 Found 5979 hits with Last Name = 'evans' and Initial = 'd'
Found 5979 hits with Last Name = 'evans' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528106

(N-{[5-methoxy-2-(1,2,3,4-tetrazol-1-yl)phenyl]meth...)Show SMILES COCc1nn(Cc2ccc(Cn3ccccc3=O)cc2)cc1C(=O)NCc1cc(OC)ccc1-n1cnnn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289410
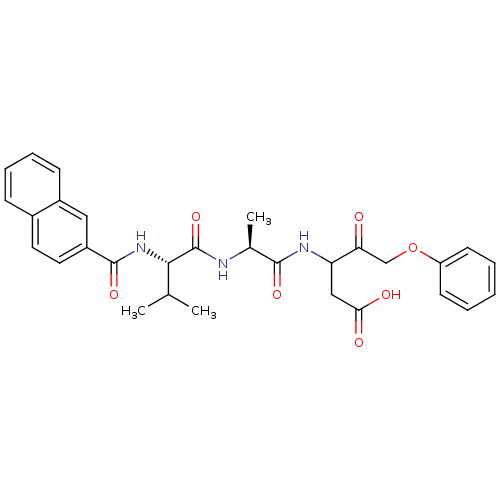
(CHEMBL26544 | Peptidic phenyl ketoether analogue)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C30H33N3O7/c1-18(2)27(33-29(38)22-14-13-20-9-7-8-10-21(20)15-22)30(39)31-19(3)28(37)32-24(16-26(35)36)25(34)17-40-23-11-5-4-6-12-23/h4-15,18-19,24,27H,16-17H2,1-3H3,(H,31,39)(H,32,37)(H,33,38)(H,35,36)/t19-,24?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289397
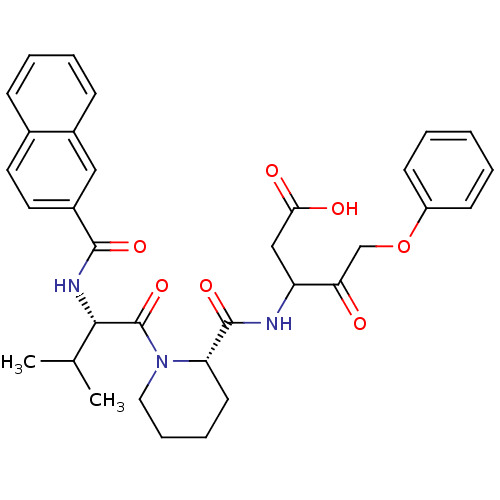
(3-[(1-{3-Methyl-2-[(naphthalene-2-carbonyl)-amino]...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N1CCCC[C@H]1C(=O)NC(CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C33H37N3O7/c1-21(2)30(35-31(40)24-16-15-22-10-6-7-11-23(22)18-24)33(42)36-17-9-8-14-27(36)32(41)34-26(19-29(38)39)28(37)20-43-25-12-4-3-5-13-25/h3-7,10-13,15-16,18,21,26-27,30H,8-9,14,17,19-20H2,1-2H3,(H,34,41)(H,35,40)(H,38,39)/t26?,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528123

(N-{[5-chloro-2-(1,2,3,4-tetrazol-1-yl)phenyl]methy...)Show SMILES COCc1nn(Cc2ccc(Cn3ccccc3=O)cc2)cc1C(=O)NCc1cc(Cl)ccc1-n1cnnn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM455297
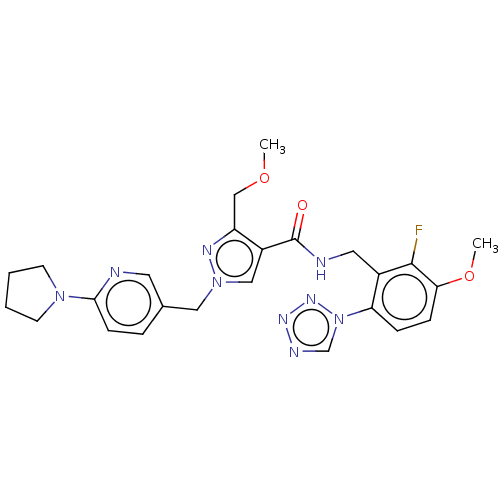
(US10730874, Compound TABLE II.2 | US11180484, Exam...)Show SMILES COCc1nn(Cc2ccc(nc2)N2CCCC2)cc1C(=O)NCc1c(F)c(OC)ccc1-n1cnnn1 Show InChI InChI=1S/C25H28FN9O3/c1-37-15-20-19(14-34(30-20)13-17-5-8-23(27-11-17)33-9-3-4-10-33)25(36)28-12-18-21(35-16-29-31-32-35)6-7-22(38-2)24(18)26/h5-8,11,14,16H,3-4,9-10,12-13,15H2,1-2H3,(H,28,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289398

