 Found 357 hits with Last Name = 'epemolu' and Initial = 'o'
Found 357 hits with Last Name = 'epemolu' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50416472

(CHEMBL1209708)Show SMILES COc1cccc2c(cn(CC3CCCCC3)c12)C(=O)N1C[C@H](C)N(C)[C@H](C)C1 |r| Show InChI InChI=1S/C24H35N3O2/c1-17-13-27(14-18(2)25(17)3)24(28)21-16-26(15-19-9-6-5-7-10-19)23-20(21)11-8-12-22(23)29-4/h8,11-12,16-19H,5-7,9-10,13-15H2,1-4H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416116

(CHEMBL1084315)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CCCNCC2)c2ccccc12 Show InChI InChI=1S/C25H27N3O4S/c1-32-23-11-12-24(20-9-3-2-8-19(20)23)33(30,31)28-17-21(18-7-4-5-10-22(18)28)25(29)27-15-6-13-26-14-16-27/h2-5,7-12,21,26H,6,13-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50416472

(CHEMBL1209708)Show SMILES COc1cccc2c(cn(CC3CCCCC3)c12)C(=O)N1C[C@H](C)N(C)[C@H](C)C1 |r| Show InChI InChI=1S/C24H35N3O2/c1-17-13-27(14-18(2)25(17)3)24(28)21-16-26(15-19-9-6-5-7-10-19)23-20(21)11-8-12-22(23)29-4/h8,11-12,16-19H,5-7,9-10,13-15H2,1-4H3/t17-,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50417843
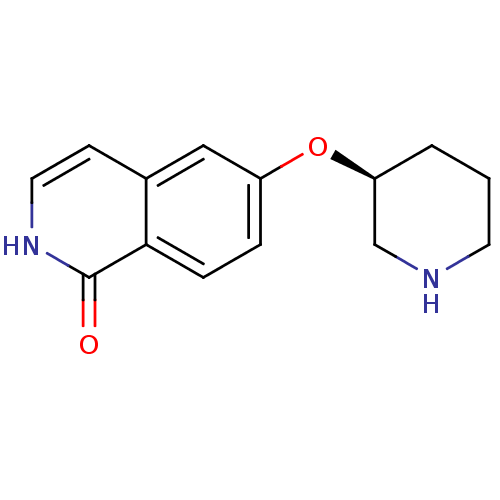
(CHEMBL1667967)Show InChI InChI=1S/C14H16N2O2/c17-14-13-4-3-11(8-10(13)5-7-16-14)18-12-2-1-6-15-9-12/h3-5,7-8,12,15H,1-2,6,9H2,(H,16,17)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Binding affinity to ROCK1 |
Bioorg Med Chem Lett 21: 1084-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.104
BindingDB Entry DOI: 10.7270/Q2DN468G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418012

(CHEMBL1682272)Show SMILES CN(CC(N)=O)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-25(11-17(22)27)12-18-23-20(24-29-18)15-10-26(9-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,10,13H,5-9,11-12H2,1H3,(H2,22,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416120
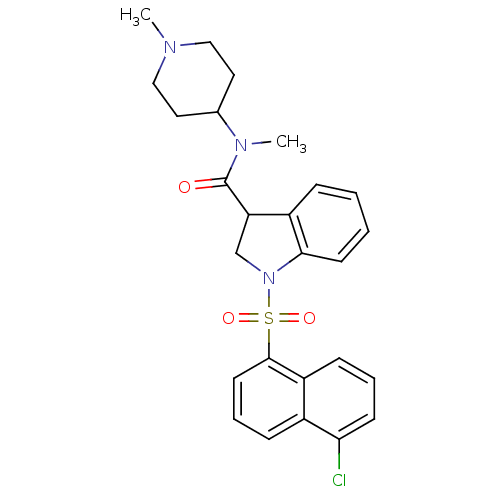
(CHEMBL1082484)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2c(Cl)cccc12 Show InChI InChI=1S/C26H28ClN3O3S/c1-28-15-13-18(14-16-28)29(2)26(31)22-17-30(24-11-4-3-7-20(22)24)34(32,33)25-12-6-8-19-21(25)9-5-10-23(19)27/h3-12,18,22H,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50418012

