 Found 1053 hits with Last Name = 'emini' and Initial = 'ea'
Found 1053 hits with Last Name = 'emini' and Initial = 'ea' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of Wild-type protease |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of Wild-type protease |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366785

(NELFINAVIR)Show SMILES Cc1c(O)cccc1C(=O)N[C@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26+,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of Wild-type protease |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of Wild-type protease |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against specific binding of [125I]-MIP-1 alpha to human CCR5 receptor |
Bioorg Med Chem Lett 11: 265-70 (2001)
BindingDB Entry DOI: 10.7270/Q2668CFZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366785

(NELFINAVIR)Show SMILES Cc1c(O)cccc1C(=O)N[C@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26+,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366785

(NELFINAVIR)Show SMILES Cc1c(O)cccc1C(=O)N[C@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26+,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50366785

(NELFINAVIR)Show SMILES Cc1c(O)cccc1C(=O)N[C@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26+,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) |
J Med Chem 43: 3386-99 (2000)
BindingDB Entry DOI: 10.7270/Q23J3DP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141931

((R)-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl-2...)Show SMILES CCn1nc(Cc2ccc(cc2)-c2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C2CCCCC2)C(O)=O)CC1 Show InChI InChI=1S/C42H51FN4O2/c1-2-47-40(26-38(44-47)24-30-16-18-32(19-17-30)31-10-5-3-6-11-31)33-20-22-45(23-21-33)27-36-28-46(29-39(36)35-14-9-15-37(43)25-35)41(42(48)49)34-12-7-4-8-13-34/h3,5-6,9-11,14-19,25-26,33-34,36,39,41H,2,4,7-8,12-13,20-24,27-29H2,1H3,(H,48,49)/t36-,39+,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141911

((R)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethyl-2H...)Show SMILES CCn1nc(Cc2ccc(cc2)C#N)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C35H44FN5O2/c1-5-41-32(19-30(38-41)17-24-9-11-25(20-37)12-10-24)26-13-15-39(16-14-26)21-28-22-40(33(34(42)43)35(2,3)4)23-31(28)27-7-6-8-29(36)18-27/h6-12,18-19,26,28,31,33H,5,13-17,21-23H2,1-4H3,(H,42,43)/t28-,31+,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141908

