13H10FNO2/c1-9(16)10-2-7-13(17)15(8-10)12-5-3-11(14)4-6-12/h2-8H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168441
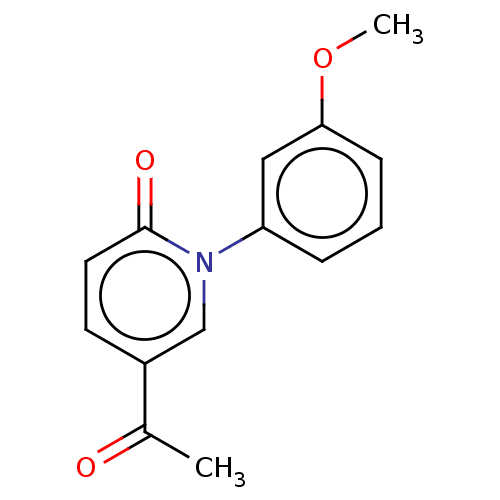
(US9675593, 14)Show InChI InChI=1S/C14H13NO3/c1-10(16)11-6-7-14(17)15(9-11)12-4-3-5-13(8-12)18-2/h3-9H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168442
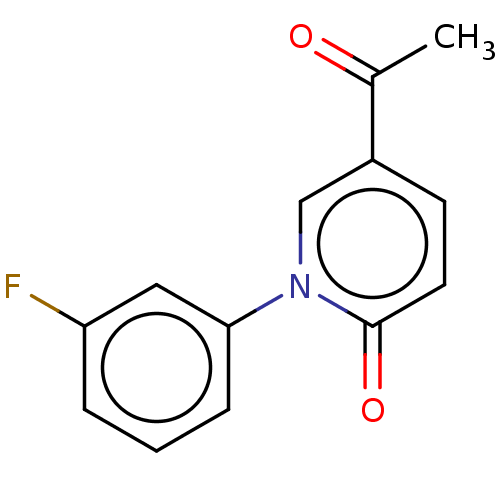
(US9675593, 15)Show InChI InChI=1S/C13H10FNO2/c1-9(16)10-5-6-13(17)15(8-10)12-4-2-3-11(14)7-12/h2-8H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168444
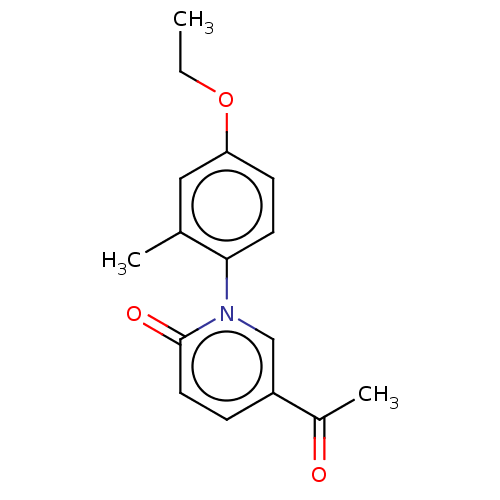
(US9675593, 17)Show InChI InChI=1S/C16H17NO3/c1-4-20-14-6-7-15(11(2)9-14)17-10-13(12(3)18)5-8-16(17)19/h5-10H,4H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168450
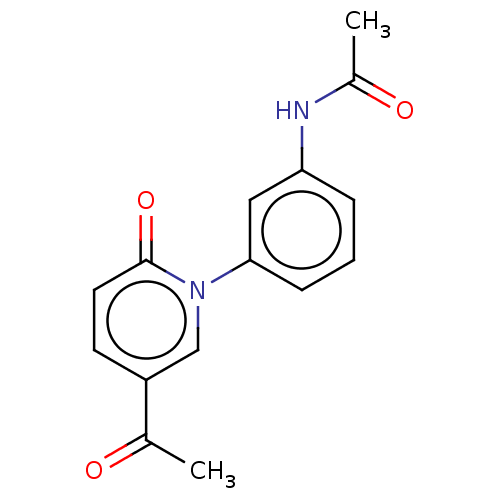
(US9675593, 18)Show InChI InChI=1S/C15H14N2O3/c1-10(18)12-6-7-15(20)17(9-12)14-5-3-4-13(8-14)16-11(2)19/h3-9H,1-2H3,(H,16,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168451
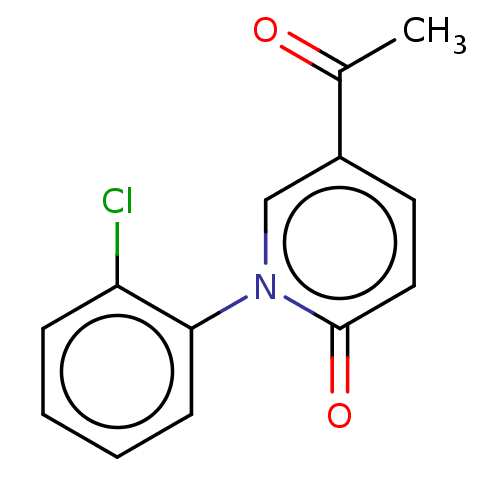
(US9675593, 19)Show InChI InChI=1S/C13H10ClNO2/c1-9(16)10-6-7-13(17)15(8-10)12-5-3-2-4-11(12)14/h2-8H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168452
