

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 preincubated for 15 mins by stopped flow CO2 dehydration method | Bioorg Med Chem 24: 104-12 (2016) Article DOI: 10.1016/j.bmc.2015.11.031 BindingDB Entry DOI: 10.7270/Q2222XRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-9 preincubated for 15 mins by stopped flow CO2 dehydration method | Bioorg Med Chem 24: 104-12 (2016) Article DOI: 10.1016/j.bmc.2015.11.031 BindingDB Entry DOI: 10.7270/Q2222XRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50517085 (CHEMBL4545711) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human CA9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111600 BindingDB Entry DOI: 10.7270/Q2542RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18778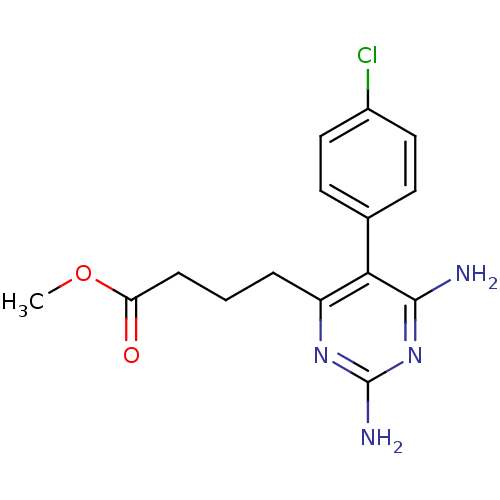 (CHEMBL22405 | P16 | methyl 4-[2,6-diamino-5-(4-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition constant against Plasmodium falciparum dihydrofolate reductase | J Med Chem 47: 4258-67 (2004) Checked by Author Article DOI: 10.1021/jm040769c BindingDB Entry DOI: 10.7270/Q2HH6JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50146823 (CHEMBL3764718) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM210938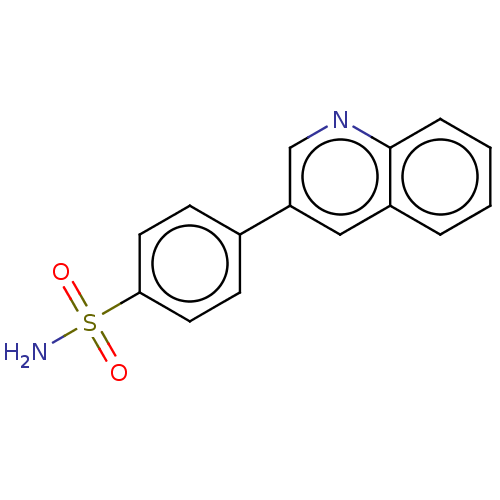 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50159077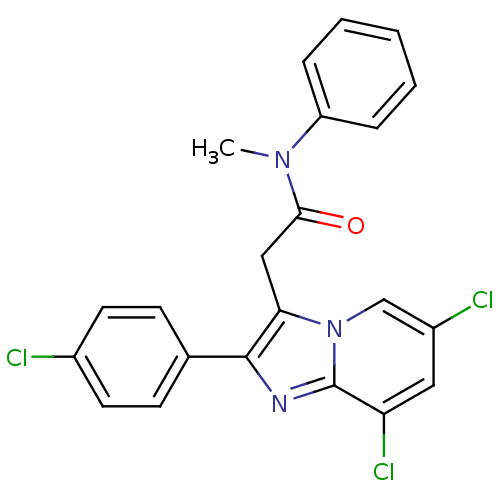 (2-[6,8-Dichloro-2-(4-chloro-phenyl)-imidazo[1,2-a]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Bari Curated by ChEMBL | Assay Description Displacement of [3H]-PK11195 from peripheral benzodiazepine receptor of rat cerebral cortex | J Med Chem 48: 292-305 (2005) Article DOI: 10.1021/jm049610q BindingDB Entry DOI: 10.7270/Q2RR1XQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50146833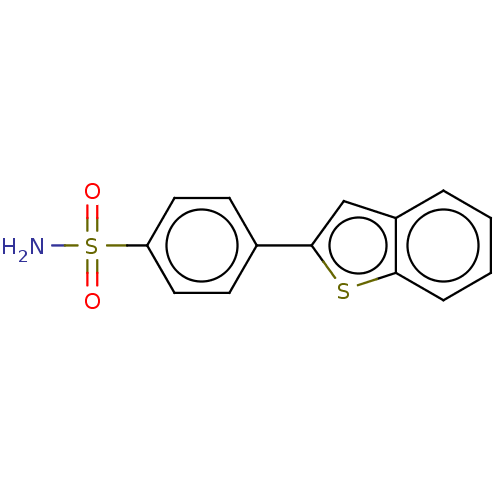 (CHEMBL3763841) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM210935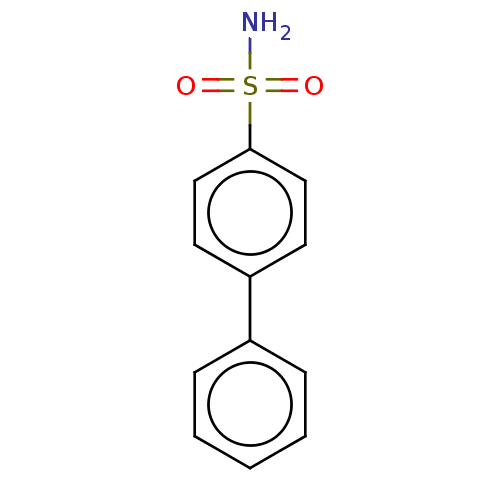 (4-(phenyl)-bezenesulfonamide (4a)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50146600 (CHEMBL3763914) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50517085 (CHEMBL4545711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human CA2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111600 BindingDB Entry DOI: 10.7270/Q2542RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50146825 (CHEMBL3765197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 preincubated for 15 mins by stopped flow CO2 dehydration method | Bioorg Med Chem 24: 104-12 (2016) Article DOI: 10.1016/j.bmc.2015.11.031 BindingDB Entry DOI: 10.7270/Q2222XRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50159075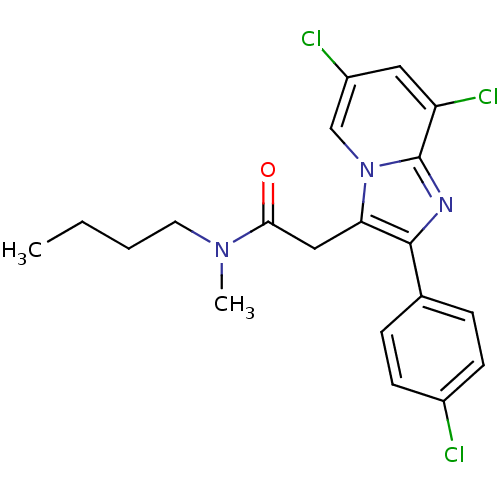 (CHEMBL180210 | N-Butyl-2-[6,8-dichloro-2-(4-chloro...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Bari Curated by ChEMBL | Assay Description Displacement of [3H]-PK11195 from peripheral benzodiazepine receptor of rat cerebral cortex | J Med Chem 48: 292-305 (2005) Article DOI: 10.1021/jm049610q BindingDB Entry DOI: 10.7270/Q2RR1XQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50146831 (CHEMBL3763167) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50517085 (CHEMBL4545711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human CA7 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111600 BindingDB Entry DOI: 10.7270/Q2542RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM85093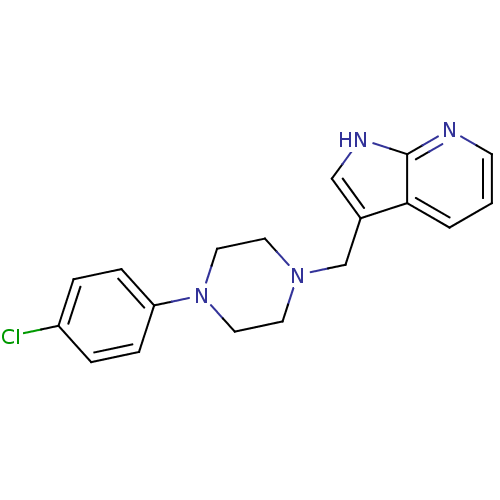 (CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D4 by [3H]-spiperone displacement. | J Med Chem 47: 2348-55 (2004) Article DOI: 10.1021/jm0305669 BindingDB Entry DOI: 10.7270/Q2M61M03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50113468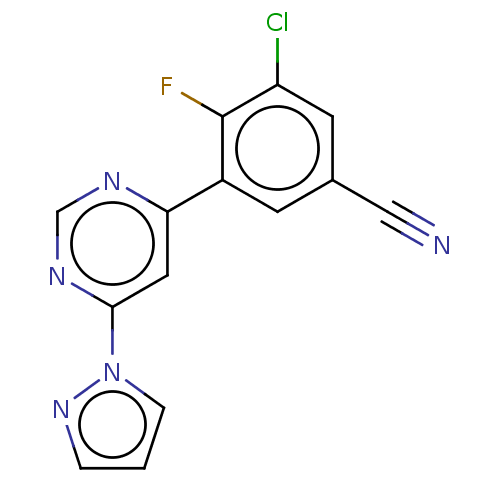 (CHEMBL3603915 | US10246432, Example 15 | US1058411...