((R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butyl-benzyl)-2-eth...)Show SMILES CCn1nc(Cc2ccc(cc2)C(C)(C)C)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H53FN4O2/c1-8-43-34(22-32(40-43)20-26-12-14-30(15-13-26)37(2,3)4)27-16-18-41(19-17-27)23-29-24-42(35(36(44)45)38(5,6)7)25-33(29)28-10-9-11-31(39)21-28/h9-15,21-22,27,29,33,35H,8,16-20,23-25H2,1-7H3,(H,44,45)/t29-,33+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141905
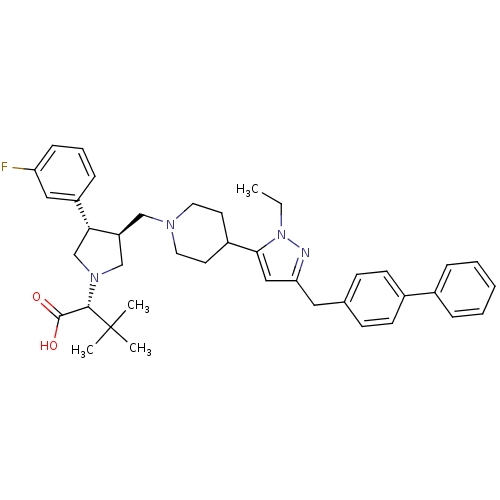
((R)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl...)Show SMILES CCn1nc(Cc2ccc(cc2)-c2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C40H49FN4O2/c1-5-45-37(24-35(42-45)22-28-14-16-30(17-15-28)29-10-7-6-8-11-29)31-18-20-43(21-19-31)25-33-26-44(38(39(46)47)40(2,3)4)27-36(33)32-12-9-13-34(41)23-32/h6-17,23-24,31,33,36,38H,5,18-22,25-27H2,1-4H3,(H,46,47)/t33-,36+,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141978
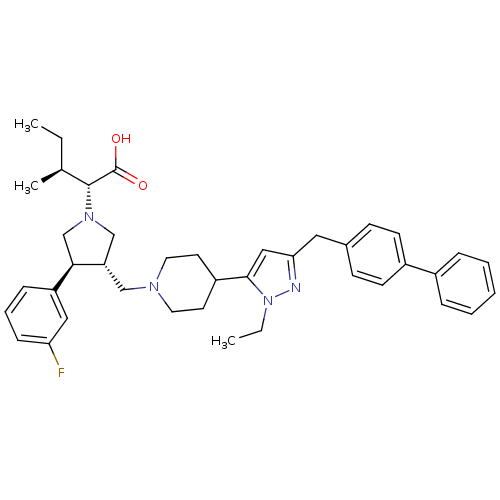
((2R,4S)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-e...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)-c3ccccc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C40H49FN4O2/c1-4-28(3)39(40(46)47)44-26-34(37(27-44)33-12-9-13-35(41)23-33)25-43-20-18-32(19-21-43)38-24-36(42-45(38)5-2)22-29-14-16-31(17-15-29)30-10-7-6-8-11-30/h6-17,23-24,28,32,34,37,39H,4-5,18-22,25-27H2,1-3H3,(H,46,47)/t28-,34-,37+,39+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141935
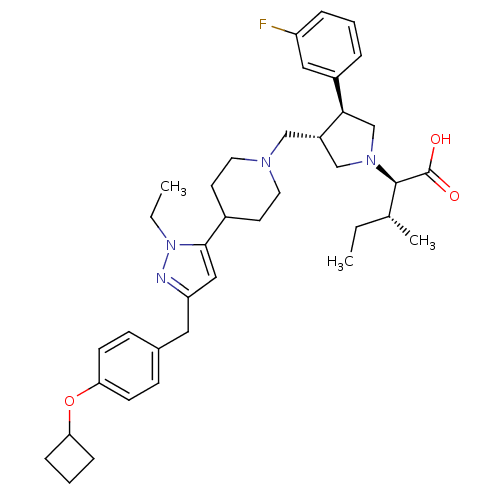
((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC4CCC4)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H51FN4O3/c1-4-26(3)37(38(44)45)42-24-30(35(25-42)29-8-6-9-31(39)21-29)23-41-18-16-28(17-19-41)36-22-32(40-43(36)5-2)20-27-12-14-34(15-13-27)46-33-10-7-11-33/h6,8-9,12-15,21-22,26,28,30,33,35,37H,4-5,7,10-11,16-20,23-25H2,1-3H3,(H,44,45)/t26-,30+,35-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141951
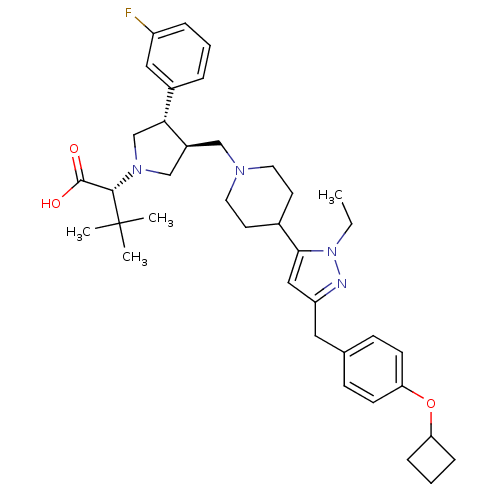
((R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-2-et...)Show SMILES CCn1nc(Cc2ccc(OC3CCC3)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H51FN4O3/c1-5-43-35(22-31(40-43)20-26-12-14-33(15-13-26)46-32-10-7-11-32)27-16-18-41(19-17-27)23-29-24-42(36(37(44)45)38(2,3)4)25-34(29)28-8-6-9-30(39)21-28/h6,8-9,12-15,21-22,27,29,32,34,36H,5,7,10-11,16-20,23-25H2,1-4H3,(H,44,45)/t29-,34+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141913
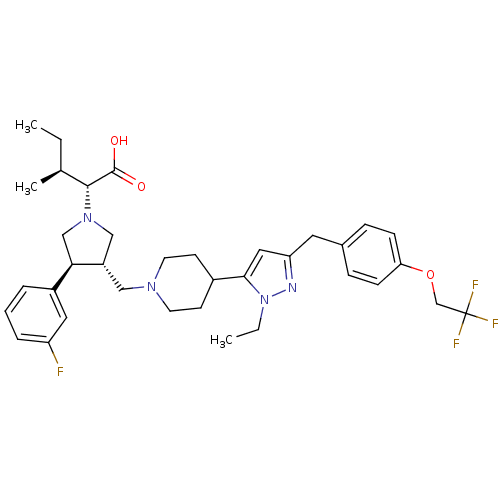
((2R,4S)-2-[(2S,3S)-3-(4-{2-Ethyl-5-[4-(2,2,2-trifl...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OCC(F)(F)F)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C36H46F4N4O3/c1-4-24(3)34(35(45)46)43-21-28(32(22-43)27-7-6-8-29(37)18-27)20-42-15-13-26(14-16-42)33-19-30(41-44(33)5-2)17-25-9-11-31(12-10-25)47-23-36(38,39)40/h6-12,18-19,24,26,28,32,34H,4-5,13-17,20-23H2,1-3H3,(H,45,46)/t24-,28-,32+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141941