(CHEMBL1682272)Show SMILES CN(CC(N)=O)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-25(11-17(22)27)12-18-23-20(24-29-18)15-10-26(9-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,10,13H,5-9,11-12H2,1H3,(H2,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416114
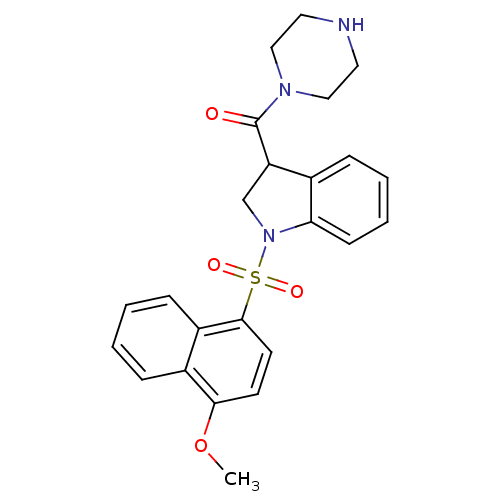
(CHEMBL1085608)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CCNCC2)c2ccccc12 Show InChI InChI=1S/C24H25N3O4S/c1-31-22-10-11-23(19-8-3-2-7-18(19)22)32(29,30)27-16-20(17-6-4-5-9-21(17)27)24(28)26-14-12-25-13-15-26/h2-11,20,25H,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416108
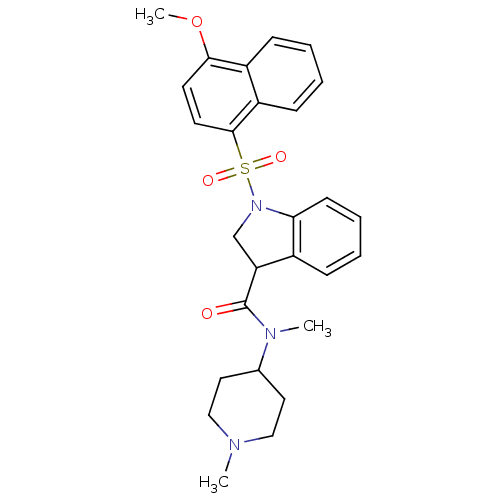
(CHEMBL1082818)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N(C)C2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C27H31N3O4S/c1-28-16-14-19(15-17-28)29(2)27(31)23-18-30(24-11-7-6-8-20(23)24)35(32,33)26-13-12-25(34-3)21-9-4-5-10-22(21)26/h4-13,19,23H,14-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416131
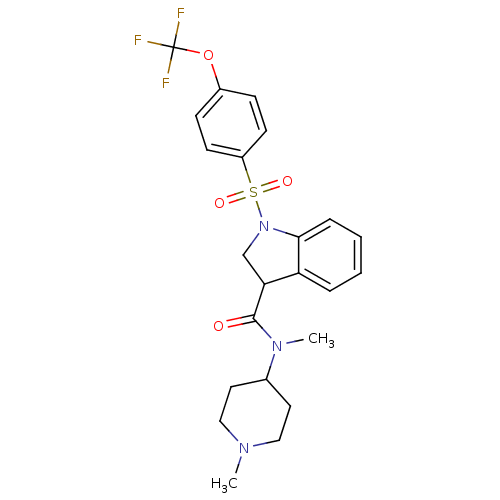
(CHEMBL1085965)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H26F3N3O4S/c1-27-13-11-16(12-14-27)28(2)22(30)20-15-29(21-6-4-3-5-19(20)21)34(31,32)18-9-7-17(8-10-18)33-23(24,25)26/h3-10,16,20H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416117
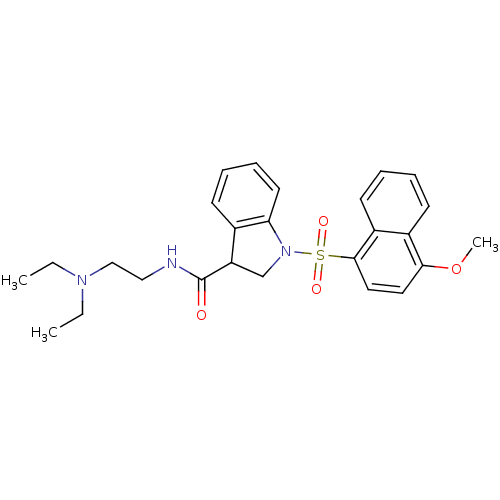
(CHEMBL1084316)Show SMILES CCN(CC)CCNC(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC)c2ccccc12 Show InChI InChI=1S/C26H31N3O4S/c1-4-28(5-2)17-16-27-26(30)22-18-29(23-13-9-8-10-19(22)23)34(31,32)25-15-14-24(33-3)20-11-6-7-12-21(20)25/h6-15,22H,4-5,16-18H2,1-3H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14029
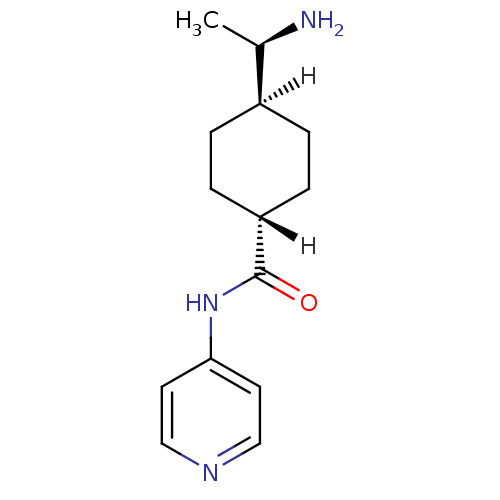
((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of ROCK-1 |
Bioorg Med Chem Lett 21: 97-101 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.060
BindingDB Entry DOI: 10.7270/Q29W0GQK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416130
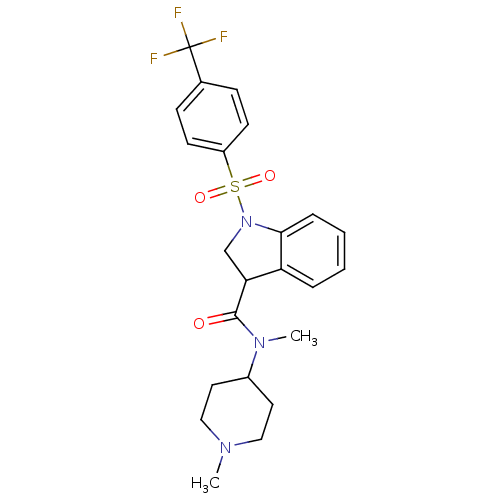