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-M-MPEP from human mGlu5 receptor expressed in HEK293 cells after 90 mins by scintillation spectroscopy analysis | J Med Chem 58: 6653-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00892 BindingDB Entry DOI: 10.7270/Q2H70HMZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50113476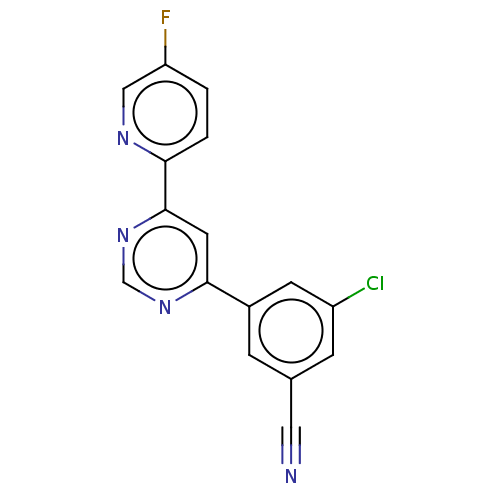 (CHEMBL3603923 | US10246432, Example 17 | US1058411...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-M-MPEP from human mGlu5 receptor expressed in HEK293 cells after 90 mins by scintillation spectroscopy analysis | J Med Chem 58: 6653-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00892 BindingDB Entry DOI: 10.7270/Q2H70HMZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50469139 (CHEMBL4292479) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | Eur J Med Chem 157: 1214-1222 (2018) Article DOI: 10.1016/j.ejmech.2018.08.096 BindingDB Entry DOI: 10.7270/Q2RR21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM210937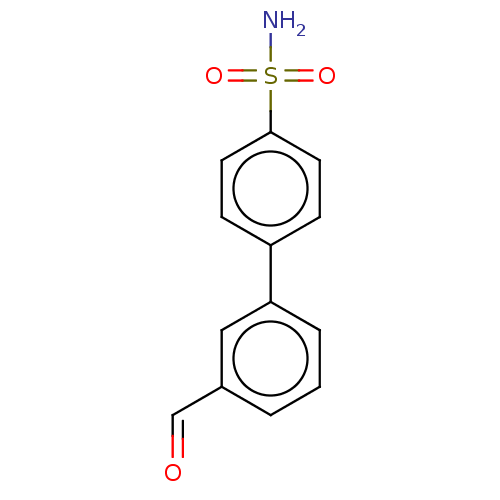 (4-(3-formylphenyl)-benzenesulfonamide (4e)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50146599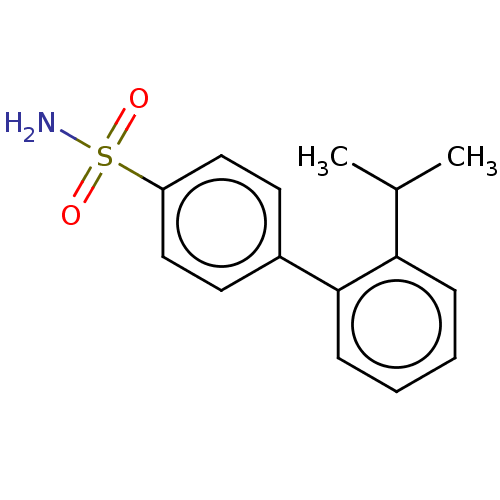 (CHEMBL3763192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50539861 (CHEMBL4638601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human CA7 pre-incubated for 15 mins by stopped flow CO2 hydrase assay | J Med Chem 63: 7392-7409 (2020) Article DOI: 10.1021/acs.jmedchem.0c00636 BindingDB Entry DOI: 10.7270/Q2F47SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50539860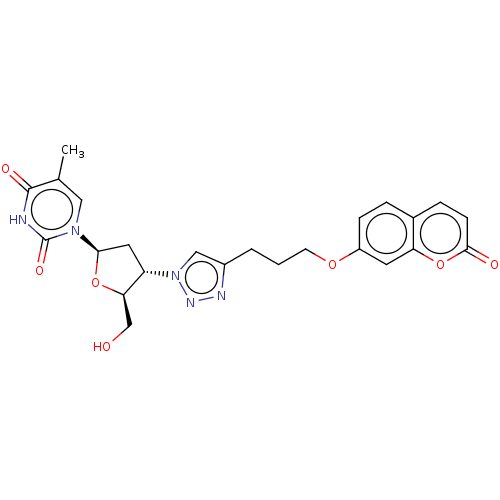 (CHEMBL4644555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human CA7 pre-incubated for 15 mins by stopped flow CO2 hydrase assay | J Med Chem 63: 7392-7409 (2020) Article DOI: 10.1021/acs.jmedchem.0c00636 BindingDB Entry DOI: 10.7270/Q2F47SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50517085 (CHEMBL4545711) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human CA1 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111600 BindingDB Entry DOI: 10.7270/Q2542RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50113477 (CHEMBL3603924 | US10246432, Example 3 | US10584111...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-M-MPEP from human mGlu5 receptor expressed in HEK293 cells after 90 mins by scintillation spectroscopy analysis | J Med Chem 58: 6653-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00892 BindingDB Entry DOI: 10.7270/Q2H70HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50030561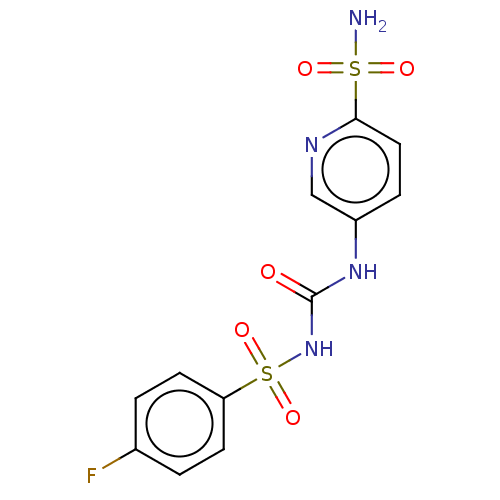 (CHEMBL3354129) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by stopped flow CO2 hydration method | J Med Chem 57: 9152-67 (2014) Article DOI: 10.1021/jm501314c BindingDB Entry DOI: 10.7270/Q2CV4K97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146825 (CHEMBL3765197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146600 (CHEMBL3763914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146831 (CHEMBL3763167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50357453 (CHEMBL1917719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma1 receptor in guinea pig brain membrane after 150 mins by liquid scintillation counting | Bioorg Med Chem 19: 6210-24 (2011) Article DOI: 10.1016/j.bmc.2011.09.016 BindingDB Entry DOI: 