 Found 281 hits with Last Name = 'emmerling' and Initial = 'mr'
Found 281 hits with Last Name = 'emmerling' and Initial = 'mr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8986
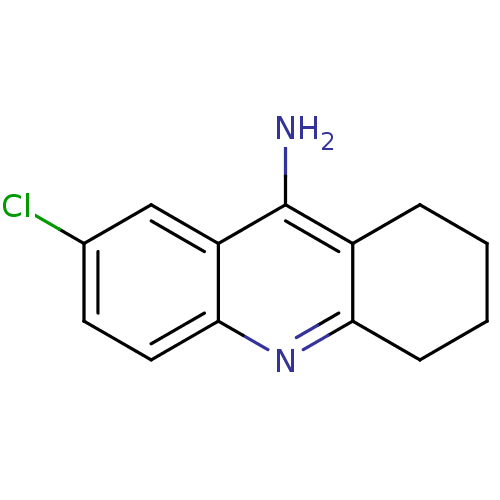
(7-chloro-1,2,3,4-tetrahydroacridin-9-amine | Tacri...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-12-10(7-8)13(15)9-3-1-2-4-11(9)16-12/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50060470
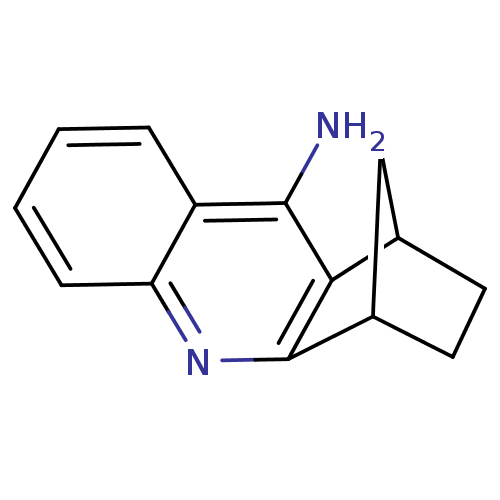
(10-azatetracyclo[10.2.1.02,11.04,9]pentadeca-2,4,6...)Show InChI InChI=1S/C14H14N2/c15-13-10-3-1-2-4-11(10)16-14-9-6-5-8(7-9)12(13)14/h1-4,8-9H,5-7H2,(H2,15,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987
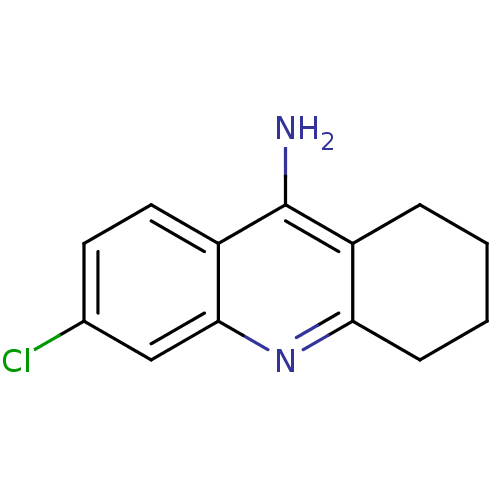
(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50280625
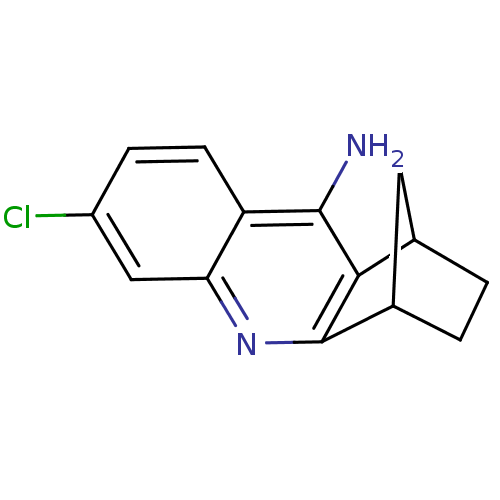
(7-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-3-4-10-11(6-9)17-14-8-2-1-7(5-8)12(14)13(10)16/h3-4,6-8H,1-2,5H2,(H2,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50279984

(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13ClN2/c14-9-5-3-7-11-12(9)13(15)8-4-1-2-6-10(8)16-11/h3,5,7H,1-2,4,6H2,(H2,15,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50280625