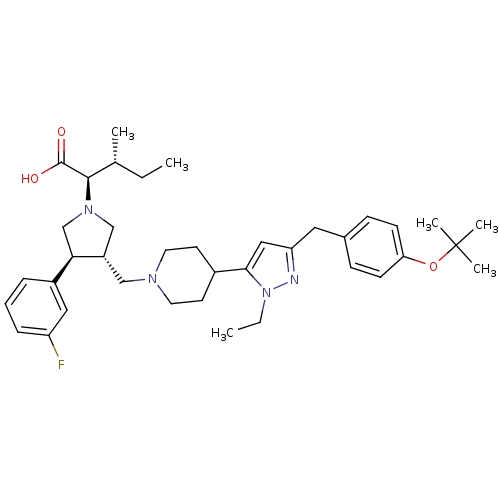
((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butoxy-benzyl)-...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC(C)(C)C)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H53FN4O3/c1-7-26(3)36(37(44)45)42-24-30(34(25-42)29-10-9-11-31(39)21-29)23-41-18-16-28(17-19-41)35-22-32(40-43(35)8-2)20-27-12-14-33(15-13-27)46-38(4,5)6/h9-15,21-22,26,28,30,34,36H,7-8,16-20,23-25H2,1-6H3,(H,44,45)/t26-,30+,34-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141906
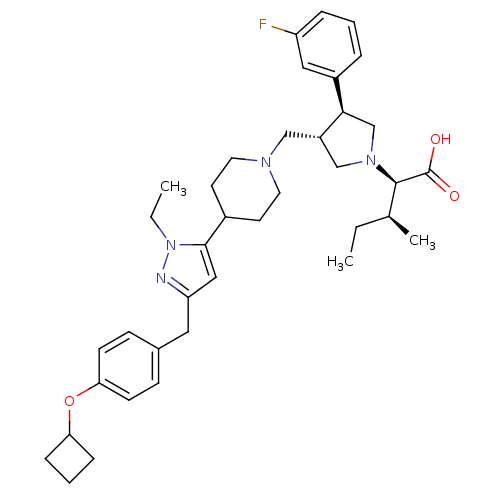
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC4CCC4)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H51FN4O3/c1-4-26(3)37(38(44)45)42-24-30(35(25-42)29-8-6-9-31(39)21-29)23-41-18-16-28(17-19-41)36-22-32(40-43(36)5-2)20-27-12-14-34(15-13-27)46-33-10-7-11-33/h6,8-9,12-15,21-22,26,28,30,33,35,37H,4-5,7,10-11,16-20,23-25H2,1-3H3,(H,44,45)/t26-,30-,35+,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141973
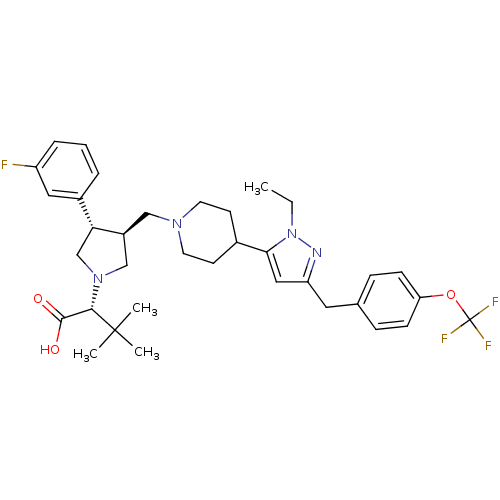
(2-[3-{4-[2-Ethyl-5-(4-trifluoromethoxy-benzyl)-2H-...)Show SMILES CCn1nc(Cc2ccc(OC(F)(F)F)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C35H44F4N4O3/c1-5-43-31(19-28(40-43)17-23-9-11-29(12-10-23)46-35(37,38)39)24-13-15-41(16-14-24)20-26-21-42(32(33(44)45)34(2,3)4)22-30(26)25-7-6-8-27(36)18-25/h6-12,18-19,24,26,30,32H,5,13-17,20-22H2,1-4H3,(H,44,45)/t26-,30+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141910
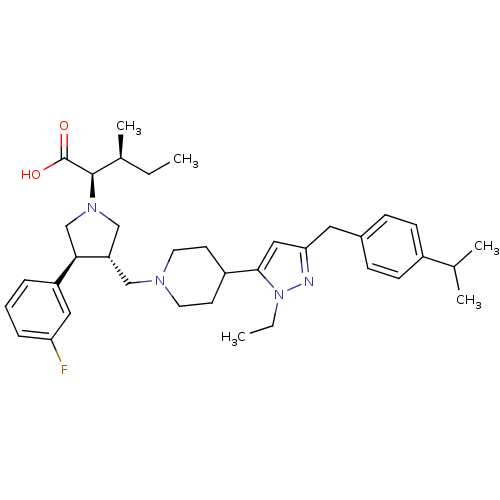
((2R,4S)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropyl-be...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)C(C)C)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C37H51FN4O2/c1-6-26(5)36(37(43)44)41-23-31(34(24-41)30-9-8-10-32(38)20-30)22-40-17-15-29(16-18-40)35-21-33(39-42(35)7-2)19-27-11-13-28(14-12-27)25(3)4/h8-14,20-21,25-26,29,31,34,36H,6-7,15-19,22-24H2,1-5H3,(H,43,44)/t26-,31-,34+,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141979
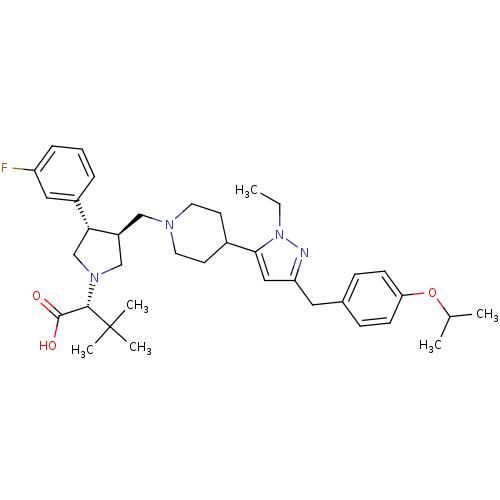
((R)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropoxy-benzy...)Show SMILES CCn1nc(Cc2ccc(OC(C)C)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C37H51FN4O3/c1-7-42-34(21-31(39-42)19-26-11-13-32(14-12-26)45-25(2)3)27-15-17-40(18-16-27)22-29-23-41(35(36(43)44)37(4,5)6)24-33(29)28-9-8-10-30(38)20-28/h8-14,20-21,25,27,29,33,35H,7,15-19,22-24H2,1-6H3,(H,43,44)/t29-,33+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141984