(CHEMBL1083909)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H26F3N3O3S/c1-27-13-11-17(12-14-27)28(2)22(30)20-15-29(21-6-4-3-5-19(20)21)33(31,32)18-9-7-16(8-10-18)23(24,25)26/h3-10,17,20H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416122
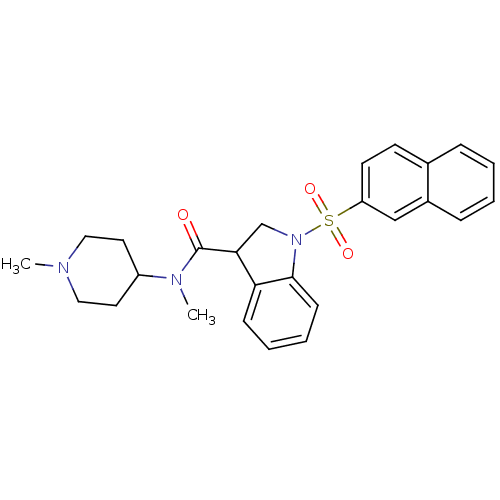
(CHEMBL1085351)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H29N3O3S/c1-27-15-13-21(14-16-27)28(2)26(30)24-18-29(25-10-6-5-9-23(24)25)33(31,32)22-12-11-19-7-3-4-8-20(19)17-22/h3-12,17,21,24H,13-16,18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416132
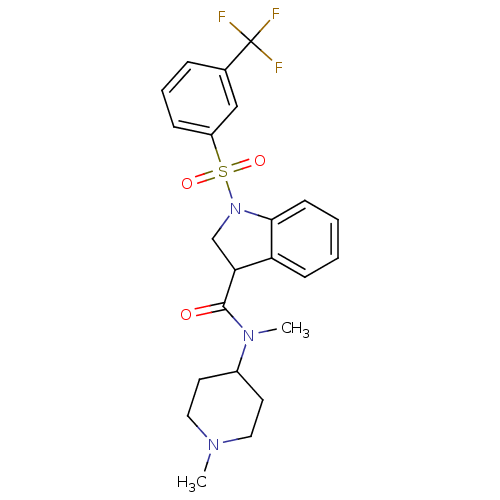
(CHEMBL1083297)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H26F3N3O3S/c1-27-12-10-17(11-13-27)28(2)22(30)20-15-29(21-9-4-3-8-19(20)21)33(31,32)18-7-5-6-16(14-18)23(24,25)26/h3-9,14,17,20H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416109
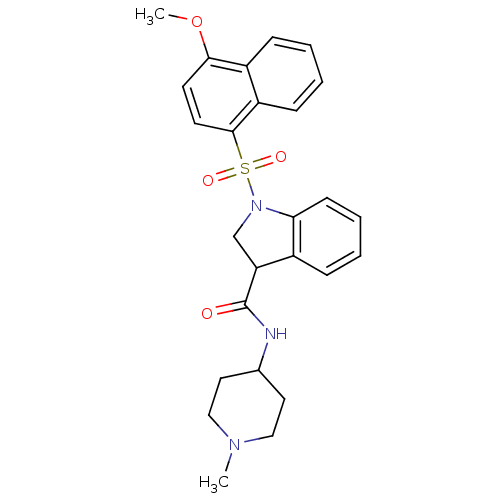
(CHEMBL1085835)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NC2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C26H29N3O4S/c1-28-15-13-18(14-16-28)27-26(30)22-17-29(23-10-6-5-7-19(22)23)34(31,32)25-12-11-24(33-2)20-8-3-4-9-21(20)25/h3-12,18,22H,13-17H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416123
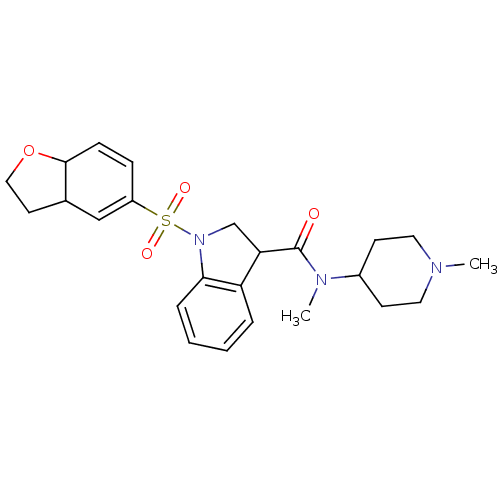
(CHEMBL1085352)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)C1=CC2CCOC2C=C1 |c:34,t:26| Show InChI InChI=1S/C24H31N3O4S/c1-25-12-9-18(10-13-25)26(2)24(28)21-16-27(22-6-4-3-5-20(21)22)32(29,30)19-7-8-23-17(15-19)11-14-31-23/h3-8,15,17-18,21,23H,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416118
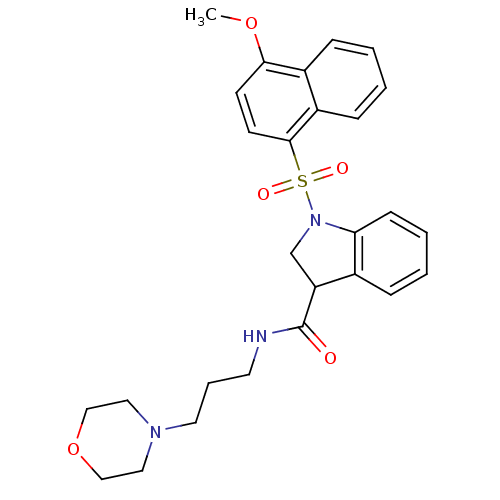
(CHEMBL1083122)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NCCCN2CCOCC2)c2ccccc12 Show InChI InChI=1S/C27H31N3O5S/c1-34-25-11-12-26(22-9-3-2-8-21(22)25)36(32,33)30-19-23(20-7-4-5-10-24(20)30)27(31)28-13-6-14-29-15-17-35-18-16-29/h2-5,7-12,23H,6,13-19H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416136
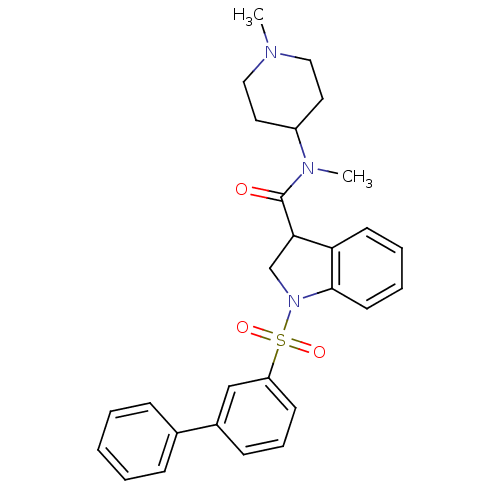