10.7270/Q2VH5P7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50517082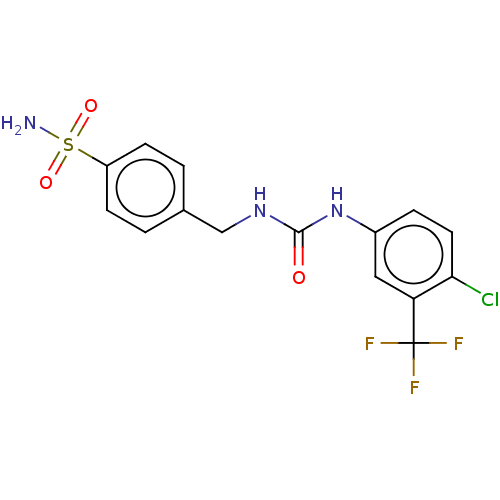 (CHEMBL4466537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human CA9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111600 BindingDB Entry DOI: 10.7270/Q2542RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50539858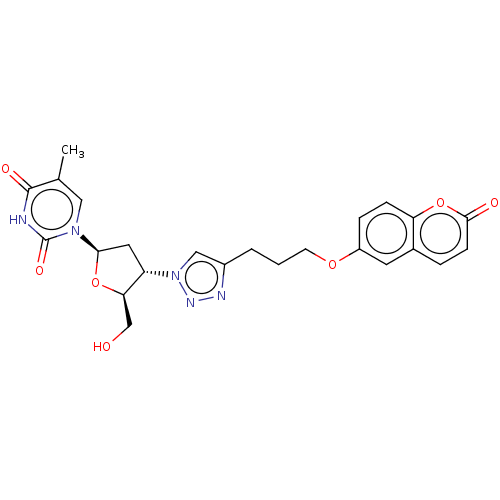 (CHEMBL4639447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human CA7 pre-incubated for 15 mins by stopped flow CO2 hydrase assay | J Med Chem 63: 7392-7409 (2020) Article DOI: 10.1021/acs.jmedchem.0c00636 BindingDB Entry DOI: 10.7270/Q2F47SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50539856 (CHEMBL4649280) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human CA7 pre-incubated for 15 mins by stopped flow CO2 hydrase assay | J Med Chem 63: 7392-7409 (2020) Article DOI: 10.1021/acs.jmedchem.0c00636 BindingDB Entry DOI: 10.7270/Q2F47SNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110772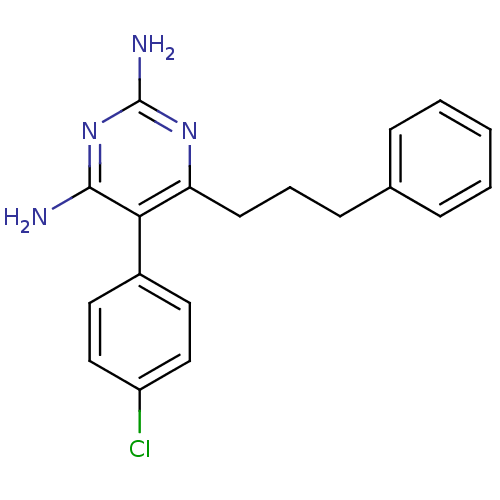 (5-(4-Chloro-phenyl)-6-(3-phenyl-propyl)-pyrimidine...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition constant against Plasmodium falciparum dihydrofolate reductase | J Med Chem 47: 4258-67 (2004) Checked by Author Article DOI: 10.1021/jm040769c BindingDB Entry DOI: 10.7270/Q2HH6JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50153255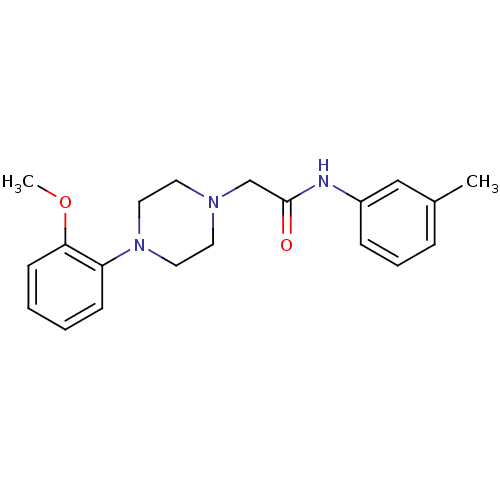 (2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-N-m-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-spiperone binding to human Dopamine receptor D4.4 allele | Bioorg Med Chem Lett 14: 5095-8 (2004) Article DOI: 10.1016/j.bmcl.2004.07.068 BindingDB Entry DOI: 10.7270/Q23X863Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18774 (5-(4-chlorophenyl)pyrimidine-2,4-diamine | CHEMBL2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition constant against Plasmodium falciparum dihydrofolate reductase | J Med Chem 47: 4258-67 (2004) Checked by Author Article DOI: 10.1021/jm040769c BindingDB Entry DOI: 10.7270/Q2HH6JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18782 (5-(4-tert-butylphenyl)-6-ethylpyrimidine-2,4-diami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition constant against Plasmodium falciparum dihydrofolate reductase | J Med Chem 47: 4258-67 (2004) Checked by Author Article DOI: 10.1021/jm040769c BindingDB Entry DOI: 10.7270/Q2HH6JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210935 (4-(phenyl)-bezenesulfonamide (4a)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18783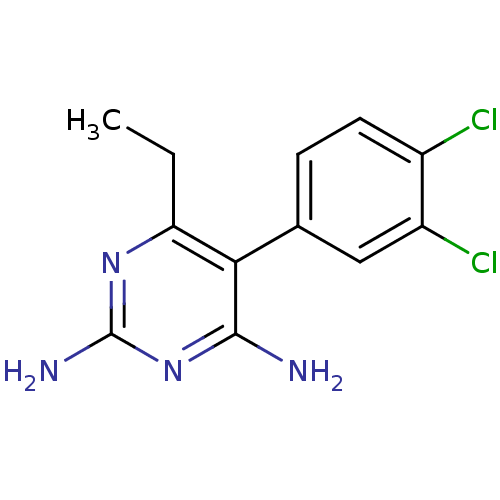 (5-(3,4-dichlorophenyl)-6-ethylpyrimidine-2,4-diami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition constant against Plasmodium falciparum dihydrofolate reductase | J Med Chem 47: 4258-67 (2004) Checked by Author Article DOI: 10.1021/jm040769c BindingDB Entry DOI: 10.7270/Q2HH6JKZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50030560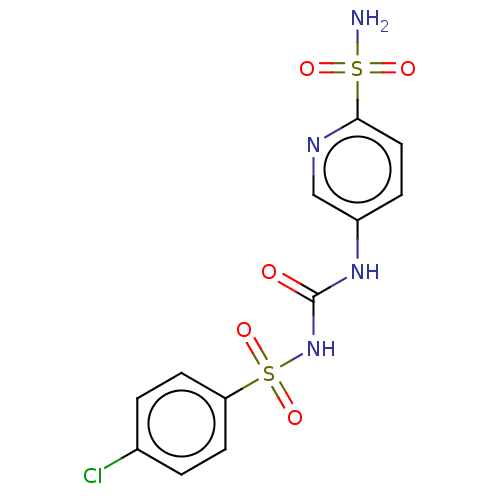 (CHEMBL3354128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by stopped flow CO2 hydration method | J Med Chem 57: 9152-67 (2014) Article DOI: 10.1021/jm501314c BindingDB Entry DOI: 10.7270/Q2CV4K97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50113479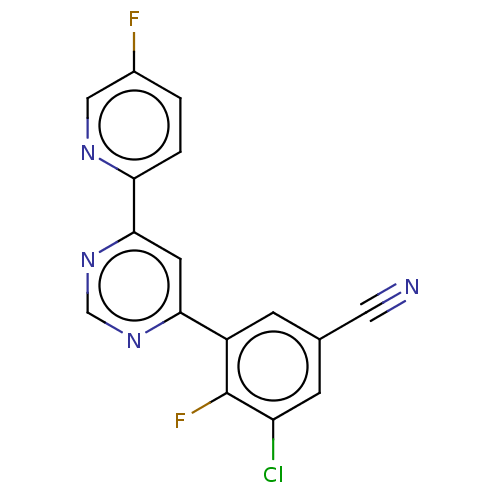 (CHEMBL3603926 | US10246432, Example 22 | US1058411...