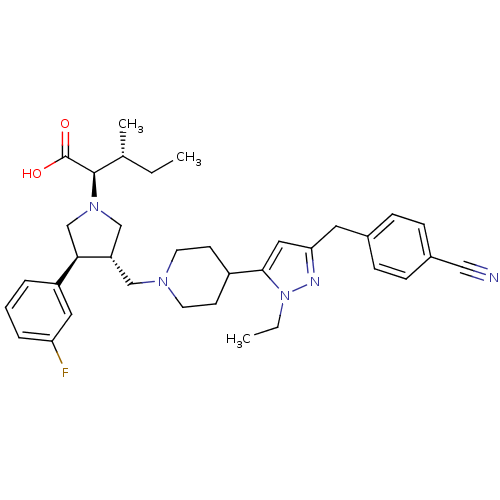
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethy...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)C#N)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C35H44FN5O2/c1-4-24(3)34(35(42)43)40-22-29(32(23-40)28-7-6-8-30(36)18-28)21-39-15-13-27(14-16-39)33-19-31(38-41(33)5-2)17-25-9-11-26(20-37)12-10-25/h6-12,18-19,24,27,29,32,34H,4-5,13-17,21-23H2,1-3H3,(H,42,43)/t24-,29+,32-,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141883
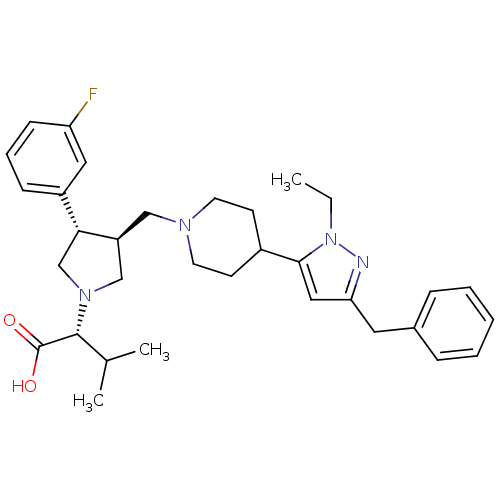
((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...)Show SMILES CCn1nc(Cc2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C(C)C)C(O)=O)CC1 |r| Show InChI InChI=1S/C33H43FN4O2/c1-4-38-31(19-29(35-38)17-24-9-6-5-7-10-24)25-13-15-36(16-14-25)20-27-21-37(32(23(2)3)33(39)40)22-30(27)26-11-8-12-28(34)18-26/h5-12,18-19,23,25,27,30,32H,4,13-17,20-22H2,1-3H3,(H,39,40)/t27-,30+,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141972
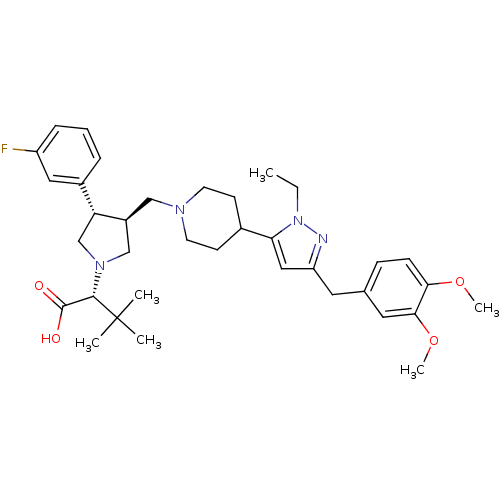
((R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-2-et...)Show SMILES CCn1nc(Cc2ccc(OC)c(OC)c2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C36H49FN4O4/c1-7-41-31(20-29(38-41)17-24-11-12-32(44-5)33(18-24)45-6)25-13-15-39(16-14-25)21-27-22-40(34(35(42)43)36(2,3)4)23-30(27)26-9-8-10-28(37)19-26/h8-12,18-20,25,27,30,34H,7,13-17,21-23H2,1-6H3,(H,42,43)/t27-,30+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141874
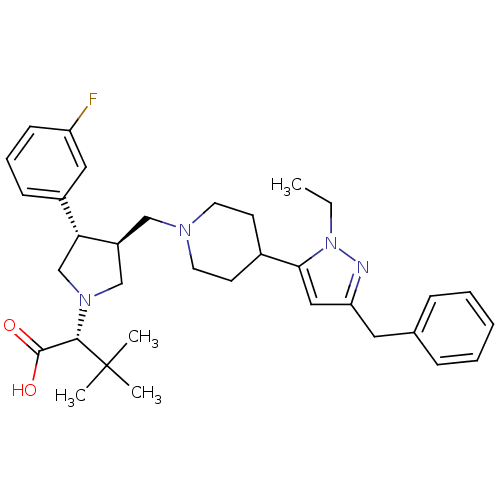
((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...)Show SMILES CCn1nc(Cc2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 |r| Show InChI InChI=1S/C34H45FN4O2/c1-5-39-31(20-29(36-39)18-24-10-7-6-8-11-24)25-14-16-37(17-15-25)21-27-22-38(32(33(40)41)34(2,3)4)23-30(27)26-12-9-13-28(35)19-26/h6-13,19-20,25,27,30,32H,5,14-18,21-23H2,1-4H3,(H,40,41)/t27-,30+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141945
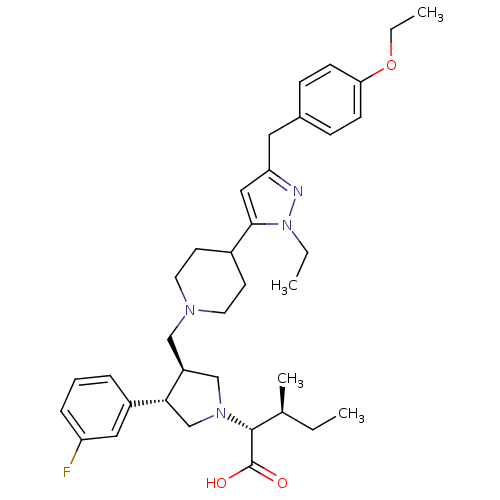
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Ethoxy-benzyl)-2-eth...)Show SMILES CCOc1ccc(Cc2cc(C3CCN(C[C@H]4CN(C[C@@H]4c4cccc(F)c4)[C@H]([C@@H](C)CC)C(O)=O)CC3)n(CC)n2)cc1 Show InChI InChI=1S/C36H49FN4O3/c1-5-25(4)35(36(42)43)40-23-29(33(24-40)28-9-8-10-30(37)20-28)22-39-17-15-27(16-18-39)34-21-31(38-41(34)6-2)19-26-11-13-32(14-12-26)44-7-3/h8-14,20-21,25,27,29,33,35H,5-7,15-19,22-24H2,1-4H3,(H,42,43)/t25-,29-,33+,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141914
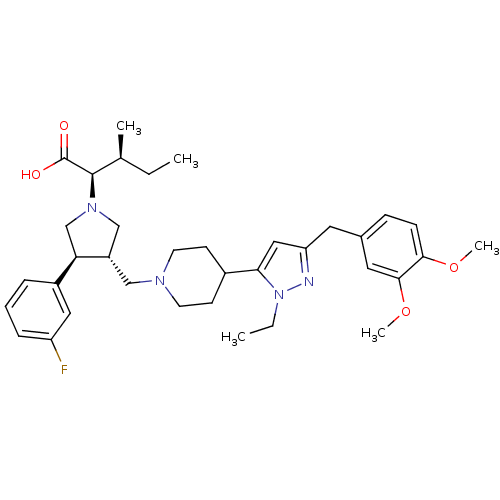