(CHEMBL1084483)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C28H31N3O3S/c1-29-17-15-23(16-18-29)30(2)28(32)26-20-31(27-14-7-6-13-25(26)27)35(33,34)24-12-8-11-22(19-24)21-9-4-3-5-10-21/h3-14,19,23,26H,15-18,20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416121
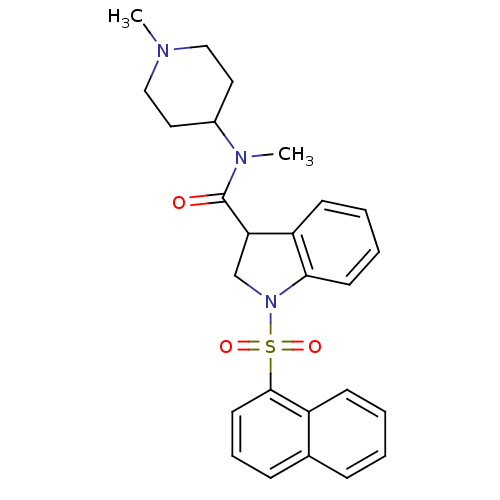
(CHEMBL1082485)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C26H29N3O3S/c1-27-16-14-20(15-17-27)28(2)26(30)23-18-29(24-12-6-5-11-22(23)24)33(31,32)25-13-7-9-19-8-3-4-10-21(19)25/h3-13,20,23H,14-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416113
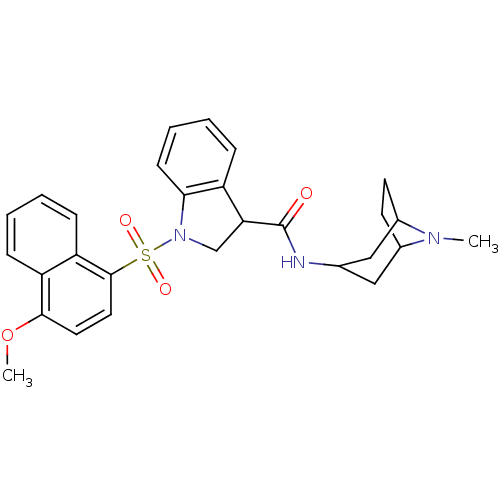
(CHEMBL1082494)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NC2CC3CCC(C2)N3C)c2ccccc12 |TLB:20:21:24.25:28| Show InChI InChI=1S/C28H31N3O4S/c1-30-19-11-12-20(30)16-18(15-19)29-28(32)24-17-31(25-10-6-5-7-21(24)25)36(33,34)27-14-13-26(35-2)22-8-3-4-9-23(22)27/h3-10,13-14,18-20,24H,11-12,15-17H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14031
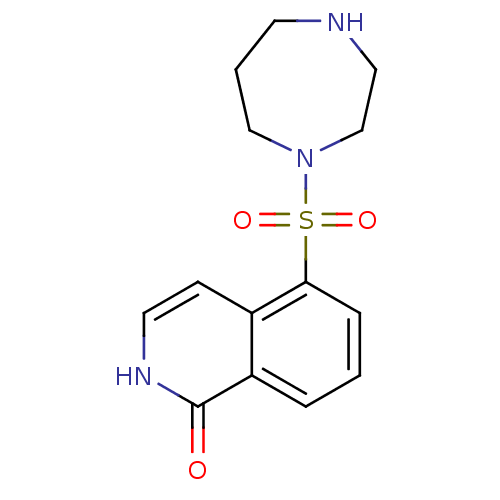
(5-(1,4-diazepane-1-sulfonyl)-1,2-dihydroisoquinoli...)Show InChI InChI=1S/C14H17N3O3S/c18-14-12-3-1-4-13(11(12)5-7-16-14)21(19,20)17-9-2-6-15-8-10-17/h1,3-5,7,15H,2,6,8-10H2,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of ROCK-1 |
Bioorg Med Chem Lett 21: 97-101 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.060
BindingDB Entry DOI: 10.7270/Q29W0GQK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50417844
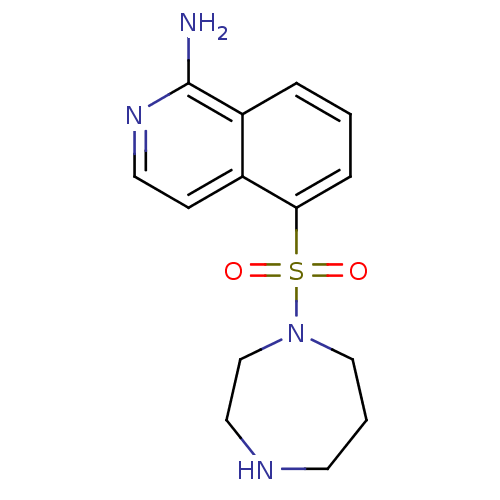
(CHEMBL1667963)Show InChI InChI=1S/C14H18N4O2S/c15-14-12-3-1-4-13(11(12)5-7-17-14)21(19,20)18-9-2-6-16-8-10-18/h1,3-5,7,16H,2,6,8-10H2,(H2,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 34.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Binding affinity to ROCK1 |
Bioorg Med Chem Lett 21: 1084-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.104
BindingDB Entry DOI: 10.7270/Q2DN468G |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50417668
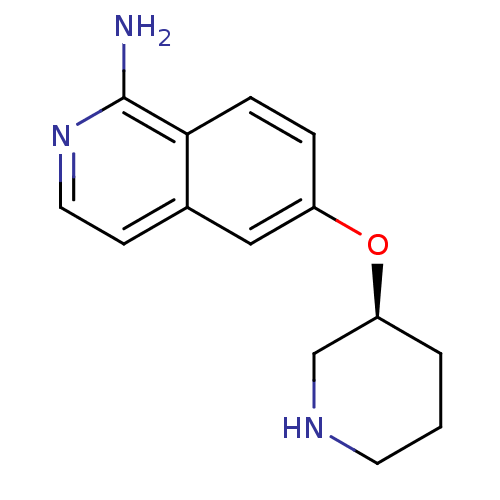
(CHEMBL1643368)Show InChI InChI=1S/C14H17N3O/c15-14-13-4-3-11(8-10(13)5-7-17-14)18-12-2-1-6-16-9-12/h3-5,7-8,12,16H,1-2,6,9H2,(H2,15,17)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Binding affinity to ROCK1 |