(CHEMBL553107 | Peptidic phenyl ketoether analogue)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N1CCC[C@H]1C(=O)NC(CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C32H35N3O7/c1-20(2)29(34-30(39)23-15-14-21-9-6-7-10-22(21)17-23)32(41)35-16-8-13-26(35)31(40)33-25(18-28(37)38)27(36)19-42-24-11-4-3-5-12-24/h3-7,9-12,14-15,17,20,25-26,29H,8,13,16,18-19H2,1-2H3,(H,33,40)(H,34,39)(H,37,38)/t25?,26-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528127

(US11180484, Example 79 | US11180484, Reference Exa...)Show SMILES COCc1nn(Cc2cnc(nc2)N2CCCC2)cc1C(=O)NCc1c(F)c(OC)ccc1-n1cnnn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135249

((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2cc(F)ccc2s1 Show InChI InChI=1S/C26H29FN2O2S/c1-16-10-22-23(28-16)4-3-5-24(22)31-15-21(30)14-29-9-8-18(11-17(29)2)26-13-19-12-20(27)6-7-25(19)32-26/h3-7,10,12-13,17-18,21,28,30H,8-9,11,14-15H2,1-2H3/t17-,18+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528105

(N-{[2-fluoro-3-methoxy-6-(1,2,3,4-tetrazol-1-yl)ph...)Show SMILES COCc1nn(Cc2ccc(Cn3ccccc3=O)cc2)cc1C(=O)NCc1c(F)c(OC)ccc1-n1cnnn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130163

((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528131

(1-{[2-(3,3-Difluoropyrrolidin-1-yl)pyrimidin-5-yl]...)Show SMILES COCc1nn(Cc2cnc(nc2)N2CCC(F)(F)C2)cc1C(=O)NCc1c(F)c(OC)ccc1-n1cnnn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528119
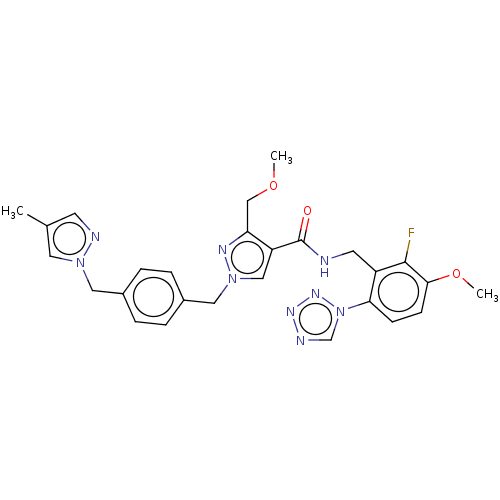
(N-{[2-fluoro-3-methoxy-6-(1,2,3,4-tetrazol-1-yl)ph...)Show SMILES COCc1nn(Cc2ccc(Cn3cc(C)cn3)cc2)cc1C(=O)NCc1c(F)c(OC)ccc1-n1cnnn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130152
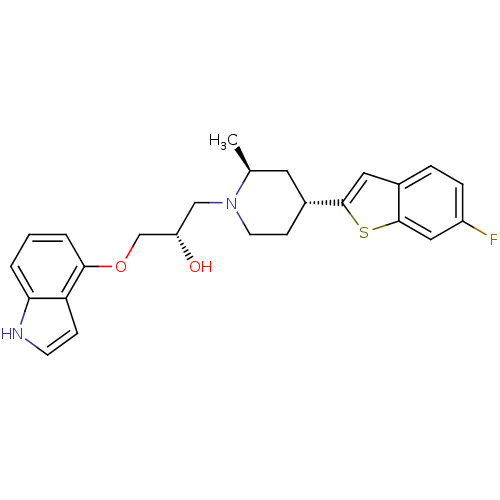
((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C25H27FN2O2S/c1-16-11-18(24-12-17-5-6-19(26)13-25(17)31-24)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16,18,20,27,29H,8,10-11,14-15H2,1H3/t16-,18+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50068972
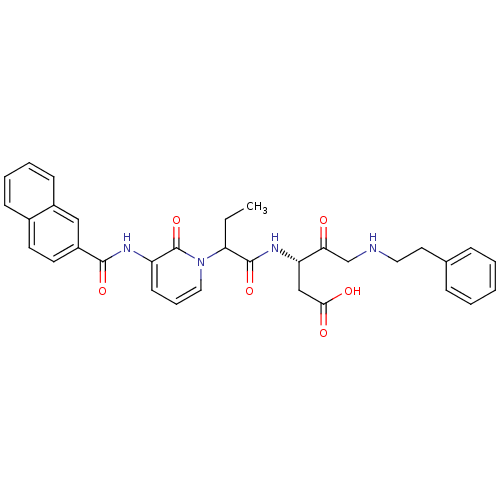
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES CCC(C(=O)N[C@@H](CC(O)=O)C(=O)CNCCc1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C33H34N4O6/c1-2-28(32(42)36-27(20-30(39)40)29(38)21-34-17-16-22-9-4-3-5-10-22)37-18-8-13-26(33(37)43)35-31(41)25-15-14-23-11-6-7-12-24(23)19-25/h3-15,18-19,27-28,34H,2,16-17,20-21H2,1H3,(H,35,41)(H,36,42)(H,39,40)/t27-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ferring Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IL-1 beta converting enzyme |
Bioorg Med Chem Lett 8: 959-64 (1999)
BindingDB Entry DOI: 10.7270/Q2ZP458C |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528127