((2R,4S)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC)c(OC)c3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C36H49FN4O4/c1-6-24(3)35(36(42)43)40-22-28(31(23-40)27-9-8-10-29(37)19-27)21-39-15-13-26(14-16-39)32-20-30(38-41(32)7-2)17-25-11-12-33(44-4)34(18-25)45-5/h8-12,18-20,24,26,28,31,35H,6-7,13-17,21-23H2,1-5H3,(H,42,43)/t24-,28-,31+,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141875
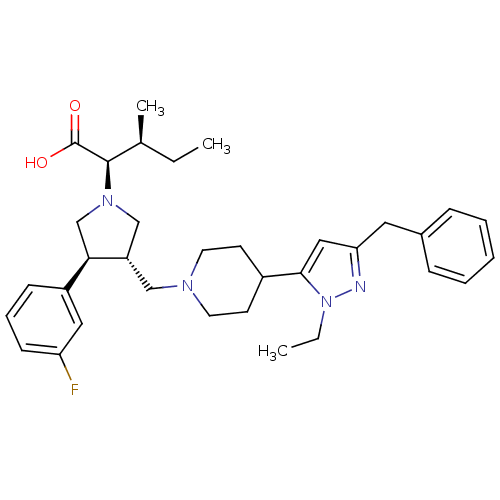
((2R,3S)-2-[(2S,3S)-3-[4-(5-Benzyl-2-ethyl-2H-pyraz...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccccc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C34H45FN4O2/c1-4-24(3)33(34(40)41)38-22-28(31(23-38)27-12-9-13-29(35)19-27)21-37-16-14-26(15-17-37)32-20-30(36-39(32)5-2)18-25-10-7-6-8-11-25/h6-13,19-20,24,26,28,31,33H,4-5,14-18,21-23H2,1-3H3,(H,40,41)/t24-,28-,31+,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141887
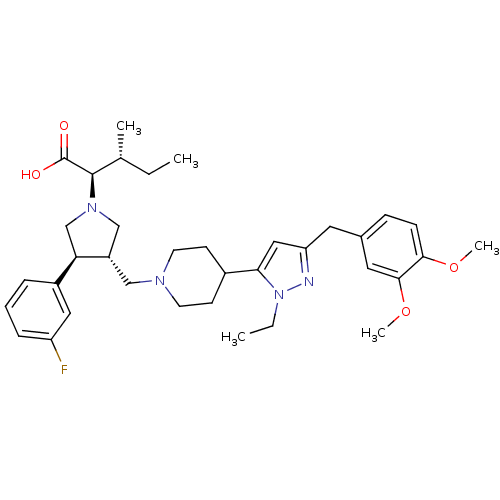
((2R,4R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC)c(OC)c3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C36H49FN4O4/c1-6-24(3)35(36(42)43)40-22-28(31(23-40)27-9-8-10-29(37)19-27)21-39-15-13-26(14-16-39)32-20-30(38-41(32)7-2)17-25-11-12-33(44-4)34(18-25)45-5/h8-12,18-20,24,26,28,31,35H,6-7,13-17,21-23H2,1-5H3,(H,42,43)/t24-,28+,31-,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037527
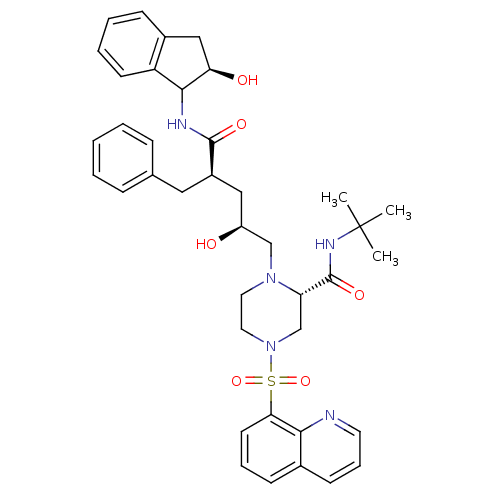
((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)NC1[C@H](O)Cc2ccccc12)S(=O)(=O)c1cccc2cccnc12 Show InChI InChI=1S/C39H47N5O6S/c1-39(2,3)42-38(48)32-25-44(51(49,50)34-17-9-14-27-15-10-18-40-35(27)34)20-19-43(32)24-30(45)22-29(21-26-11-5-4-6-12-26)37(47)41-36-31-16-8-7-13-28(31)23-33(36)46/h4-18,29-30,32-33,36,45-46H,19-25H2,1-3H3,(H,41,47)(H,42,48)/t29-,30+,32+,33-,36?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Evaluated for the inhibition of HIV protease |
J Med Chem 37: 3443-51 (1994)
BindingDB Entry DOI: 10.7270/Q24J0D5K |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9113
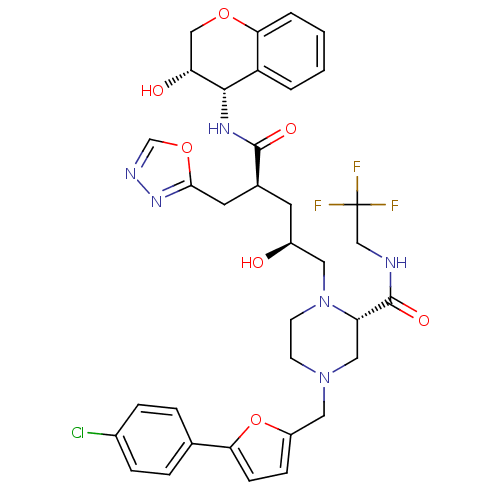
((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...)Show SMILES O[C@@H](C[C@@H](Cc1nnco1)C(=O)N[C@@H]1[C@H](O)COc2ccccc12)CN1CCN(Cc2ccc(o2)-c2ccc(Cl)cc2)C[C@H]1C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C35H38ClF3N6O7/c36-23-7-5-21(6-8-23)29-10-9-25(52-29)16-44-11-12-45(27(17-44)34(49)40-19-35(37,38)39)15-24(46)13-22(14-31-43-41-20-51-31)33(48)42-32-26-3-1-2-4-30(26)50-18-28(32)47/h1-10,20,22,24,27-28,32,46-47H,11-19H2,(H,40,49)(H,42,48)/t22-,24-,27-,28+,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 14: 4651-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.092