Bioorg Med Chem Lett 21: 1084-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.104
BindingDB Entry DOI: 10.7270/Q2DN468G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416110

(CHEMBL1085836)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NC2CCN(Cc3ccccc3)CC2)c2ccccc12 Show InChI InChI=1S/C32H33N3O4S/c1-39-30-15-16-31(27-13-6-5-12-26(27)30)40(37,38)35-22-28(25-11-7-8-14-29(25)35)32(36)33-24-17-19-34(20-18-24)21-23-9-3-2-4-10-23/h2-16,24,28H,17-22H2,1H3,(H,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416124
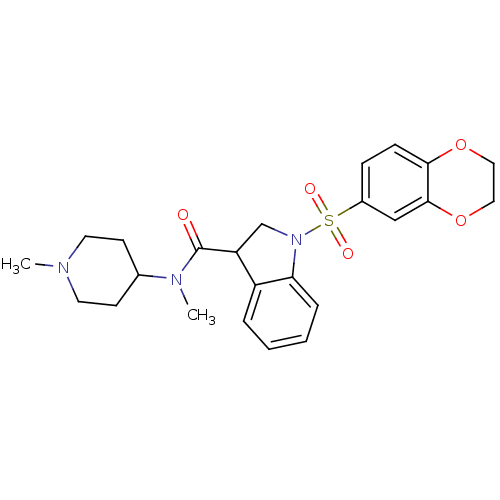
(CHEMBL1085353)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc2OCCOc2c1 Show InChI InChI=1S/C24H29N3O5S/c1-25-11-9-17(10-12-25)26(2)24(28)20-16-27(21-6-4-3-5-19(20)21)33(29,30)18-7-8-22-23(15-18)32-14-13-31-22/h3-8,15,17,20H,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416115
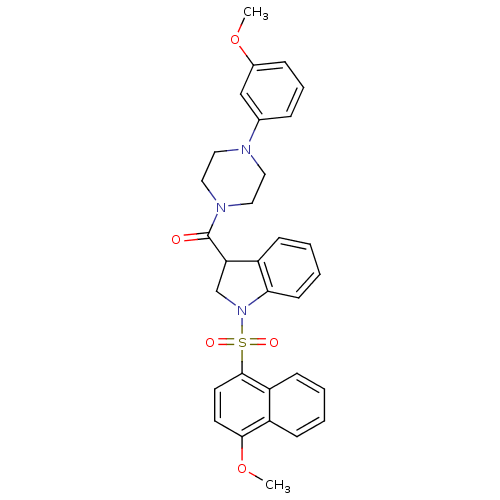
(CHEMBL1084614)Show SMILES COc1cccc(c1)N1CCN(CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC)c2ccccc12 Show InChI InChI=1S/C31H31N3O5S/c1-38-23-9-7-8-22(20-23)32-16-18-33(19-17-32)31(35)27-21-34(28-13-6-5-10-24(27)28)40(36,37)30-15-14-29(39-2)25-11-3-4-12-26(25)30/h3-15,20,27H,16-19,21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50417674
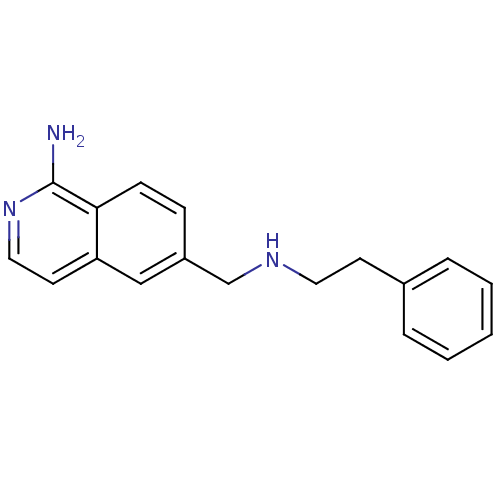
(CHEMBL1643361)Show InChI InChI=1S/C18H19N3/c19-18-17-7-6-15(12-16(17)9-11-21-18)13-20-10-8-14-4-2-1-3-5-14/h1-7,9,11-12,20H,8,10,13H2,(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of ROCK-1 |
Bioorg Med Chem Lett 21: 97-101 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.060
BindingDB Entry DOI: 10.7270/Q29W0GQK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416127
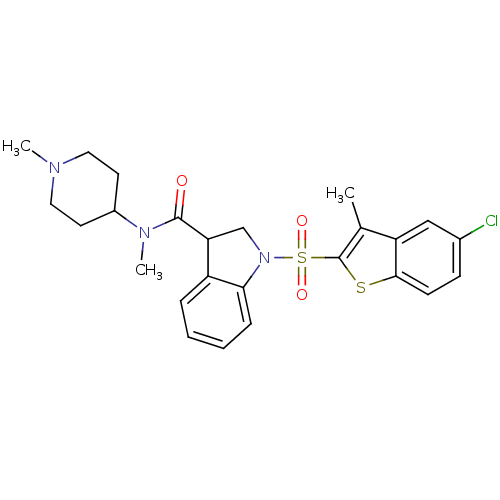
(CHEMBL1082765)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1sc2ccc(Cl)cc2c1C Show InChI InChI=1S/C25H28ClN3O3S2/c1-16-20-14-17(26)8-9-23(20)33-25(16)34(31,32)29-15-21(19-6-4-5-7-22(19)29)24(30)28(3)18-10-12-27(2)13-11-18/h4-9,14,18,21H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416119