(US11180484, Example 79 | US11180484, Reference Exa...)Show SMILES COCc1nn(Cc2cnc(nc2)N2CCCC2)cc1C(=O)NCc1c(F)c(OC)ccc1-n1cnnn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130168
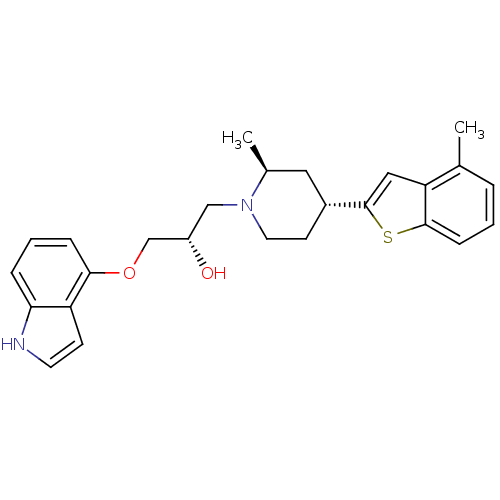
((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2c(C)cccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-5-3-8-25-22(17)14-26(31-25)19-10-12-28(18(2)13-19)15-20(29)16-30-24-7-4-6-23-21(24)9-11-27-23/h3-9,11,14,18-20,27,29H,10,12-13,15-16H2,1-2H3/t18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130157
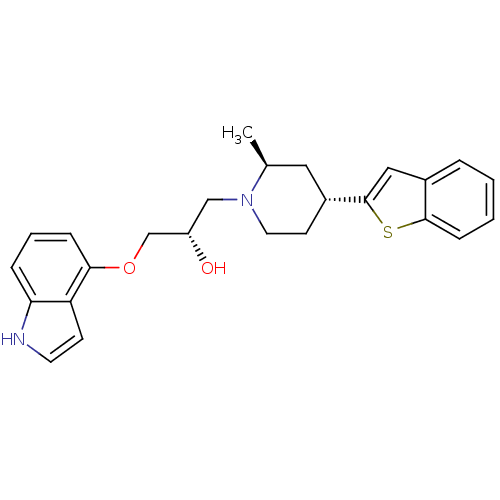
((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccccc2s1 Show InChI InChI=1S/C25H28N2O2S/c1-17-13-19(25-14-18-5-2-3-8-24(18)30-25)10-12-27(17)15-20(28)16-29-23-7-4-6-22-21(23)9-11-26-22/h2-9,11,14,17,19-20,26,28H,10,12-13,15-16H2,1H3/t17-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528074