BindingDB Entry DOI: 10.7270/Q22Z13QX |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283729
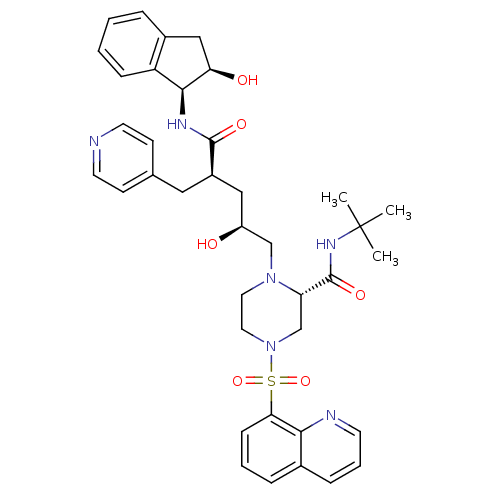
((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(CCN1C[C@@H](O)C[C@@H](Cc1ccncc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)S(=O)(=O)c1cccc2cccnc12 Show InChI InChI=1S/C38H46N6O6S/c1-38(2,3)42-37(48)31-24-44(51(49,50)33-12-6-9-26-10-7-15-40-34(26)33)19-18-43(31)23-29(45)21-28(20-25-13-16-39-17-14-25)36(47)41-35-30-11-5-4-8-27(30)22-32(35)46/h4-17,28-29,31-32,35,45-46H,18-24H2,1-3H3,(H,41,47)(H,42,48)/t28-,29+,31+,32-,35+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Human Immunodeficiency virus-1 protease |
Bioorg Med Chem Lett 4: 2769-2774 (1994)
Article DOI: 10.1016/S0960-894X(01)80592-8
BindingDB Entry DOI: 10.7270/Q25B02F8 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9114
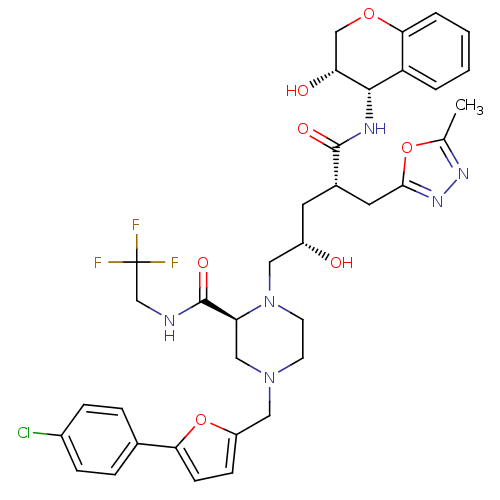
((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...)Show SMILES Cc1nnc(C[C@H](C[C@H](O)CN2CCN(Cc3ccc(o3)-c3ccc(Cl)cc3)C[C@H]2C(=O)NCC(F)(F)F)C(=O)N[C@@H]2[C@H](O)COc3ccccc23)o1 |r| Show InChI InChI=1S/C36H40ClF3N6O7/c1-21-43-44-32(52-21)15-23(34(49)42-33-27-4-2-3-5-31(27)51-19-29(33)48)14-25(47)16-46-13-12-45(18-28(46)35(50)41-20-36(38,39)40)17-26-10-11-30(53-26)22-6-8-24(37)9-7-22/h2-11,23,25,28-29,33,47-48H,12-20H2,1H3,(H,41,50)(H,42,49)/t23-,25-,28-,29+,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 14: 4651-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.092
BindingDB Entry DOI: 10.7270/Q22Z13QX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9109
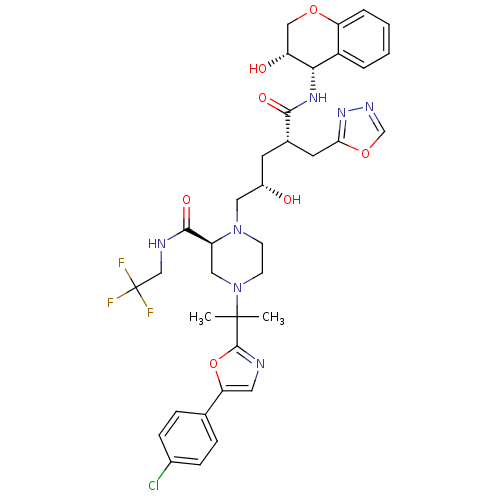
((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...)Show SMILES CC(C)(N1CCN(C[C@@H](O)C[C@@H](Cc2nnco2)C(=O)N[C@@H]2[C@H](O)COc3ccccc23)[C@@H](C1)C(=O)NCC(F)(F)F)c1ncc(o1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C36H41ClF3N7O7/c1-35(2,34-41-15-29(54-34)21-7-9-23(37)10-8-21)47-12-11-46(26(17-47)33(51)42-19-36(38,39)40)16-24(48)13-22(14-30-45-43-20-53-30)32(50)44-31-25-5-3-4-6-28(25)52-18-27(31)49/h3-10,15,20,22,24,26-27,31,48-49H,11-14,16-19H2,1-2H3,(H,42,51)(H,44,50)/t22-,24-,26-,27+,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 14: 4651-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.092
BindingDB Entry DOI: 10.7270/Q22Z13QX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9115
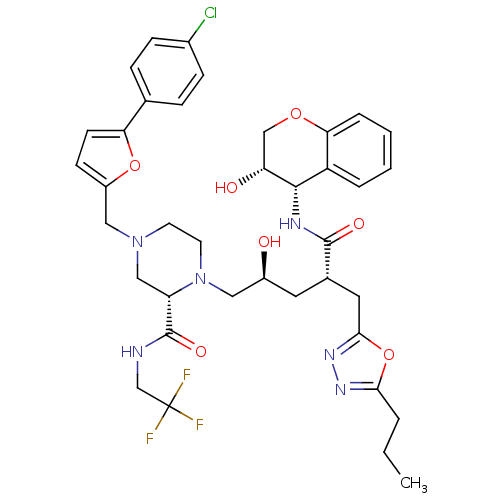