(CHEMBL1082483)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NCCCn2ccnc2)c2ccccc12 Show InChI InChI=1S/C26H26N4O4S/c1-34-24-11-12-25(21-9-3-2-8-20(21)24)35(32,33)30-17-22(19-7-4-5-10-23(19)30)26(31)28-13-6-15-29-16-14-27-18-29/h2-5,7-12,14,16,18,22H,6,13,15,17H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416138
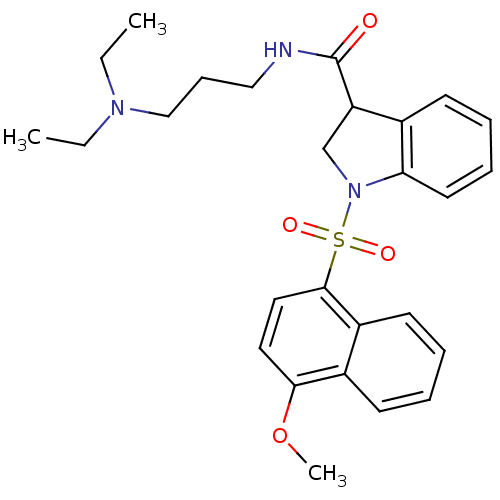
(CHEMBL1084317)Show SMILES CCN(CC)CCCNC(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC)c2ccccc12 Show InChI InChI=1S/C27H33N3O4S/c1-4-29(5-2)18-10-17-28-27(31)23-19-30(24-14-9-8-11-20(23)24)35(32,33)26-16-15-25(34-3)21-12-6-7-13-22(21)26/h6-9,11-16,23H,4-5,10,17-19H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416112
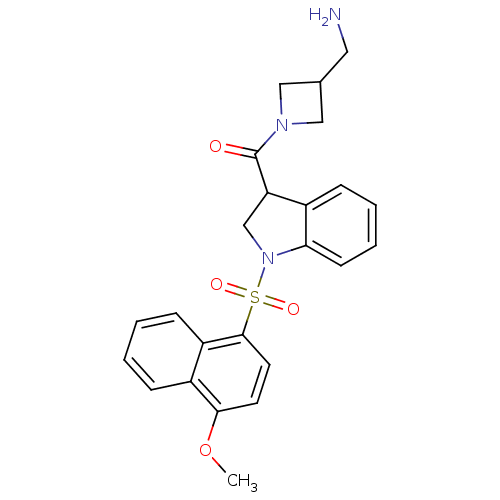
(CHEMBL1085362)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CC(CN)C2)c2ccccc12 Show InChI InChI=1S/C24H25N3O4S/c1-31-22-10-11-23(19-8-3-2-7-18(19)22)32(29,30)27-15-20(17-6-4-5-9-21(17)27)24(28)26-13-16(12-25)14-26/h2-11,16,20H,12-15,25H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416133

(CHEMBL1083298)Show SMILES COc1ccc(c(C)c1C)S(=O)(=O)N1CC(C(=O)N(C)C2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C25H33N3O4S/c1-17-18(2)24(11-10-23(17)32-5)33(30,31)28-16-21(20-8-6-7-9-22(20)28)25(29)27(4)19-12-14-26(3)15-13-19/h6-11,19,21H,12-16H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416134
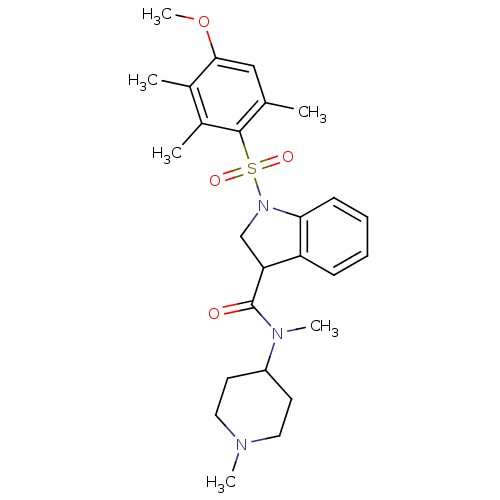
(CHEMBL1084214)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N1CC(C(=O)N(C)C2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C26H35N3O4S/c1-17-15-24(33-6)18(2)19(3)25(17)34(31,32)29-16-22(21-9-7-8-10-23(21)29)26(30)28(5)20-11-13-27(4)14-12-20/h7-10,15,20,22H,11-14,16H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14027
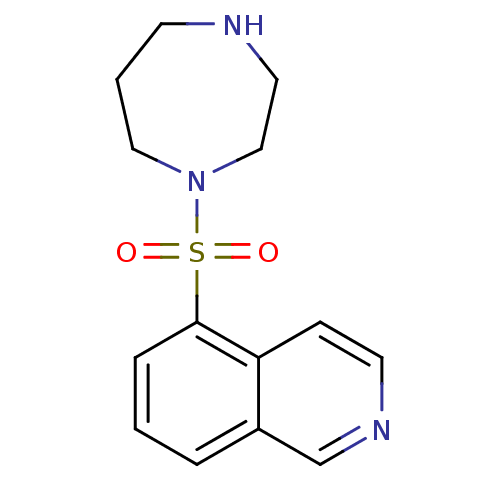
(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of ROCK-1 |
Bioorg Med Chem Lett 21: 97-101 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.060
BindingDB Entry DOI: 10.7270/Q29W0GQK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416126
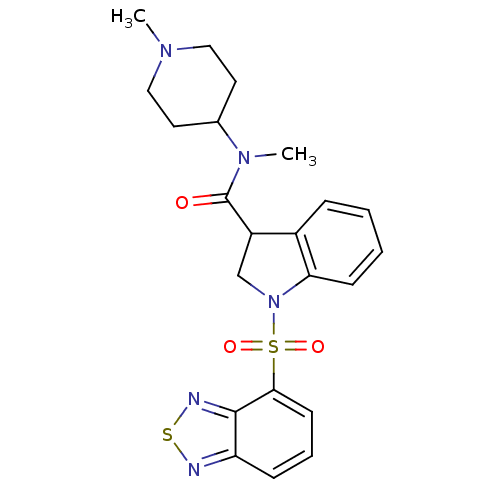