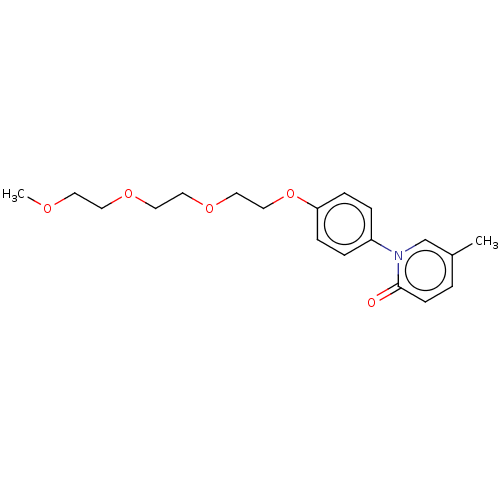
(US9675593, 21)Show InChI InChI=1S/C19H25NO5/c1-16-3-8-19(21)20(15-16)17-4-6-18(7-5-17)25-14-13-24-12-11-23-10-9-22-2/h3-8,15H,9-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168453
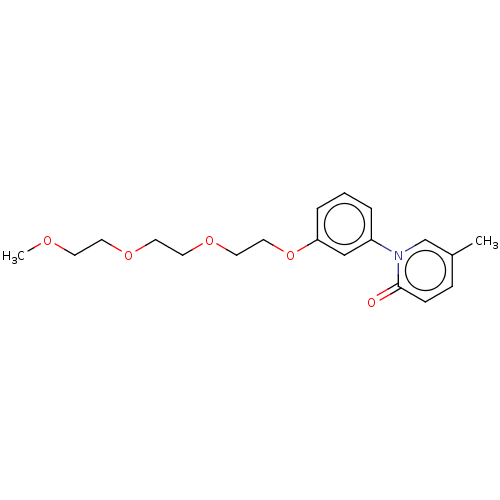
(US9675593, 22)Show InChI InChI=1S/C19H25NO5/c1-16-6-7-19(21)20(15-16)17-4-3-5-18(14-17)25-13-12-24-11-10-23-9-8-22-2/h3-7,14-15H,8-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168454
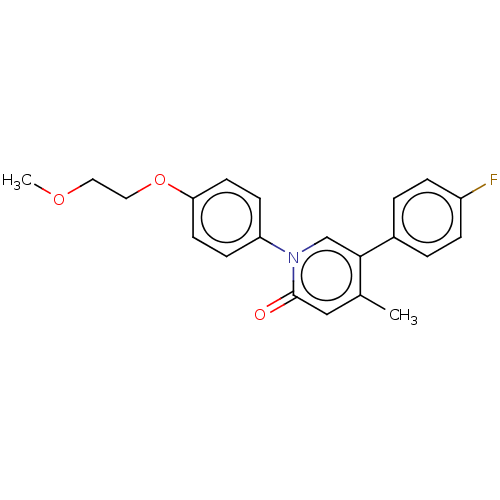
(US9675593, 23)Show SMILES COCCOc1ccc(cc1)-n1cc(c(C)cc1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C21H20FNO3/c1-15-13-21(24)23(14-20(15)16-3-5-17(22)6-4-16)18-7-9-19(10-8-18)26-12-11-25-2/h3-10,13-14H,11-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168462
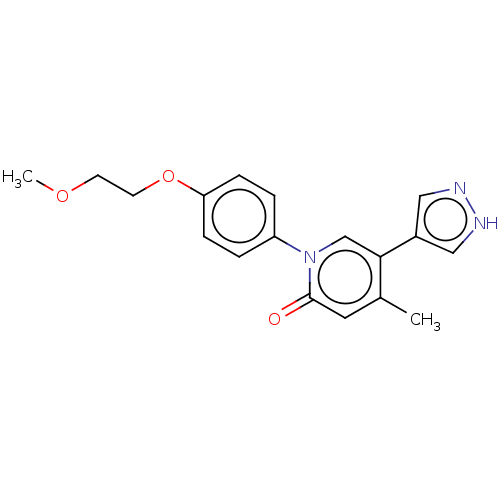
(US9675593, 24)Show InChI InChI=1S/C18H19N3O3/c1-13-9-18(22)21(12-17(13)14-10-19-20-11-14)15-3-5-16(6-4-15)24-8-7-23-2/h3-6,9-12H,7-8H2,1-2H3,(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168465
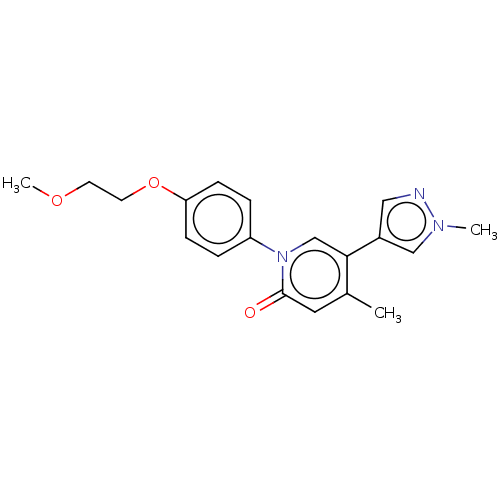
(US9675593, 25)Show InChI InChI=1S/C19H21N3O3/c1-14-10-19(23)22(13-18(14)15-11-20-21(2)12-15)16-4-6-17(7-5-16)25-9-8-24-3/h4-7,10-13H,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168466
