

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cruzipain (Trypanosoma cruzi) | BDBM50306596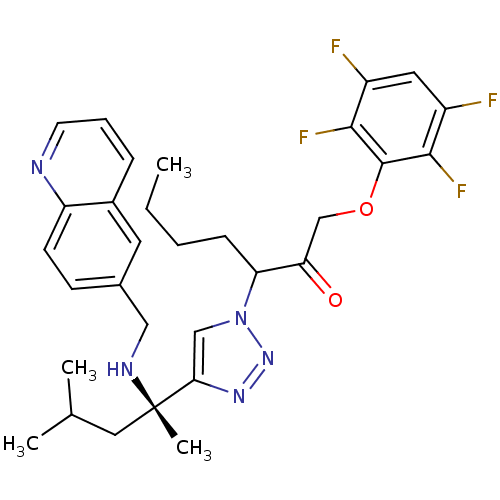 (3-(4-((S)-4-methyl-2-(quinolin-6-ylmethylamino)pen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50306600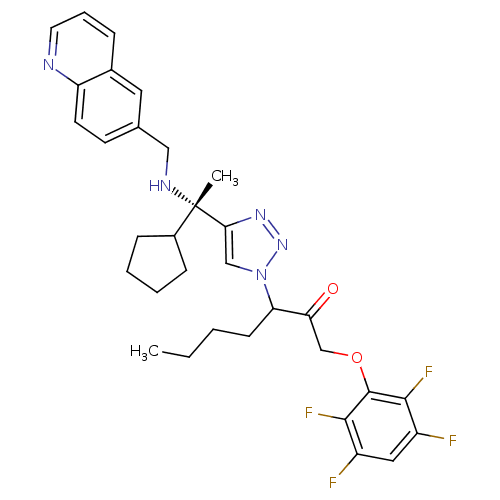 (3-(4-((S)-1-cyclopentyl-1-(quinolin-6-ylmethylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50306598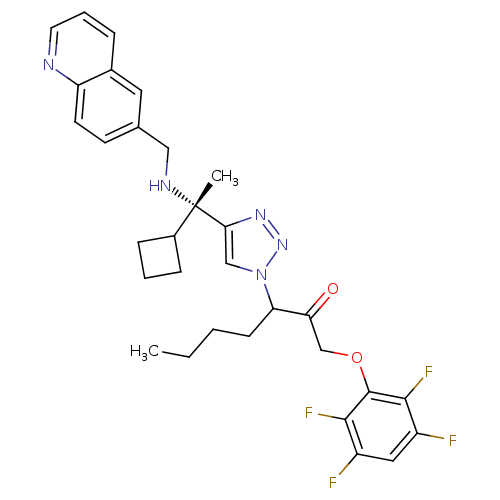 (3-(4-((S)-1-cyclobutyl-1-(quinolin-6-ylmethylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50306597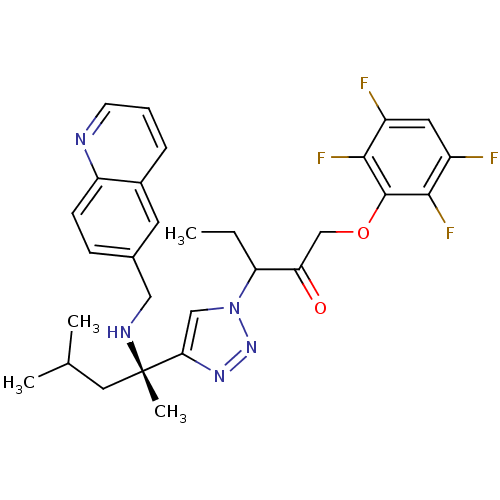 (3-(4-((S)-4-methyl-2-(quinolin-6-ylmethylamino)pen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50306599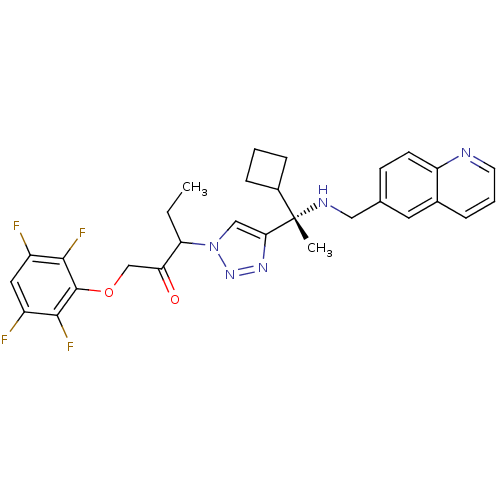 (3-(4-((S)-1-cyclobutyl-1-(quinolin-6-ylmethylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50306592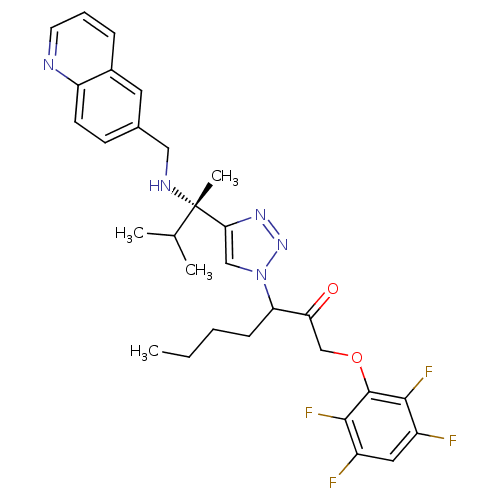 (3-(4-((S)-3-methyl-2-(quinolin-6-ylmethylamino)but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128869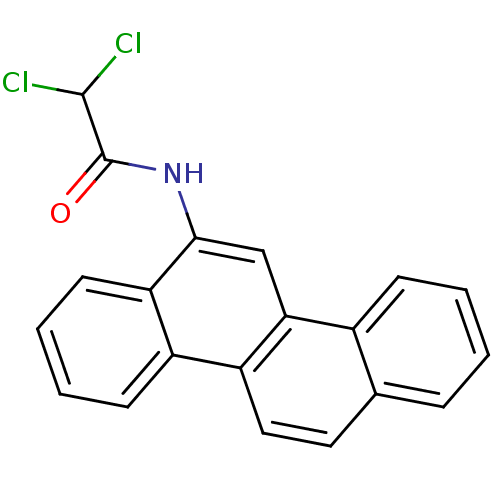 (2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128871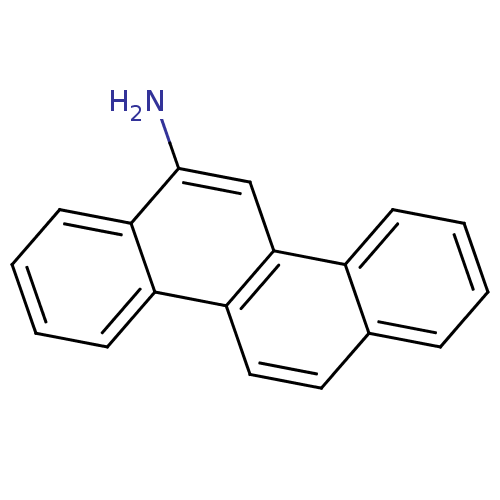 (CHEMBL313154 | Chrysen-6-ylamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50306595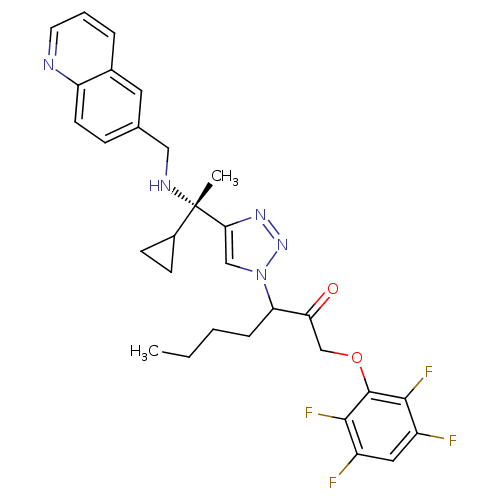 (3-(4-((S)-1-cyclopropyl-1-(quinolin-6-ylmethylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50306593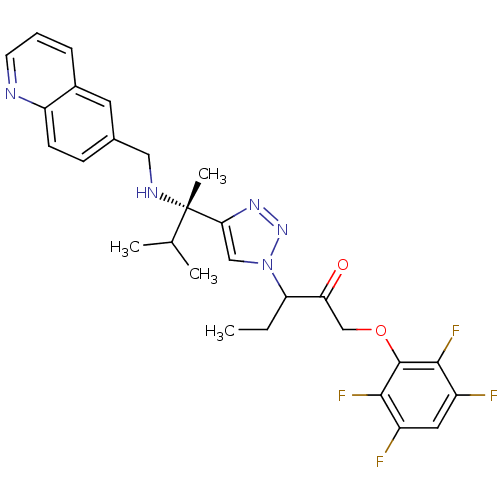 (3-(4-((S)-3-methyl-2-(quinolin-6-ylmethylamino)but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50306601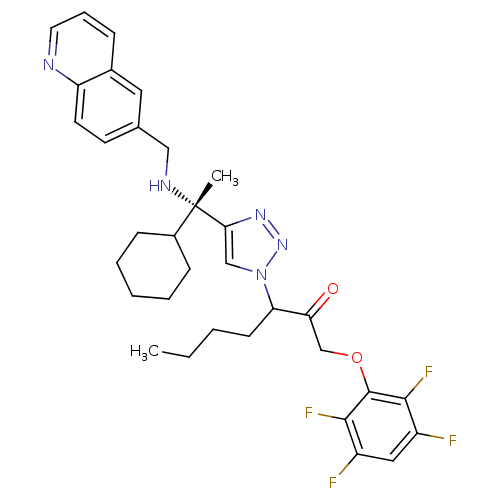 (3-(4-((S)-1-cyclohexyl-1-(quinolin-6-ylmethylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128867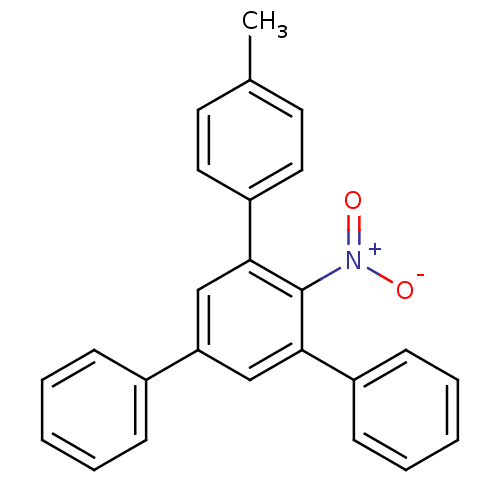 (3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128866 (2-(2,4,7-Trinitro-fluoren-9-ylidene)-malononitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128872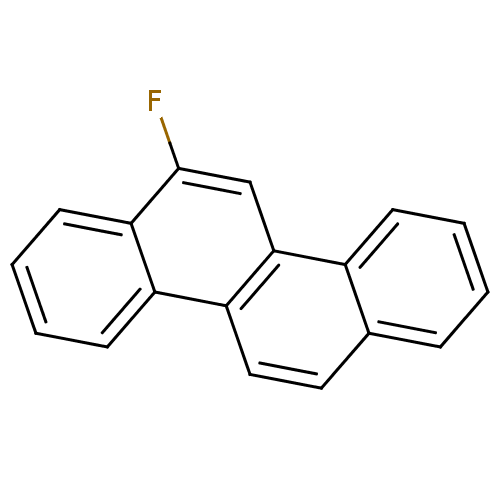 (6-Fluoro-chrysene | CHEMBL83242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128866 (2-(2,4,7-Trinitro-fluoren-9-ylidene)-malononitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128865 (6-Nitro-chrysene | CHEMBL82858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50306594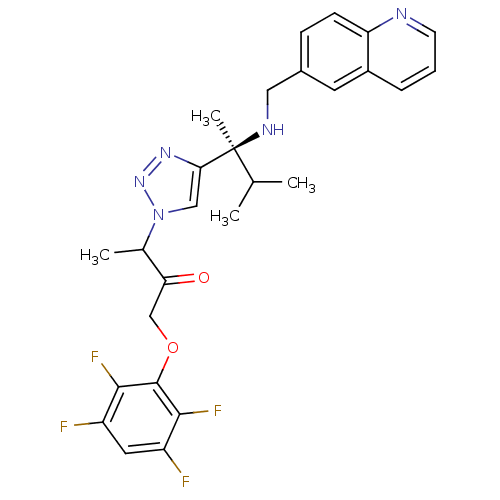 (3-(4-((S)-3-methyl-2-(quinolin-6-ylmethylamino)but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | J Med