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-M-MPEP from human mGlu5 receptor expressed in HEK293 cells after 90 mins by scintillation spectroscopy analysis | J Med Chem 58: 6653-64 (2015) Article DOI: 10.1021/acs.jmedchem.5b00892 BindingDB Entry DOI: 10.7270/Q2H70HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50517088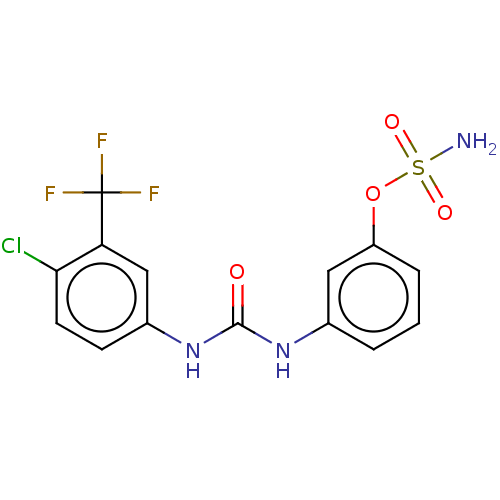 (CHEMBL4543156) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human CA9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111600 BindingDB Entry DOI: 10.7270/Q2542RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50197809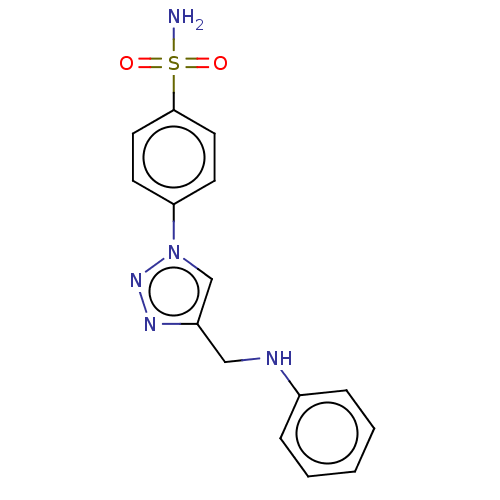 (CHEMBL3908714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 pre-incubated for 15 mins by stopped flow carbon dioxide hydrase assay | J Med Chem 59: 10692-10704 (2016) Article DOI: 10.1021/acs.jmedchem.6b01389 BindingDB Entry DOI: 10.7270/Q2Q81G1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18775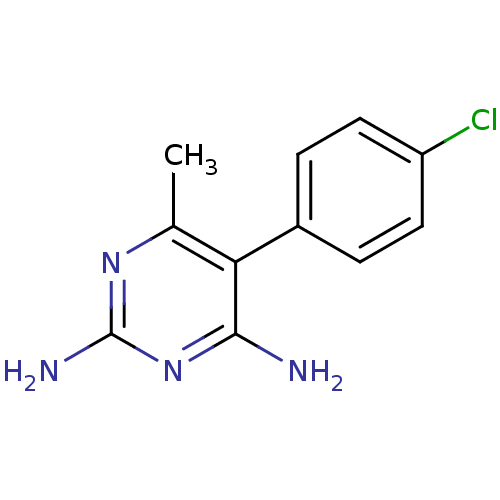 (5-(4-chlorophenyl)-6-methylpyrimidine-2,4-diamine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition constant against Plasmodium falciparum dihydrofolate reductase | J Med Chem 47: 4258-67 (2004) Checked by Author Article DOI: 10.1021/jm040769c BindingDB Entry DOI: 10.7270/Q2HH6JKZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146833 (CHEMBL3763841) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by CO2 hydration-based stopped flow assay | J Med Chem 59: 721-32 (2016) Article DOI: 10.1021/acs.jmedchem.5b01771 BindingDB Entry DOI: 10.7270/Q2K0764T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18781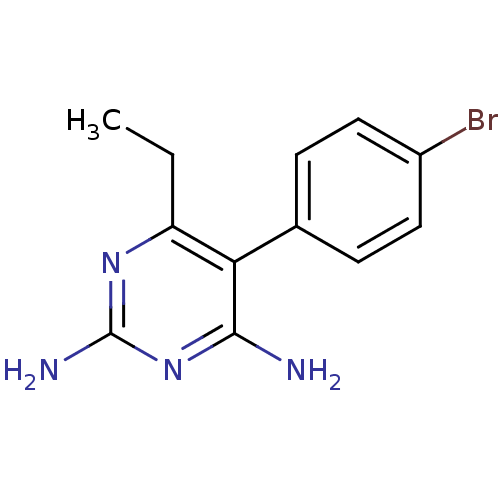 (5-(4-bromophenyl)-6-ethylpyrimidine-2,4-diamine | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition constant against Plasmodium falciparum dihydrofolate reductase | J Med Chem 47: 4258-67 (2004) Checked by Author Article DOI: 10.1021/jm040769c BindingDB Entry DOI: 10.7270/Q2HH6JKZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 8507 total ) | Next | Last >> |