(CHEMBL1082764)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2nsnc12 Show InChI InChI=1S/C22H25N5O3S2/c1-25-12-10-15(11-13-25)26(2)22(28)17-14-27(19-8-4-3-6-16(17)19)32(29,30)20-9-5-7-18-21(20)24-31-23-18/h3-9,15,17H,10-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416135
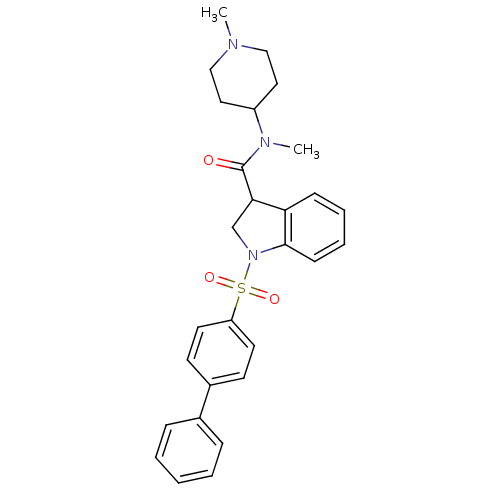
(CHEMBL1084482)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H31N3O3S/c1-29-18-16-23(17-19-29)30(2)28(32)26-20-31(27-11-7-6-10-25(26)27)35(33,34)24-14-12-22(13-15-24)21-8-4-3-5-9-21/h3-15,23,26H,16-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416111
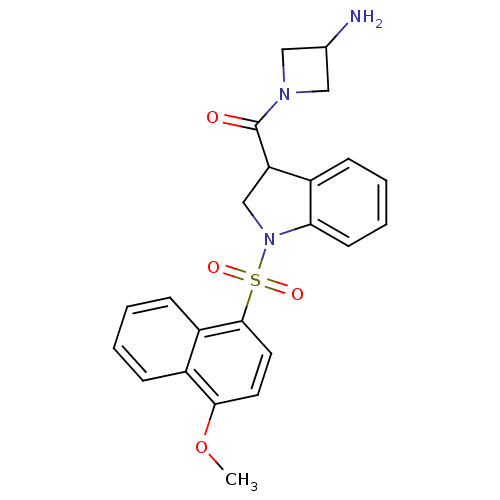
(CHEMBL1085361)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CC(N)C2)c2ccccc12 Show InChI InChI=1S/C23H23N3O4S/c1-30-21-10-11-22(18-8-3-2-7-17(18)21)31(28,29)26-14-19(16-6-4-5-9-20(16)26)23(27)25-12-15(24)13-25/h2-11,15,19H,12-14,24H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418117

(CHEMBL1762815)Show SMILES CCc1sc(nc1CN(CCO)C(C)C)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C25H34ClN3O2S/c1-4-23-22(16-28(10-11-30)17(2)3)27-25(32-23)20-15-29(14-18-8-12-31-13-9-18)24-19(20)6-5-7-21(24)26/h5-7,15,17-18,30H,4,8-14,16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 2541-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.023
BindingDB Entry DOI: 10.7270/Q2NP24RP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418110
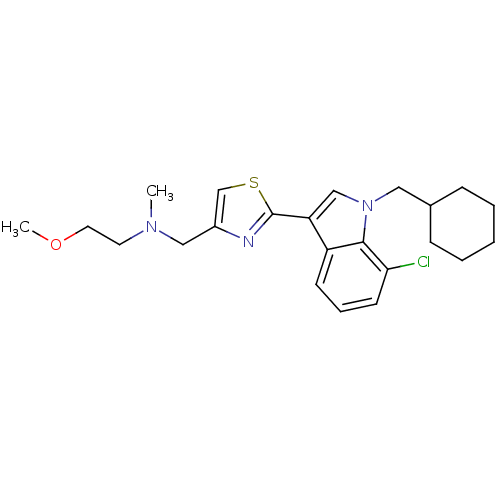
(CHEMBL1762804)Show SMILES COCCN(C)Cc1csc(n1)-c1cn(CC2CCCCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C23H30ClN3OS/c1-26(11-12-28-2)14-18-16-29-23(25-18)20-15-27(13-17-7-4-3-5-8-17)22-19(20)9-6-10-21(22)24/h6,9-10,15-17H,3-5,7-8,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 2541-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.023
BindingDB Entry DOI: 10.7270/Q2NP24RP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416125
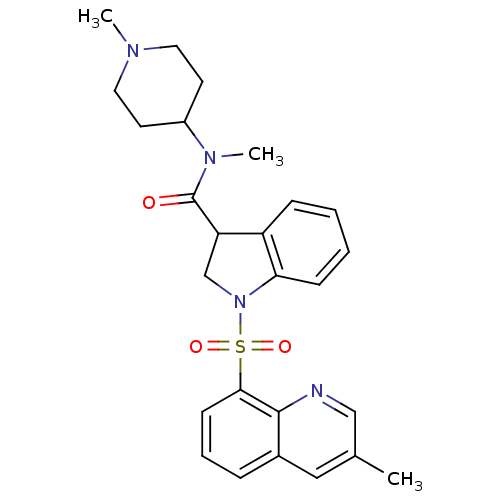
