

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100983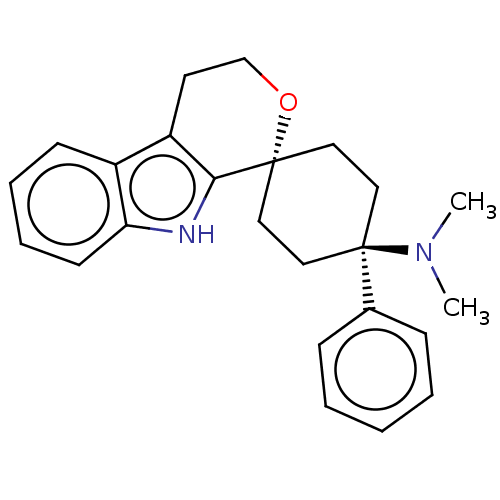 (CHEMBL3326224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239934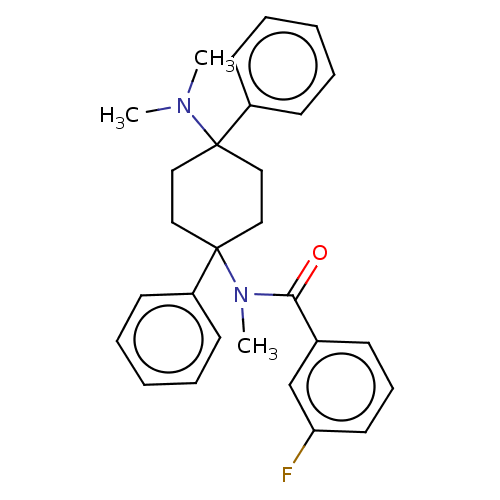 (US9403767, 117) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101099 (CHEMBL3326228) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101306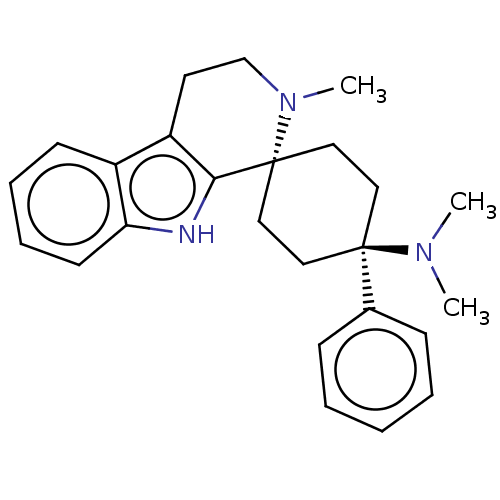 (CHEMBL3326229) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177911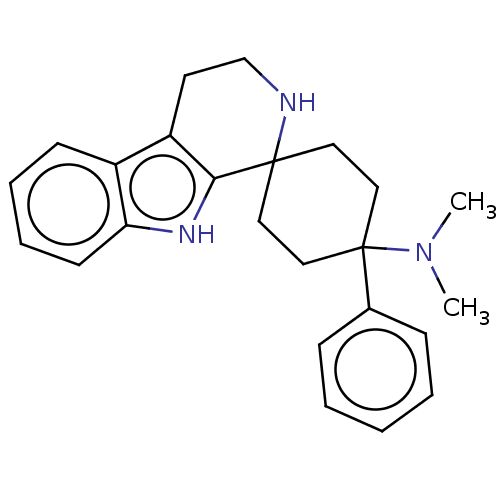 (US9120797, 10 | US9120797, 9) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.260 | -54.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50100983 (CHEMBL3326224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101088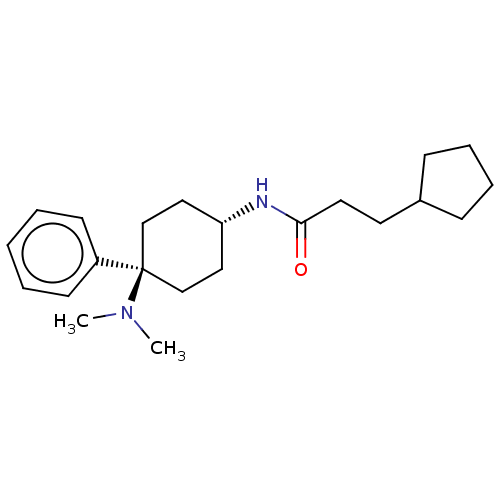 (CHEMBL3326220) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100983 (CHEMBL3326224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50316622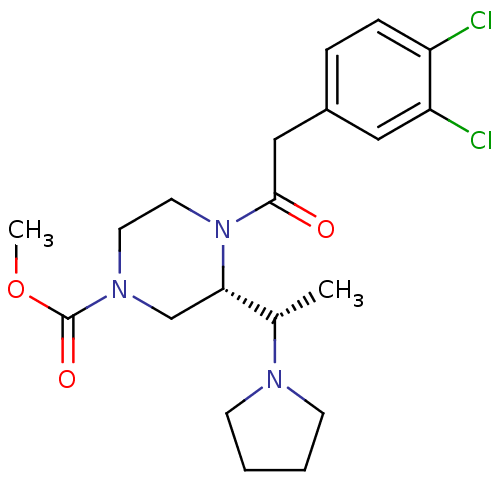 ((S)-methyl 4-(2-(3,4-dichlorophenyl)acetyl)-3-((S)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westf£lische Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs by scintillation counting analysis | J Med Chem 57: 6845-60 (2014) Article DOI: 10.1021/jm500940q BindingDB Entry DOI: 10.7270/Q2G73GDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000296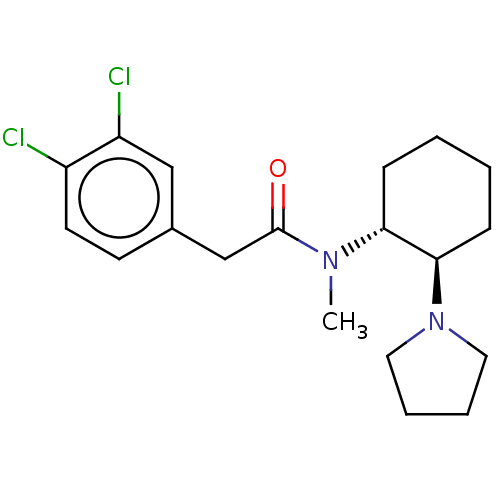 