

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024874 (CHEMBL3335066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024893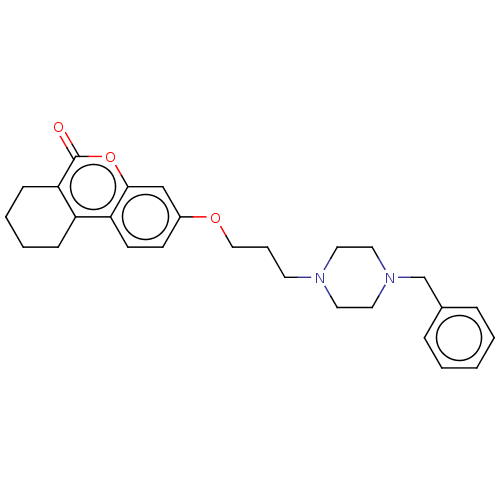 (CHEMBL3335048) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024881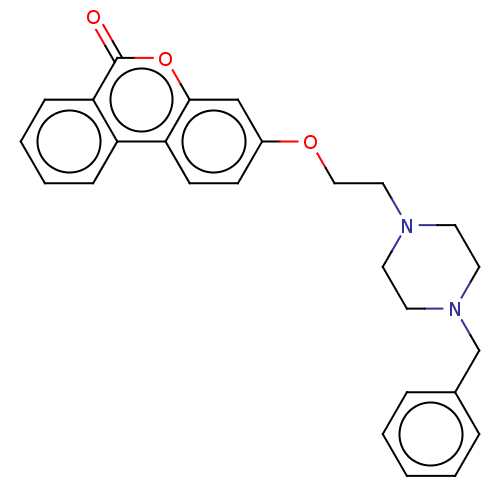 (CHEMBL3335060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024891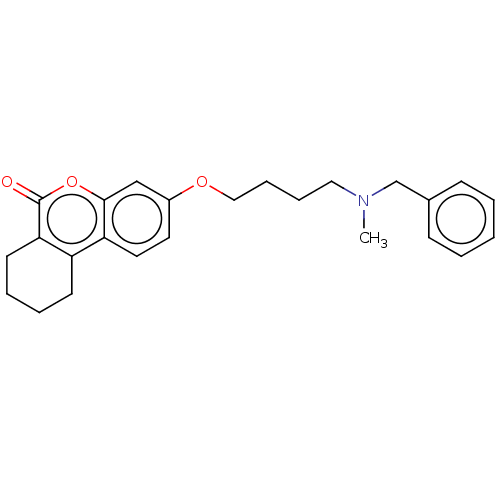 (CHEMBL3335050) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024872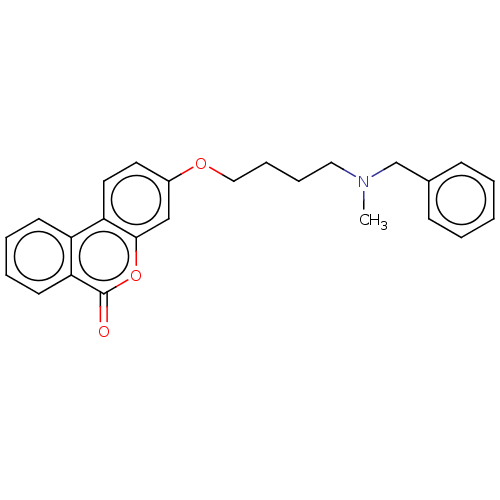 (CHEMBL3335068) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024900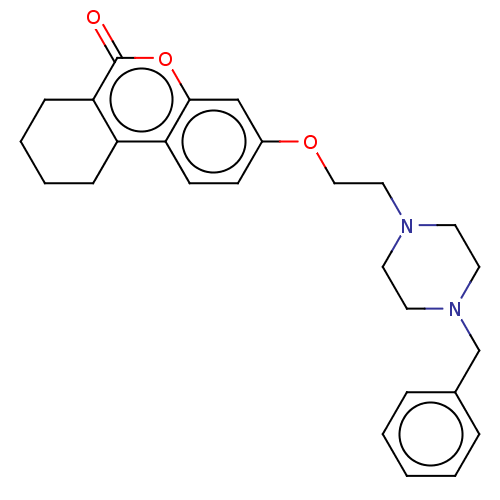 (CHEMBL3335029) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024890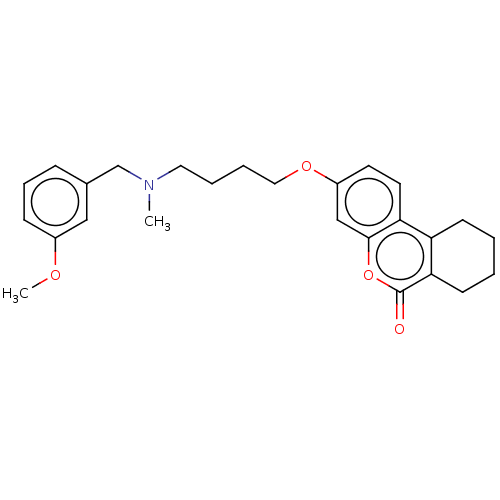 (CHEMBL3335051) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024898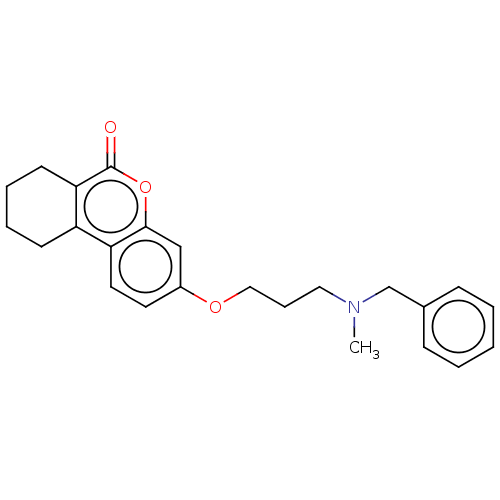 (CHEMBL3335031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024897 (CHEMBL3335032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024862 (CHEMBL3335019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM294221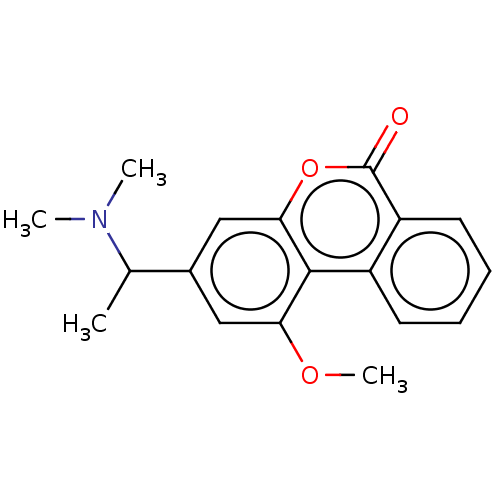 ((±)-3-(1-(dimethylamino)ethyl)-1-methoxy-6H-benzo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024953 (CHEMBL3335025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50024893 (CHEMBL3335048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50024874 (CHEMBL3335066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024876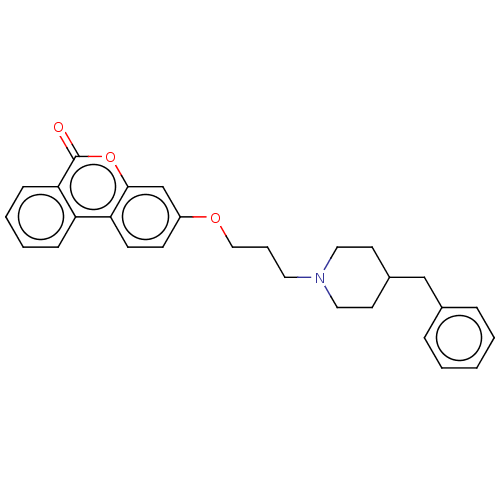 (CHEMBL3335064) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM294223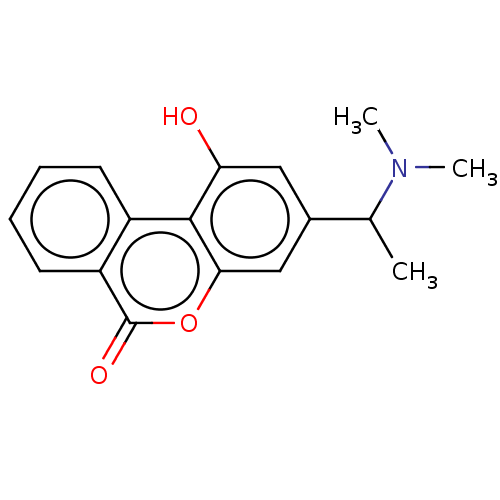 ((±)-3-(1-(dimethylamino)ethyl)-1-hydroxy-6H-benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50024957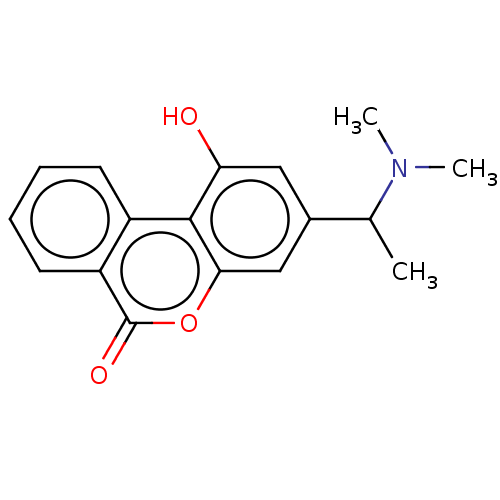 (CHEMBL3335021) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024896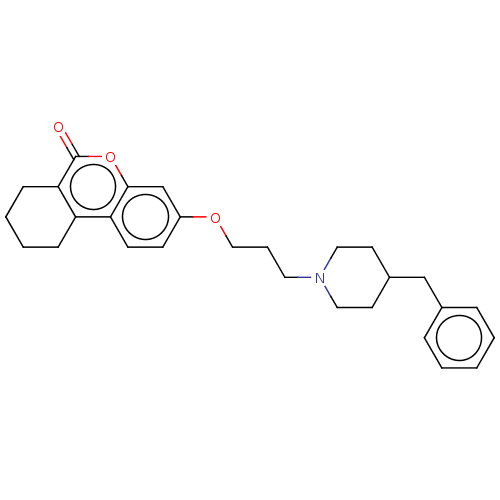 (CHEMBL3335046) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024895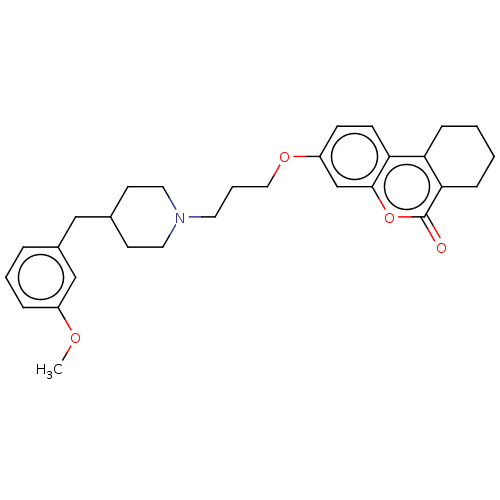 (CHEMBL3335047) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024933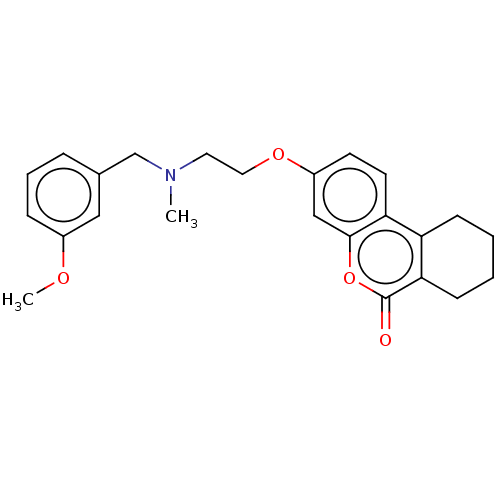 (CHEMBL3335026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024892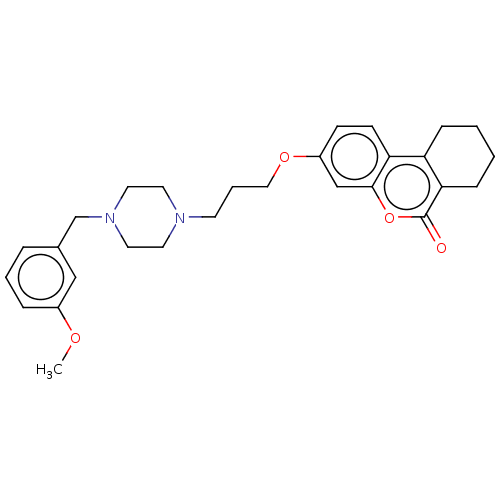 (CHEMBL3335049) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024887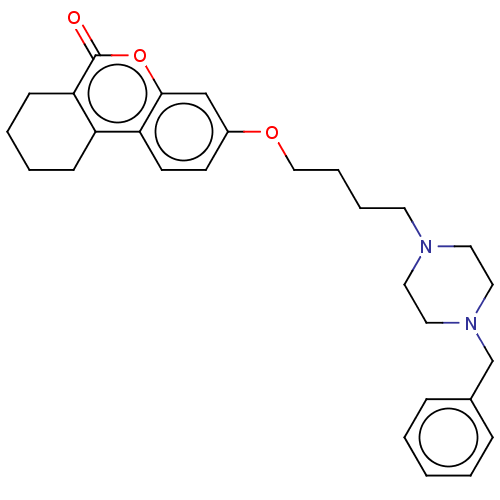 (CHEMBL3335054) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50024872 (CHEMBL3335068) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024885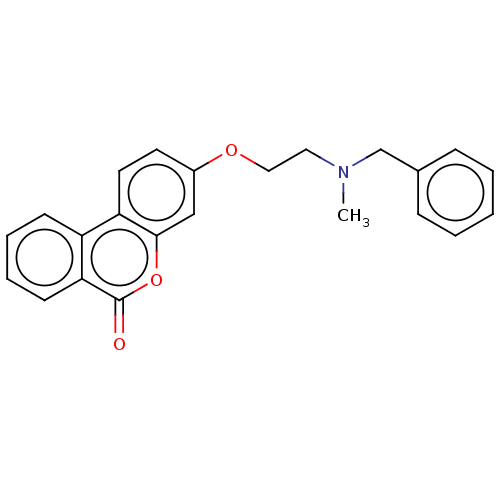 (CHEMBL3335056) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024899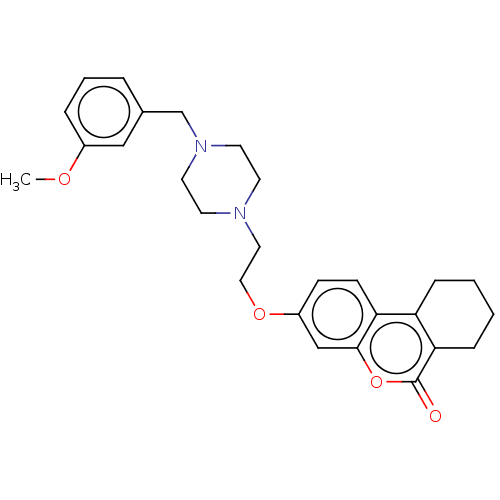 (CHEMBL3335030) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024864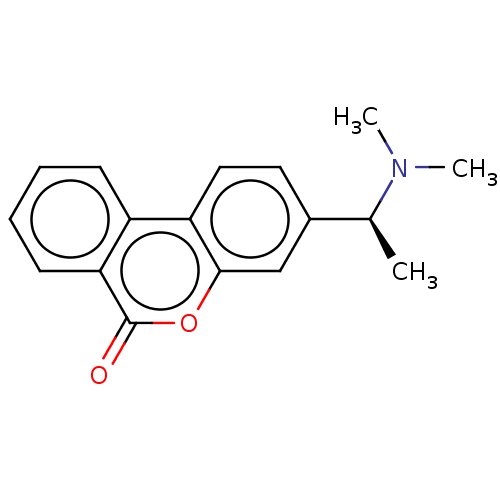 (CHEMBL3335018) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM294220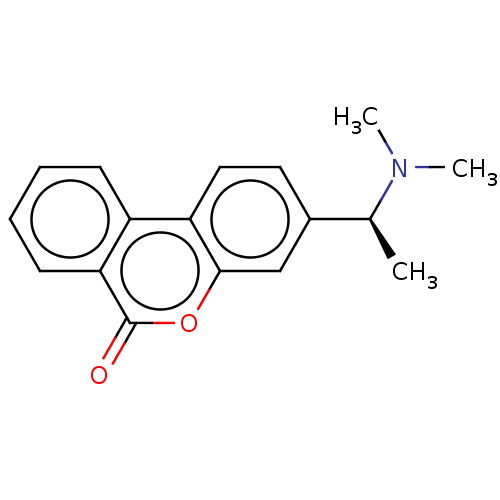 ((S)-3-(1-(dimethylamino)ethyl)-6H-benzo[c]chromen-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024924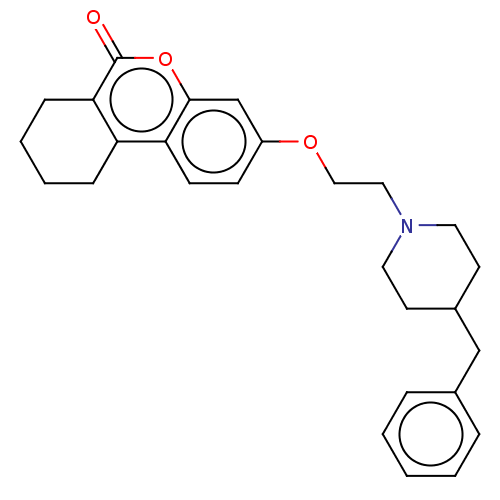 (CHEMBL3335027) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024868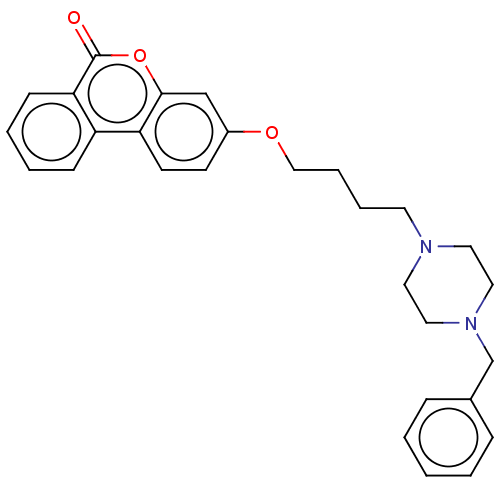 (CHEMBL3335071) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024871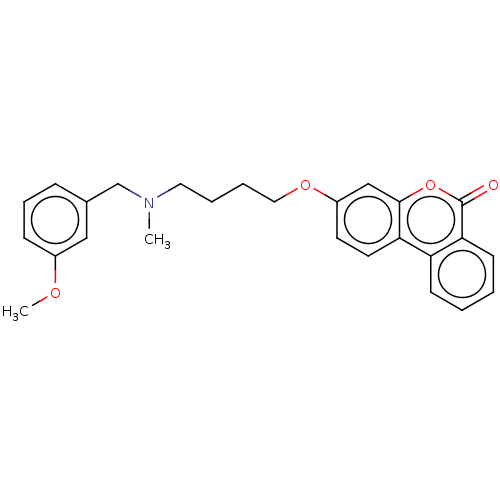 (CHEMBL3335069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024879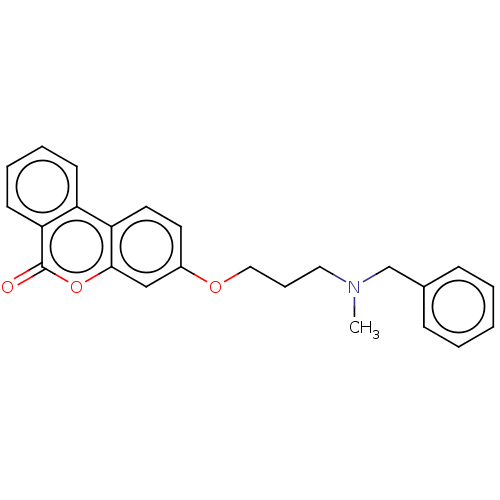 (CHEMBL3335062) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM294226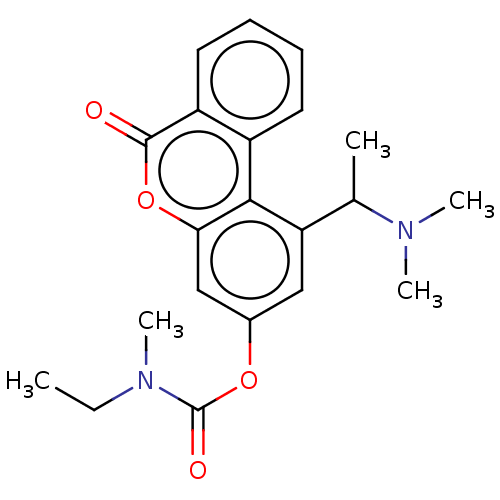 ((±)-1-(1-(dimethylamino)ethyl)-6-oxo-6H-benzo[c]ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50024954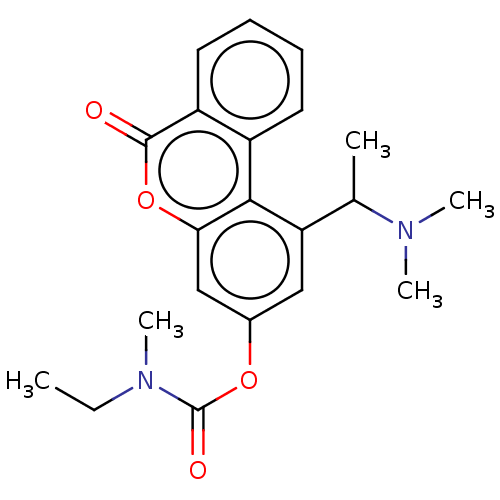 (CHEMBL3335024) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024886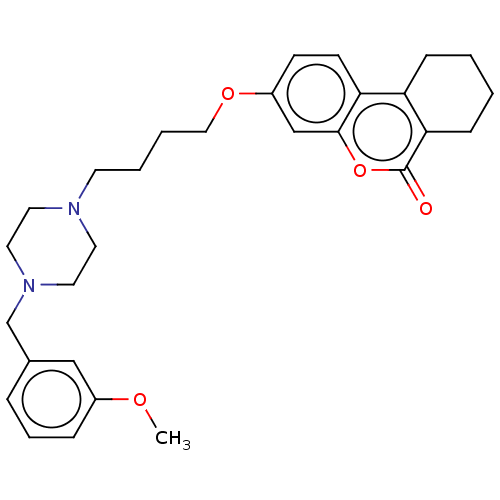 (CHEMBL3335055) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024889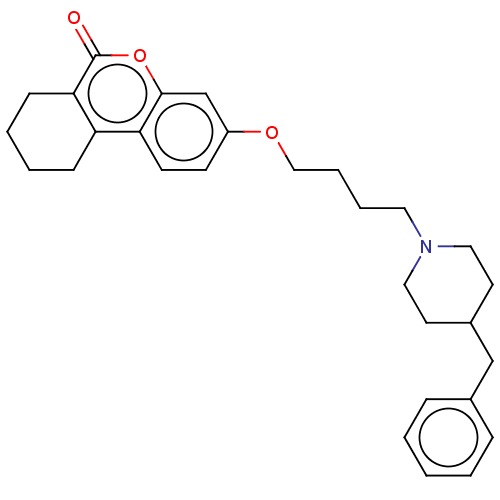 (CHEMBL3335052) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024865 (CHEMBL3335017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM294219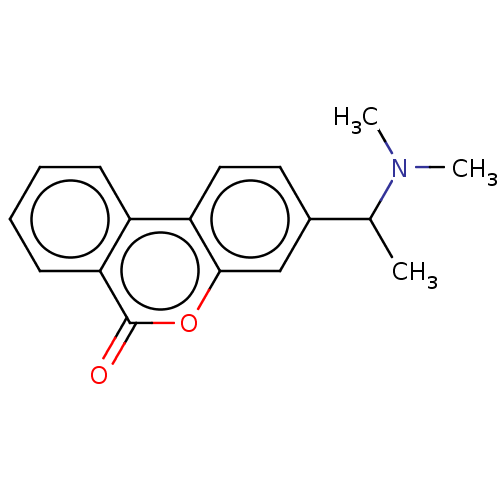 ((±)-3-(1-(dimethylamino)ethyl)-6H-benzo[c]chromen-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50024866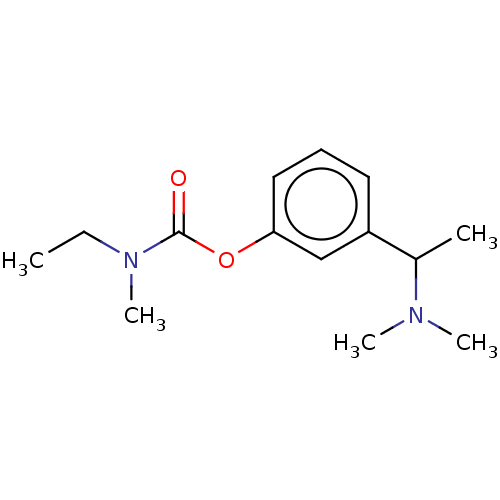 (CHEMBL151763) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024880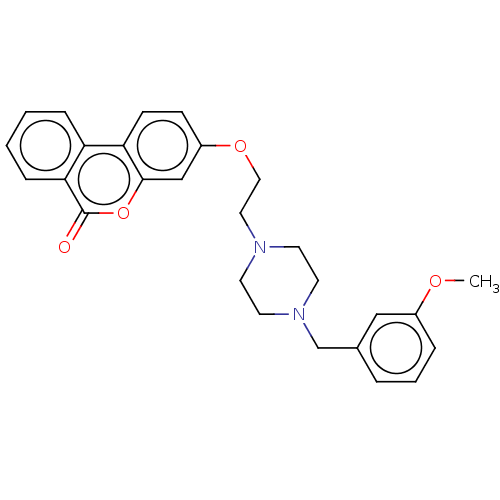 (CHEMBL3335061) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50024881 (CHEMBL3335060) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM294225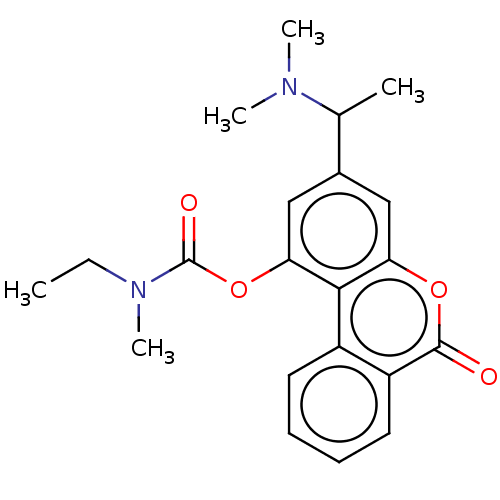 ((±)-3-(1-(dimethylamino)ethyl)-6-oxo-6H-benzo[c]ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NOBEL ILAÇ SANAYII VE TICARET A.S. US Patent | Assay Description The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th... | US Patent US9586925 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50024955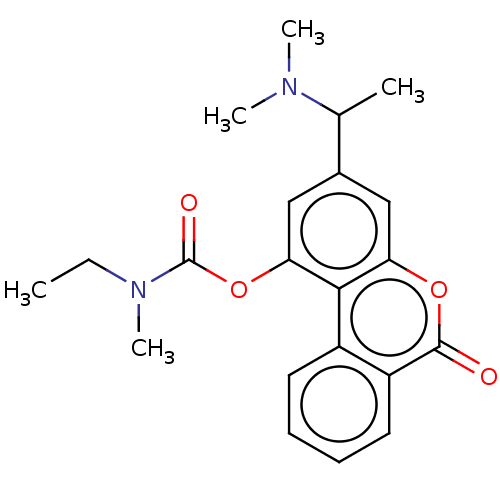 (CHEMBL3335023) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50024888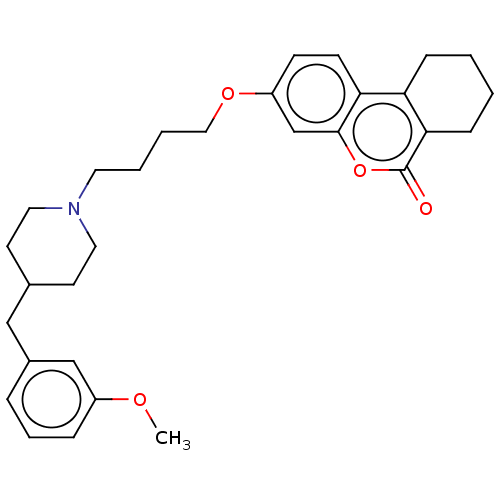 (CHEMBL3335053) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 5141-54 (2014) Article DOI: 10.1016/j.bmc.2014.08.016 BindingDB Entry DOI: 10.7270/Q2WH2RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 116 total ) | Next | Last >> |