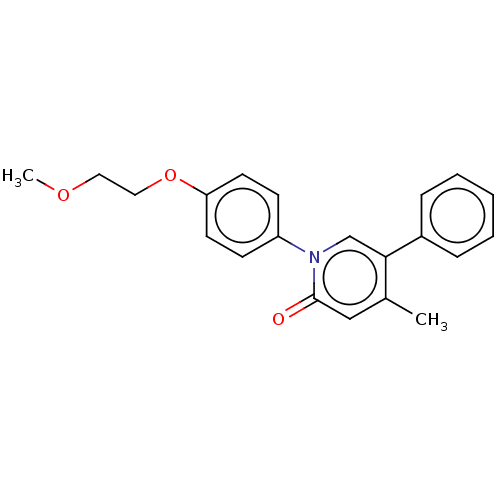
(US9675593, 26)Show InChI InChI=1S/C21H21NO3/c1-16-14-21(23)22(15-20(16)17-6-4-3-5-7-17)18-8-10-19(11-9-18)25-13-12-24-2/h3-11,14-15H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | <5.00E+4 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168467
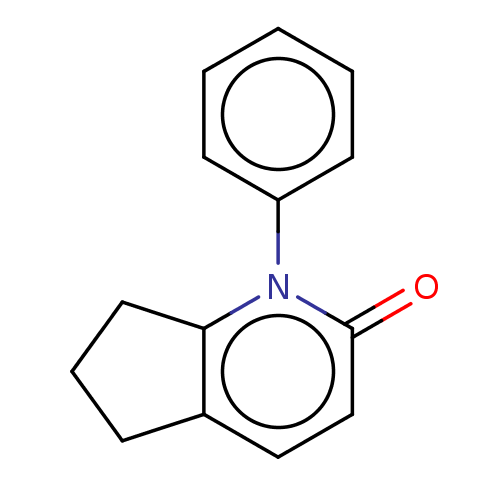
(US9675593, 27)Show InChI InChI=1S/C14H13NO/c16-14-10-9-11-5-4-8-13(11)15(14)12-6-2-1-3-7-12/h1-3,6-7,9-10H,4-5,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |
Endothelin-1
(Homo sapiens (Human)) | BDBM168468
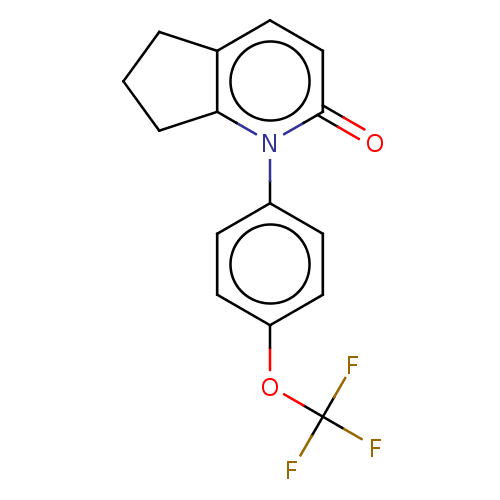
(US9675593, 28)Show InChI InChI=1S/C15H12F3NO2/c16-15(17,18)21-12-7-5-11(6-8-12)19-13-3-1-2-10(13)4-9-14(19)20/h4-9H,1-3H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a |
SRMLSC
US Patent
| Assay Description
Fibroblasts (primary human lung and dermal, HFL-1, 3T3 etc) are seeded in 96-well plates at ~15000 cells/well and serum starved for 0-48 hours. After... |
US Patent US9675593 (2017)
BindingDB Entry DOI: 10.7270/Q2319T24 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data