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membrane after 150 mins by scintillation counting | J Med Chem 53: 4212-22 (2010) Article DOI: 10.1021/jm100182p BindingDB Entry DOI: 10.7270/Q2PZ59RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50316622 ((S)-methyl 4-(2-(3,4-dichlorophenyl)acetyl)-3-((S)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membrane after 150 mins by scintillation counting | J Med Chem 53: 4212-22 (2010) Article DOI: 10.1021/jm100182p BindingDB Entry DOI: 10.7270/Q2PZ59RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551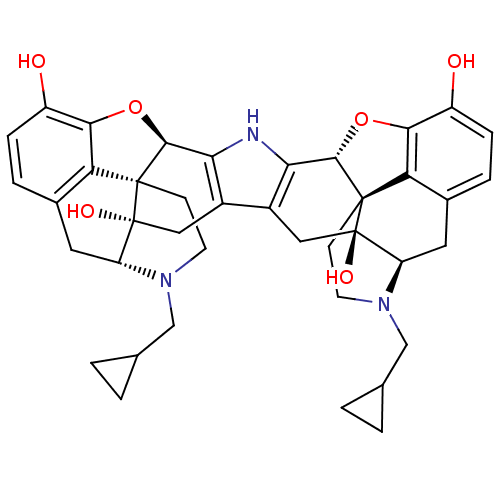 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Agonist activity at human recombinant kappa opioid receptor expressed in HEK293 cells assessed as stimulation of [35S]GTPgammaS binding after 45 mins... | J Med Chem 53: 4212-22 (2010) Article DOI: 10.1021/jm100182p BindingDB Entry DOI: 10.7270/Q2PZ59RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westf£lische Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs by scintillation counting analysis | J Med Chem 57: 6845-60 (2014) Article DOI: 10.1021/jm500940q BindingDB Entry DOI: 10.7270/Q2G73GDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239934 (US9403767, 117) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | -54.