Chem 53: 1763-73 (2010) Article DOI: 10.1021/jm901633v BindingDB Entry DOI: 10.7270/Q21N8172 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128870 (CHEMBL85685 | chrysene) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 8.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128867 (3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128868 (5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128867 (3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128869 (2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128869 (2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128868 (5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128868 (5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 4.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol 14-alpha demethylase (Trypanosoma cruzi) | BDBM29342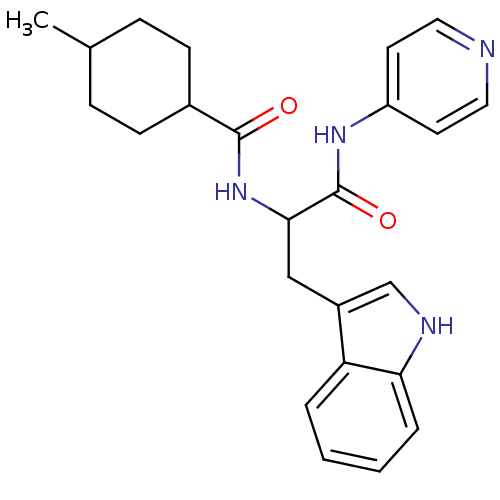 (ChemDiv C155-0123, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity to Trypanosoma cruzi sterol 14alpha-demethylase by UV-Spectrophotometry | Antimicrob Agents Chemother 54: 2480-8 (2010) Article DOI: 10.1128/AAC.00281-10 BindingDB Entry DOI: 10.7270/Q2R49QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||