(N-{[2-Fluoro-6-(1,2,3,4-tetrazol-1-yl)phenyl]methy...)Show SMILES COCc1nn(Cc2ccc(Cn3ccccc3=O)cc2)cc1C(=O)NCc1c(F)cccc1-n1cnnn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135246
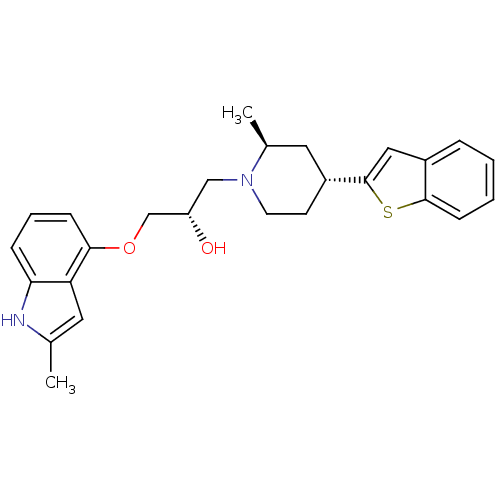
((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2ccccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-12-22-23(27-17)7-5-8-24(22)30-16-21(29)15-28-11-10-20(13-18(28)2)26-14-19-6-3-4-9-25(19)31-26/h3-9,12,14,18,20-21,27,29H,10-11,13,15-16H2,1-2H3/t18-,20+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528130
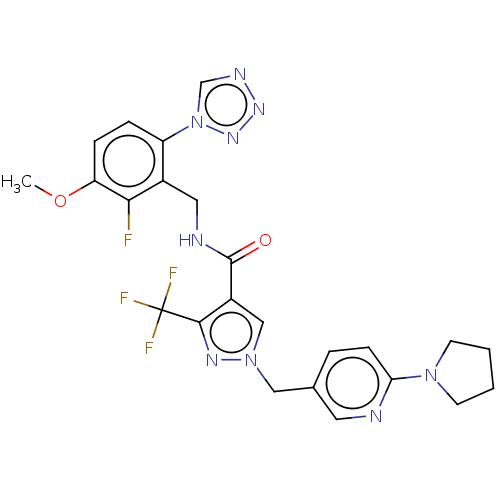
(N-{[2-fluoro-3-methoxy-6-(1,2,3,4-tetrazol-1-yl)ph...)Show SMILES COc1ccc(c(CNC(=O)c2cn(Cc3ccc(nc3)N3CCCC3)nc2C(F)(F)F)c1F)-n1cnnn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130169
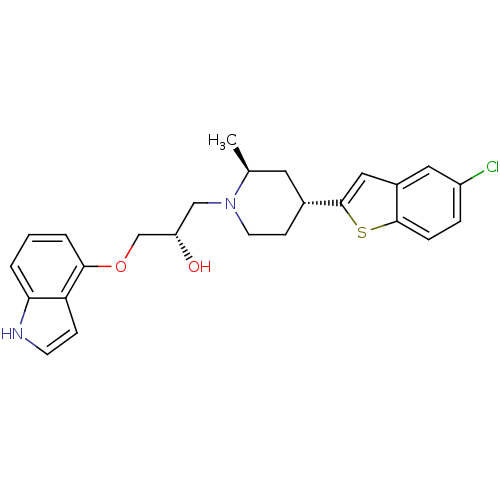
((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2cc(Cl)ccc2s1 Show InChI InChI=1S/C25H27ClN2O2S/c1-16-11-17(25-13-18-12-19(26)5-6-24(18)31-25)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16-17,20,27,29H,8,10-11,14-15H2,1H3/t16-,17+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50017519
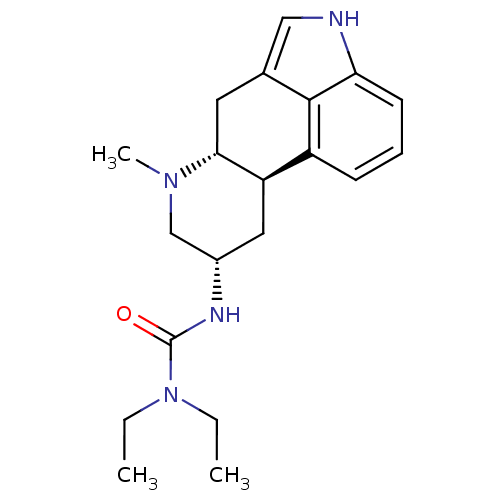
(1,1-Diethyl-3-(7-methyl-4,6,6a,7,8,9,10,10a-octahy...)Show SMILES CCN(CC)C(=O)N[C@H]1C[C@H]2[C@@H](Cc3c[nH]c4cccc2c34)N(C)C1 Show InChI InChI=1S/C20H28N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,11,14,16,18,21H,4-5,9-10,12H2,1-3H3,(H,22,25)/t14-,16+,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Maryland Psychiatric Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 236: 483-6 (1993)
Article DOI: 10.1016/0014-2999(93)90488-4
BindingDB Entry DOI: 10.7270/Q25Q4TKG |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50068954