((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...)Show SMILES CCCc1nnc(C[C@H](C[C@H](O)CN2CCN(Cc3ccc(o3)-c3ccc(Cl)cc3)C[C@H]2C(=O)NCC(F)(F)F)C(=O)N[C@@H]2[C@H](O)COc3ccccc23)o1 |r| Show InChI InChI=1S/C38H44ClF3N6O7/c1-2-5-33-45-46-34(55-33)17-24(36(51)44-35-28-6-3-4-7-32(28)53-21-30(35)50)16-26(49)18-48-15-14-47(20-29(48)37(52)43-22-38(40,41)42)19-27-12-13-31(54-27)23-8-10-25(39)11-9-23/h3-4,6-13,24,26,29-30,35,49-50H,2,5,14-22H2,1H3,(H,43,52)(H,44,51)/t24-,26-,29-,30+,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 14: 4651-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.092
BindingDB Entry DOI: 10.7270/Q22Z13QX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9111
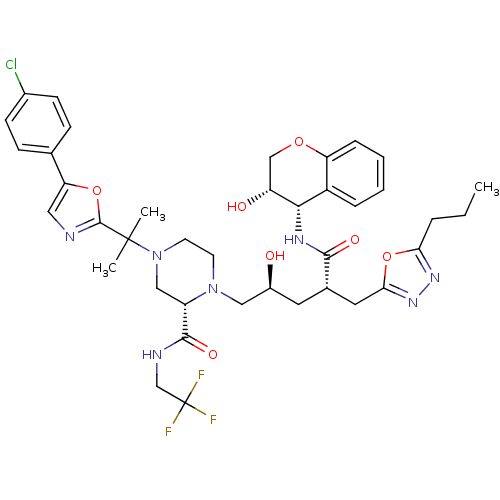
((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...)Show SMILES CCCc1nnc(C[C@H](C[C@H](O)CN2CCN(C[C@H]2C(=O)NCC(F)(F)F)C(C)(C)c2ncc(o2)-c2ccc(Cl)cc2)C(=O)N[C@@H]2[C@H](O)COc3ccccc23)o1 |r| Show InChI InChI=1S/C39H47ClF3N7O7/c1-4-7-32-47-48-33(57-32)17-24(35(53)46-34-27-8-5-6-9-30(27)55-21-29(34)52)16-26(51)19-49-14-15-50(20-28(49)36(54)45-22-39(41,42)43)38(2,3)37-44-18-31(56-37)23-10-12-25(40)13-11-23/h5-6,8-13,18,24,26,28-29,34,51-52H,4,7,14-17,19-22H2,1-3H3,(H,45,54)(H,46,53)/t24-,26-,28-,29+,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 14: 4651-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.092
BindingDB Entry DOI: 10.7270/Q22Z13QX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9112
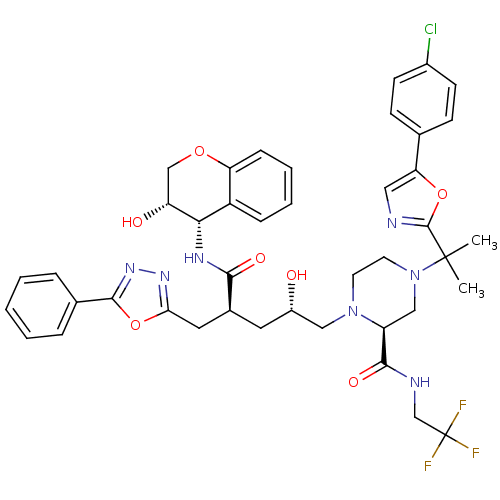
((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...)Show SMILES CC(C)(N1CCN(C[C@@H](O)C[C@@H](Cc2nnc(o2)-c2ccccc2)C(=O)N[C@@H]2[C@H](O)COc3ccccc23)[C@@H](C1)C(=O)NCC(F)(F)F)c1ncc(o1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C42H45ClF3N7O7/c1-41(2,40-47-20-34(59-40)25-12-14-28(43)15-13-25)53-17-16-52(31(22-53)38(57)48-24-42(44,45)46)21-29(54)18-27(19-35-50-51-39(60-35)26-8-4-3-5-9-26)37(56)49-36-30-10-6-7-11-33(30)58-23-32(36)55/h3-15,20,27,29,31-32,36,54-55H,16-19,21-24H2,1-2H3,(H,48,57)(H,49,56)/t27-,29-,31-,32+,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 14: 4651-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.092
BindingDB Entry DOI: 10.7270/Q22Z13QX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9107
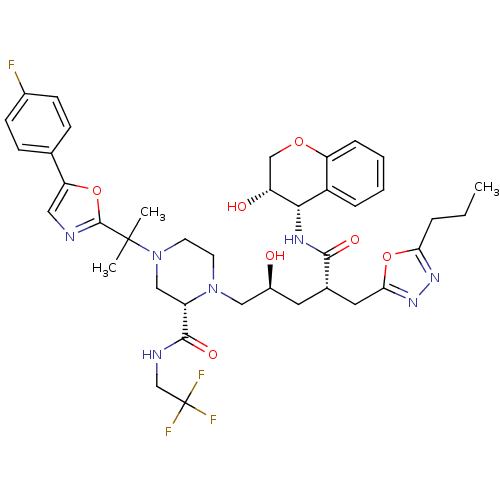
((2S)-4-{2-[5-(4-fluorophenyl)-1,3-oxazol-2-yl]prop...)Show SMILES CCCc1nnc(C[C@H](C[C@H](O)CN2CCN(C[C@H]2C(=O)NCC(F)(F)F)C(C)(C)c2ncc(o2)-c2ccc(F)cc2)C(=O)N[C@@H]2[C@H](O)COc3ccccc23)o1 |r| Show InChI InChI=1S/C39H47F4N7O7/c1-4-7-32-47-48-33(57-32)17-24(35(53)46-34-27-8-5-6-9-30(27)55-21-29(34)52)16-26(51)19-49-14-15-50(20-28(49)36(54)45-22-39(41,42)43)38(2,3)37-44-18-31(56-37)23-10-12-25(40)13-11-23/h5-6,8-13,18,24,26,28-29,34,51-52H,4,7,14-17,19-22H2,1-3H3,(H,45,54)(H,46,53)/t24-,26-,28-,29+,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 14: 4651-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.092