(7-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-3-4-10-11(6-9)17-14-8-2-1-7(5-8)12(14)13(10)16/h3-4,6-8H,1-2,5H2,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50280627
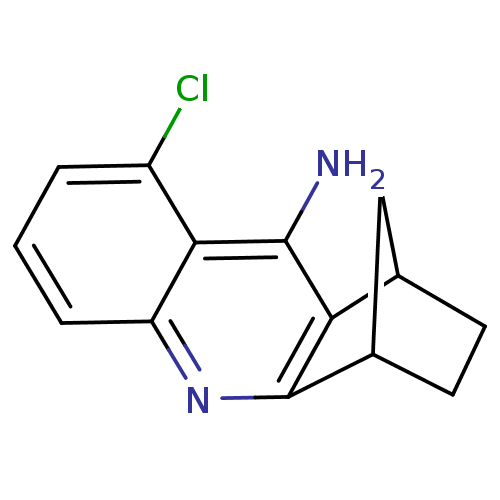
(5-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-2-1-3-10-12(9)13(16)11-7-4-5-8(6-7)14(11)17-10/h1-3,7-8H,4-6H2,(H2,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279984

(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13ClN2/c14-9-5-3-7-11-12(9)13(15)8-4-1-2-6-10(8)16-11/h3,5,7H,1-2,4,6H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50060470

(10-azatetracyclo[10.2.1.02,11.04,9]pentadeca-2,4,6...)Show InChI InChI=1S/C14H14N2/c15-13-10-3-1-2-4-11(10)16-14-9-6-5-8(7-9)12(13)14/h1-4,8-9H,5-7H2,(H2,15,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50279984

(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13ClN2/c14-9-5-3-7-11-12(9)13(15)8-4-1-2-6-10(8)16-11/h3,5,7H,1-2,4,6H2,(H2,15,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50280627

(5-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-2-1-3-10-12(9)13(16)11-7-4-5-8(6-7)14(11)17-10/h1-3,7-8H,4-6H2,(H2,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50280624
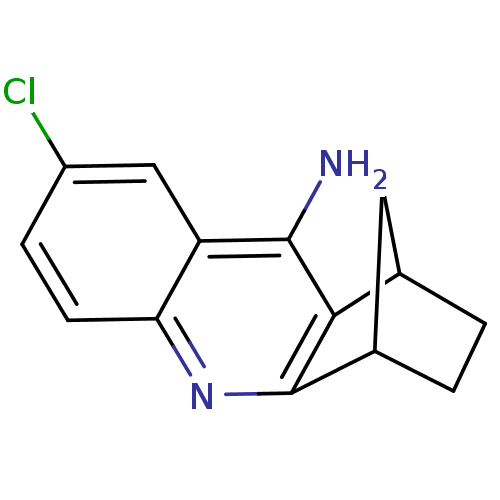
(6-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-3-4-11-10(6-9)13(16)12-7-1-2-8(5-7)14(12)17-11/h3-4,6-8H,1-2,5H2,(H2,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50063698

(4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccc2cc(ccc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C19H17N5O2/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23/h1-10H,(H3,20,21)(H4,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro for inhibition of purified bovine trypsin. |
Bioorg Med Chem Lett 9: 815-20 (1999)
BindingDB Entry DOI: 10.7270/Q29G5M0K |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50063698

(4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccc2cc(ccc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C19H17N5O2/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23/h1-10H,(H3,20,21)(H4,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
The compound was evaluated to inhibit trypsinand is expressed in IC50 (The concentration required to inhibit 50% of the enzyme). |
J Med Chem 41: 1060-7 (1998)
Article DOI: 10.1021/jm970394d
BindingDB Entry DOI: 10.7270/Q2PZ57ZT |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50063698

(4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccc2cc(ccc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C19H17N5O2/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23/h1-10H,(H3,20,21)(H4,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Trypsin. |
Bioorg Med Chem Lett 6: 679-682 (1996)
Article DOI: 10.1016/0960-894X(96)00094-7
BindingDB Entry DOI: 10.7270/Q2P84BVF |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50280625

(7-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-3-4-10-11(6-9)17-14-8-2-1-7(5-8)12(14)13(10)16/h3-4,6-8H,1-2,5H2,(H2,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50060470