(CHEMBL1085354)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2cc(C)cnc12 Show InChI InChI=1S/C26H30N4O3S/c1-18-15-19-7-6-10-24(25(19)27-16-18)34(32,33)30-17-22(21-8-4-5-9-23(21)30)26(31)29(3)20-11-13-28(2)14-12-20/h4-10,15-16,20,22H,11-14,17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416128
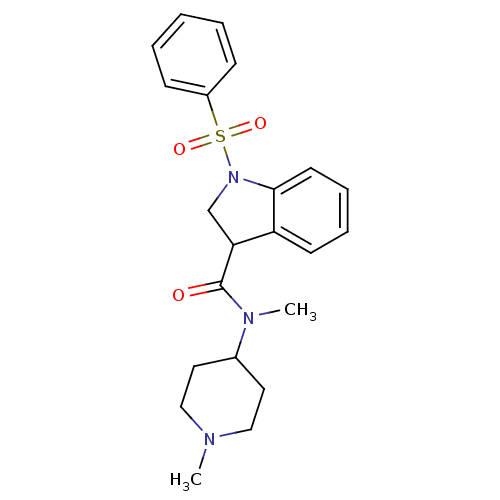
(CHEMBL1082767)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C22H27N3O3S/c1-23-14-12-17(13-15-23)24(2)22(26)20-16-25(21-11-7-6-10-19(20)21)29(27,28)18-8-4-3-5-9-18/h3-11,17,20H,12-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418122

(CHEMBL1762814)Show SMILES CCN(CCO)Cc1nc(sc1CC)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C24H32ClN3O2S/c1-3-22-21(16-27(4-2)10-11-29)26-24(31-22)19-15-28(14-17-8-12-30-13-9-17)23-18(19)6-5-7-20(23)25/h5-7,15,17,29H,3-4,8-14,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 2541-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.023
BindingDB Entry DOI: 10.7270/Q2NP24RP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416129
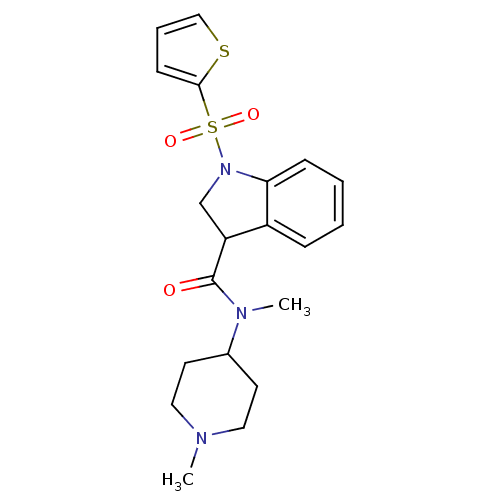
(CHEMBL1082766)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccs1 Show InChI InChI=1S/C20H25N3O3S2/c1-21-11-9-15(10-12-21)22(2)20(24)17-14-23(18-7-4-3-6-16(17)18)28(25,26)19-8-5-13-27-19/h3-8,13,15,17H,9-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418116

(CHEMBL1762813)Show SMILES CCc1sc(nc1CN(C)CCO)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C23H30ClN3O2S/c1-3-21-20(15-26(2)9-10-28)25-23(30-21)18-14-27(13-16-7-11-29-12-8-16)22-17(18)5-4-6-19(22)24/h4-6,14,16,28H,3,7-13,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 2541-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.023
BindingDB Entry DOI: 10.7270/Q2NP24RP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418109

(CHEMBL1760028)Show SMILES CCN(CC)Cc1csc(n1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C22H28ClN3OS/c1-3-25(4-2)13-17-15-28-22(24-17)19-14-26(12-16-8-10-27-11-9-16)21-18(19)6-5-7-20(21)23/h5-7,14-16H,3-4,8-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 2541-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.023
BindingDB Entry DOI: 10.7270/Q2NP24RP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data