BindingDB Entry DOI: 10.7270/Q22Z13QX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9110
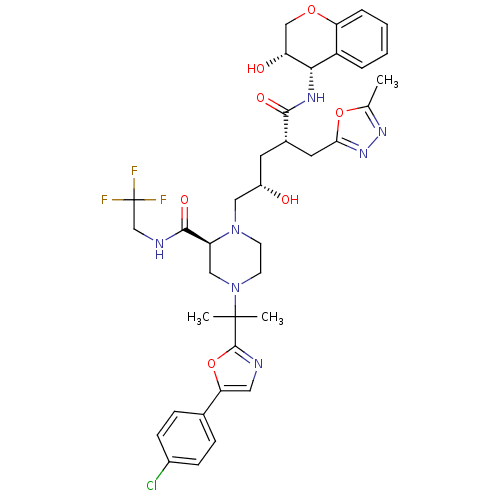
((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...)Show SMILES Cc1nnc(C[C@H](C[C@H](O)CN2CCN(C[C@H]2C(=O)NCC(F)(F)F)C(C)(C)c2ncc(o2)-c2ccc(Cl)cc2)C(=O)N[C@@H]2[C@H](O)COc3ccccc23)o1 |r| Show InChI InChI=1S/C37H43ClF3N7O7/c1-21-45-46-31(54-21)15-23(33(51)44-32-26-6-4-5-7-29(26)53-19-28(32)50)14-25(49)17-47-12-13-48(18-27(47)34(52)43-20-37(39,40)41)36(2,3)35-42-16-30(55-35)22-8-10-24(38)11-9-22/h4-11,16,23,25,27-28,32,49-50H,12-15,17-20H2,1-3H3,(H,43,52)(H,44,51)/t23-,25-,27-,28+,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 14: 4651-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.092
BindingDB Entry DOI: 10.7270/Q22Z13QX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9116

((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...)Show SMILES O[C@@H](C[C@@H](Cc1nnc(o1)-c1ccccc1)C(=O)N[C@@H]1[C@H](O)COc2ccccc12)CN1CCN(Cc2ccc(o2)-c2ccc(Cl)cc2)C[C@H]1C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C41H42ClF3N6O7/c42-28-12-10-25(11-13-28)34-15-14-30(57-34)21-50-16-17-51(32(22-50)39(55)46-24-41(43,44)45)20-29(52)18-27(19-36-48-49-40(58-36)26-6-2-1-3-7-26)38(54)47-37-31-8-4-5-9-35(31)56-23-33(37)53/h1-15,27,29,32-33,37,52-53H,16-24H2,(H,46,55)(H,47,54)/t27-,29-,32-,33+,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 14: 4651-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.092
BindingDB Entry DOI: 10.7270/Q22Z13QX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,Q496K]
(Human immunodeficiency virus type 1) | BDBM9121

((2S)-1-[(2S,4R)-2-hydroxy-4-{[(3S,4S)-3-hydroxy-3,...)Show SMILES O[C@@H](C[C@@H](Cc1cccnc1)C(=O)N[C@@H]1[C@H](O)COc2ccccc12)CN1CCN(Cc2ccc(o2)-c2ccccc2)C[C@H]1C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C38H42F3N5O6/c39-38(40,41)24-43-37(50)31-22-45(21-29-12-13-33(52-29)26-8-2-1-3-9-26)15-16-46(31)20-28(47)18-27(17-25-7-6-14-42-19-25)36(49)44-35-30-10-4-5-11-34(30)51-23-32(35)48/h1-14,19,27-28,31-32,35,47-48H,15-18,20-24H2,(H,43,50)(H,44,49)/t27-,28+,31+,32-,35+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Bioorg Med Chem Lett 13: 4027-30 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.049
BindingDB Entry DOI: 10.7270/Q2Z60M7Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data