(10-azatetracyclo[10.2.1.02,11.04,9]pentadeca-2,4,6...)Show InChI InChI=1S/C14H14N2/c15-13-10-3-1-2-4-11(10)16-14-9-6-5-8(7-9)12(13)14/h1-4,8-9H,5-7H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50280623
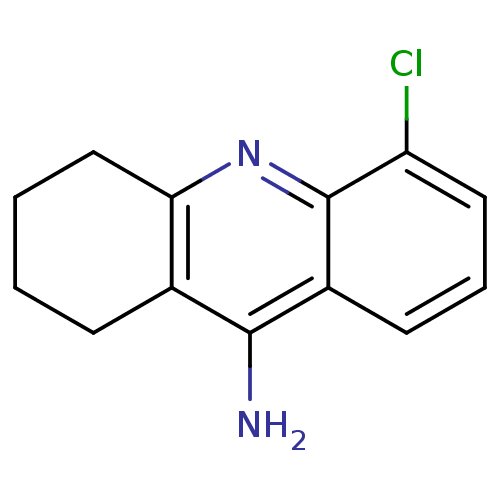
(5-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13ClN2/c14-10-6-3-5-9-12(15)8-4-1-2-7-11(8)16-13(9)10/h3,5-6H,1-2,4,7H2,(H2,15,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8986

(7-chloro-1,2,3,4-tetrahydroacridin-9-amine | Tacri...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-12-10(7-8)13(15)9-3-1-2-4-11(9)16-12/h5-7H,1-4H2,(H2,15,16) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50280627

(5-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-2-1-3-10-12(9)13(16)11-7-4-5-8(6-7)14(11)17-10/h1-3,7-8H,4-6H2,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50280623

(5-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13ClN2/c14-10-6-3-5-9-12(15)8-4-1-2-7-11(8)16-13(9)10/h3,5-6H,1-2,4,7H2,(H2,15,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in rat red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM9347

((2Z)-but-2-enedioic acid; 9-amino-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C13H14N2O/c14-13-8-4-1-2-5-9(8)15-10-6-3-7-11(16)12(10)13/h1-2,4-5,11,16H,3,6-7H2,(H2,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM9347

((2Z)-but-2-enedioic acid; 9-amino-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C13H14N2O/c14-13-8-4-1-2-5-9(8)15-10-6-3-7-11(16)12(10)13/h1-2,4-5,11,16H,3,6-7H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50280623

(5-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13ClN2/c14-10-6-3-5-9-12(15)8-4-1-2-7-11(8)16-13(9)10/h3,5-6H,1-2,4,7H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in monkey red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50280624

(6-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-3-4-11-10(6-9)13(16)12-7-1-2-8(5-7)14(12)17-11/h3-4,6-8H,1-2,5H2,(H2,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in human red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50280626
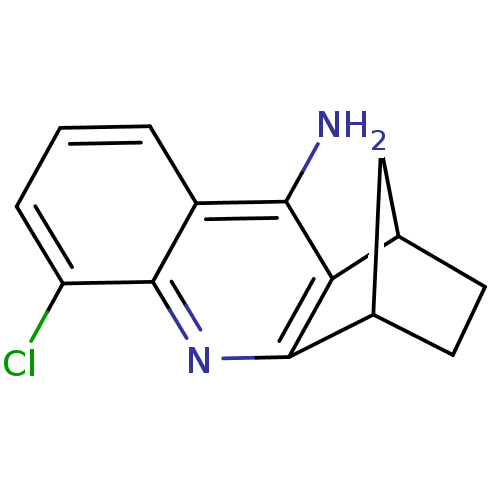
(8-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-10-3-1-2-9-12(16)11-7-4-5-8(6-7)13(11)17-14(9)10/h1-3,7-8H,4-6H2,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50063698

(4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccc2cc(ccc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C19H17N5O2/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23/h1-10H,(H3,20,21)(H4,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against C1s serine protease . |
Bioorg Med Chem Lett 6: 679-682 (1996)
Article DOI: 10.1016/0960-894X(96)00094-7
BindingDB Entry DOI: 10.7270/Q2P84BVF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in mouse red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9347