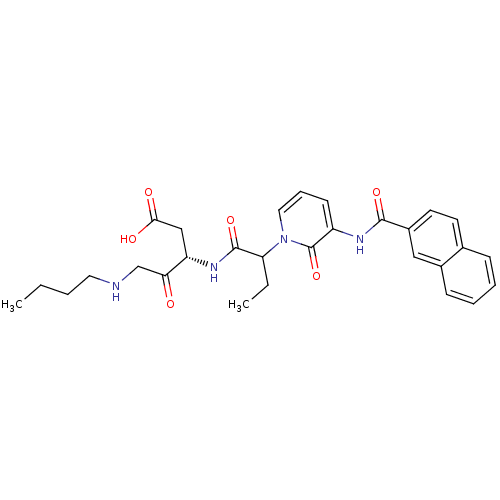
((S)-5-Butylamino-3-(2-{3-[(naphthalene-2-carbonyl)...)Show SMILES CCCCNCC(=O)[C@H](CC(O)=O)NC(=O)C(CC)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C29H34N4O6/c1-3-5-14-30-18-25(34)23(17-26(35)36)32-28(38)24(4-2)33-15-8-11-22(29(33)39)31-27(37)21-13-12-19-9-6-7-10-20(19)16-21/h6-13,15-16,23-24,30H,3-5,14,17-18H2,1-2H3,(H,31,37)(H,32,38)(H,35,36)/t23-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ferring Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IL-1 beta converting enzyme |
Bioorg Med Chem Lett 8: 959-64 (1999)
BindingDB Entry DOI: 10.7270/Q2ZP458C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50136680
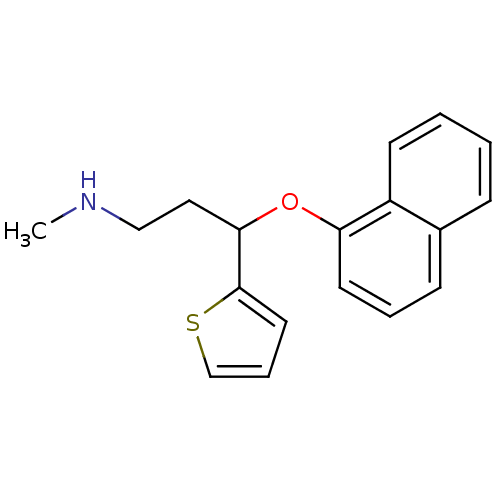
(CHEMBL424660 | N-methyl-3-(1-naphthyloxy)-3-(2-thi...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropharmacology 45: 935-44 (2003)
Article DOI: 10.1016/s0028-3908(03)00268-5
BindingDB Entry DOI: 10.7270/Q2VQ318R |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50068949
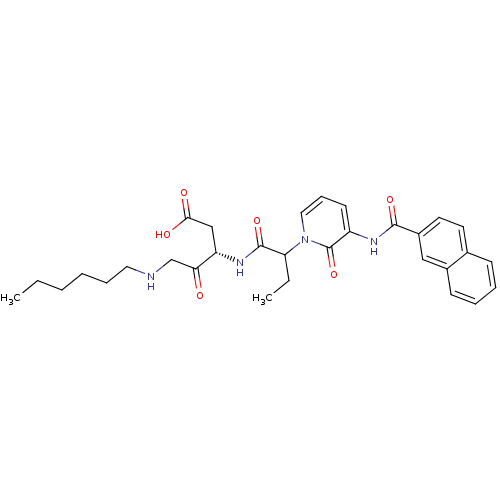
((S)-5-Hexylamino-3-(2-{3-[(naphthalene-2-carbonyl)...)Show SMILES CCCCCCNCC(=O)[C@H](CC(O)=O)NC(=O)C(CC)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C31H38N4O6/c1-3-5-6-9-16-32-20-27(36)25(19-28(37)38)34-30(40)26(4-2)35-17-10-13-24(31(35)41)33-29(39)23-15-14-21-11-7-8-12-22(21)18-23/h7-8,10-15,17-18,25-26,32H,3-6,9,16,19-20H2,1-2H3,(H,33,39)(H,34,40)(H,37,38)/t25-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ferring Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IL-1 beta converting enzyme |
Bioorg Med Chem Lett 8: 959-64 (1999)
BindingDB Entry DOI: 10.7270/Q2ZP458C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135256