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101152 (CHEMBL3326232) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177911 (US9120797, 10 | US9120797, 9) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.360 | -53.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027433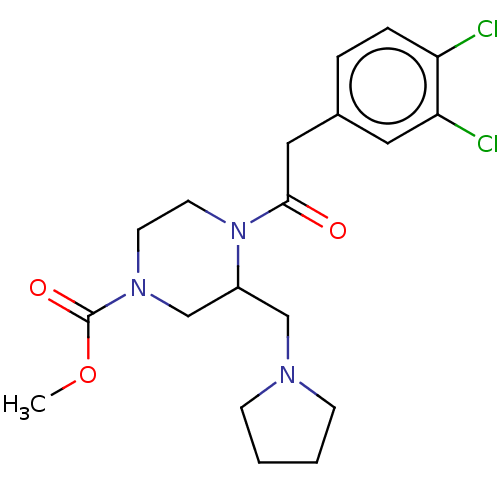 (CHEMBL603370 | GR-89696) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [(3)H]U69593 from mu opioid receptor | J Med Chem 53: 4212-22 (2010) Article DOI: 10.1021/jm100182p BindingDB Entry DOI: 10.7270/Q2PZ59RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101099 (CHEMBL3326228) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101091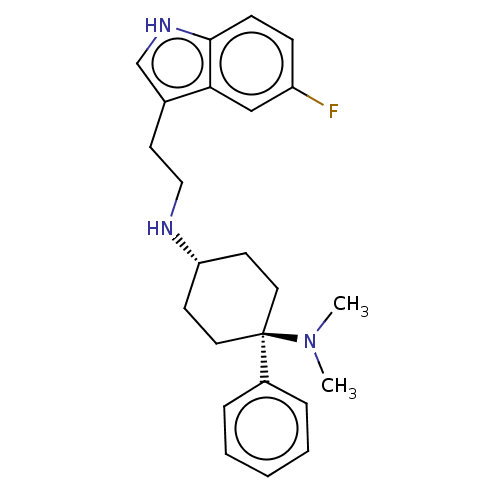 (CHEMBL3326223) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239911 (US9403767, 76 | US9403767, 77) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101096 (CHEMBL3325961) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101088 (CHEMBL3326220) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100991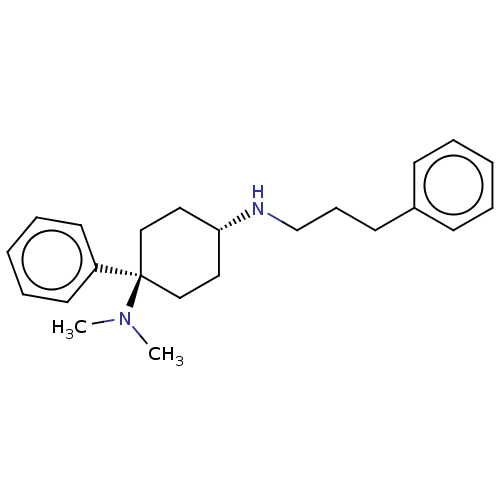 (CHEMBL3325879) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177935 (US9120797, 33) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177935 (US9120797, 33) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239921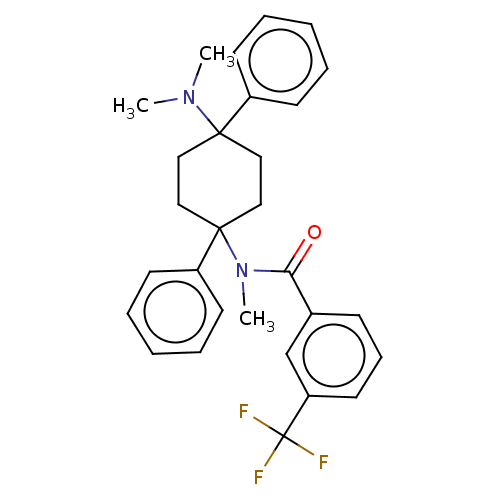 (US9403767, 99) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101306 (CHEMBL3326229) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177955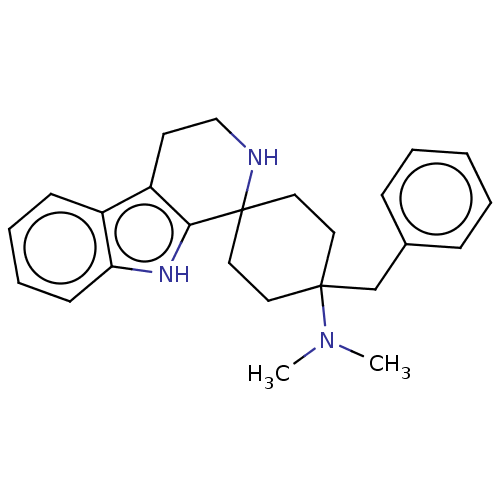 (US9120797, 53) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50100983 (CHEMBL3326224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239925 (US9403767, 104 | US9403767, 105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101095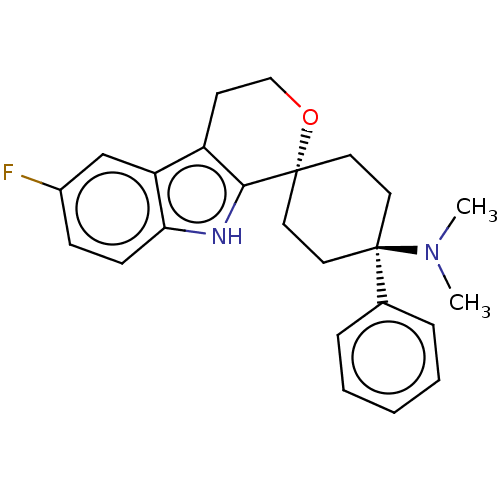 (CHEMBL3325957) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177936 (US9120797, 34) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50100993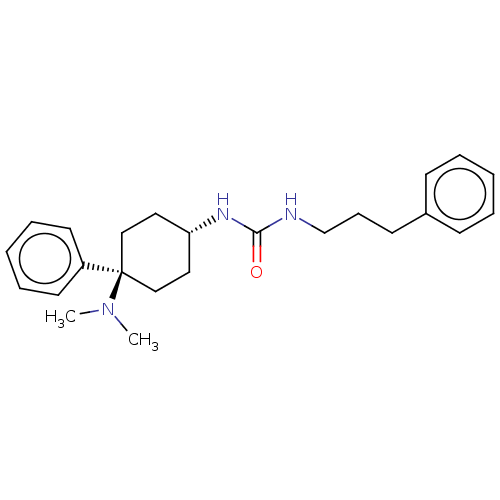 (CHEMBL3325881) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239911 (US9403767, 76 | US9403767, 77) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239914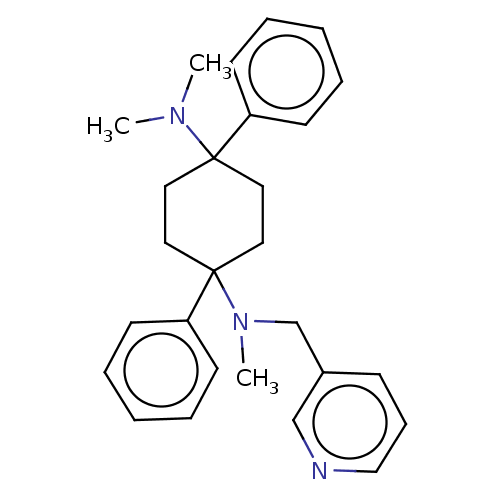 (US9403767, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177948 (US9120797, 46) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101095 (CHEMBL3325957) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50381677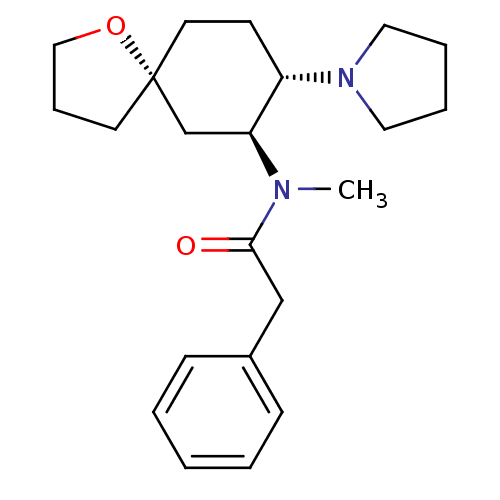 (CHEMBL1256748 | U-69593) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westf£lische Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs by scintillation counting analysis | J Med Chem 57: 6845-60 (2014) Article DOI: 10.1021/jm500940q BindingDB Entry DOI: 