((2Z)-but-2-enedioic acid; 9-amino-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C13H14N2O/c14-13-8-4-1-2-5-9(8)15-10-6-3-7-11(16)12(10)13/h1-2,4-5,11,16H,3,6-7H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Complement C1r subcomponent
(Homo sapiens (Human)) | BDBM50063701

(2-(2-Iodo-phenylamino)-naphtho[2,3-d][1,3]oxazin-4...)Show InChI InChI=1S/C18H11IN2O2/c19-14-7-3-4-8-15(14)20-18-21-16-10-12-6-2-1-5-11(12)9-13(16)17(22)23-18/h1-10H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate |
J Med Chem 41: 1060-7 (1998)
Article DOI: 10.1021/jm970394d
BindingDB Entry DOI: 10.7270/Q2PZ57ZT |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50280626

(8-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-10-3-1-2-9-12(16)11-7-4-5-8(6-7)13(11)17-14(9)10/h1-3,7-8H,4-6H2,(H2,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of (BChE) Butyrylcholinesterase of horse serum |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50063698

(4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccc2cc(ccc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C19H17N5O2/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23/h1-10H,(H3,20,21)(H4,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against plasmin. |
Bioorg Med Chem Lett 6: 679-682 (1996)
Article DOI: 10.1016/0960-894X(96)00094-7
BindingDB Entry DOI: 10.7270/Q2P84BVF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay |
Bioorg Med Chem Lett 2: 861-864 (1992)
Article DOI: 10.1016/S0960-894X(00)80545-4
BindingDB Entry DOI: 10.7270/Q29886XS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50063698

(4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccc2cc(ccc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C19H17N5O2/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23/h1-10H,(H3,20,21)(H4,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against Thrombin. |
Bioorg Med Chem Lett 6: 679-682 (1996)
Article DOI: 10.1016/0960-894X(96)00094-7
BindingDB Entry DOI: 10.7270/Q2P84BVF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM9347

((2Z)-but-2-enedioic acid; 9-amino-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C13H14N2O/c14-13-8-4-1-2-5-9(8)15-10-6-3-7-11(16)12(10)13/h1-2,4-5,11,16H,3,6-7H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 361 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in rat red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Complement C1r subcomponent
(Homo sapiens (Human)) | BDBM50063745
(2-(2-Iodo-phenylamino)-7-trifluoromethyl-benzo[d][...)Show InChI InChI=1S/C15H8F3IN2O2/c16-15(17,18)8-5-6-9-12(7-8)21-14(23-13(9)22)20-11-4-2-1-3-10(11)19/h1-7H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibitory activity against purified human C1r protease protease |
Bioorg Med Chem Lett 9: 815-20 (1999)
BindingDB Entry DOI: 10.7270/Q29G5M0K |
More data for this
Ligand-Target Pair | |
Complement C1r subcomponent
(Homo sapiens (Human)) | BDBM50063726

(7-Chloro-2-(2,6-dichloro-phenylamino)-benzo[d][1,3...)Show InChI InChI=1S/C14H7Cl3N2O2/c15-7-4-5-8-11(6-7)18-14(21-13(8)20)19-12-9(16)2-1-3-10(12)17/h1-6H,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate |
J Med Chem 41: 1060-7 (1998)
Article DOI: 10.1021/jm970394d
BindingDB Entry DOI: 10.7270/Q2PZ57ZT |
More data for this
Ligand-Target Pair | |
Complement C1r subcomponent
(Homo sapiens (Human)) | BDBM50063745

(2-(2-Iodo-phenylamino)-7-trifluoromethyl-benzo[d][...)Show InChI InChI=1S/C15H8F3IN2O2/c16-15(17,18)8-5-6-9-12(7-8)21-14(23-13(9)22)20-11-4-2-1-3-10(11)19/h1-7H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate |
J Med Chem 41: 1060-7 (1998)
Article DOI: 10.1021/jm970394d
BindingDB Entry DOI: 10.7270/Q2PZ57ZT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50052410

(9-Amino-1,2,3,4-tetrahydro-acridin-4-ol | CHEMBL31...)Show InChI InChI=1S/C13H14N2O/c14-12-8-4-1-2-6-10(8)15-13-9(12)5-3-7-11(13)16/h1-2,4,6,11,16H,3,5,7H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in rat red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data