10.7270/Q2G73GDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50381677 (CHEMBL1256748 | U-69593) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membrane after 150 mins by scintillation counting | J Med Chem 53: 4212-22 (2010) Article DOI: 10.1021/jm100182p BindingDB Entry DOI: 10.7270/Q2PZ59RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50316615 ((+)-Methyl(1S,2R,5R)-8-[2-(3,4-Dichlorophenyl)acet...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membrane after 150 mins by scintillation counting | J Med Chem 53: 4212-22 (2010) Article DOI: 10.1021/jm100182p BindingDB Entry DOI: 10.7270/Q2PZ59RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101096 (CHEMBL3325961) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101152 (CHEMBL3326232) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101100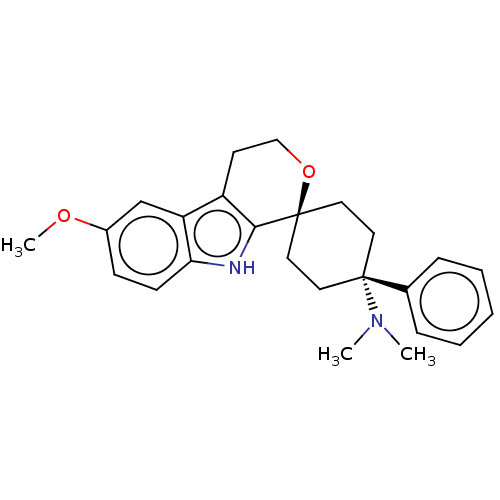 (CHEMBL3325962) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177947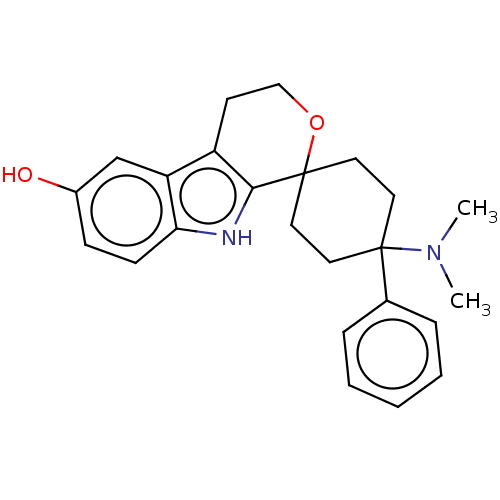 (US9120797, 45) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177955 (US9120797, 53) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177934 (US9120797, 32) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239867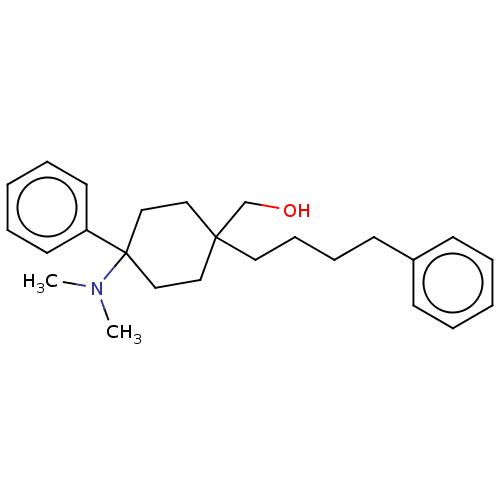 (US9403767, 20 | US9403767, 21) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 528 total ) | Next | Last >> |