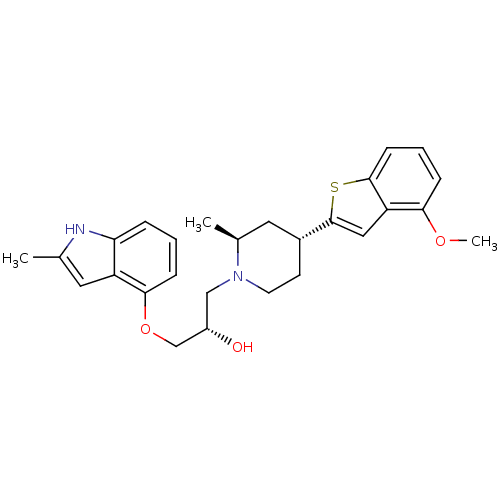
((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]c(C)cc23)[C@@H](C)C1 Show InChI InChI=1S/C27H32N2O3S/c1-17-12-21-23(28-17)6-4-8-25(21)32-16-20(30)15-29-11-10-19(13-18(29)2)27-14-22-24(31-3)7-5-9-26(22)33-27/h4-9,12,14,18-20,28,30H,10-11,13,15-16H2,1-3H3/t18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Maryland Psychiatric Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 236: 483-6 (1993)
Article DOI: 10.1016/0014-2999(93)90488-4
BindingDB Entry DOI: 10.7270/Q25Q4TKG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50047138
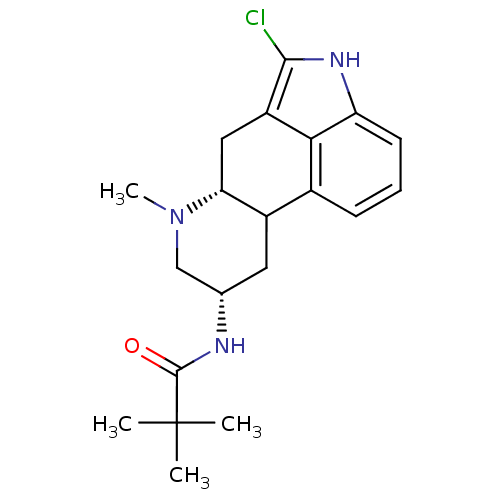
(CHEMBL25074 | N-(5-Chloro-7-methyl-4,6,6a,7,8,9,10...)Show SMILES CN1C[C@H](CC2[C@H]1Cc1c(Cl)[nH]c3cccc2c13)NC(=O)C(C)(C)C Show InChI InChI=1S/C20H26ClN3O/c1-20(2,3)19(25)22-11-8-13-12-6-5-7-15-17(12)14(18(21)23-15)9-16(13)24(4)10-11/h5-7,11,13,16,23H,8-10H2,1-4H3,(H,22,25)/t11-,13?,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Maryland Psychiatric Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 236: 483-6 (1993)
Article DOI: 10.1016/0014-2999(93)90488-4
BindingDB Entry DOI: 10.7270/Q25Q4TKG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM86421
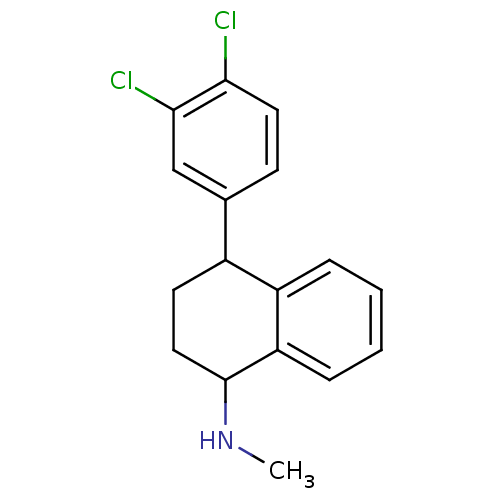
(CAS_79617-96-2 | NSC_68617 | SERTRALINE)Show InChI InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropharmacology 45: 935-44 (2003)
Article DOI: 10.1016/s0028-3908(03)00268-5
BindingDB Entry DOI: 10.7270/Q2VQ318R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082406

(1-(2-fluorophenoxy)-3-spiro[1,3-dihydrobenzo[e]iso...)Show SMILES O[C@H](COc1ccccc1F)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C25H26FNO3/c26-23-7-3-4-8-24(23)29-16-19(28)15-27-13-11-25(12-14-27)22-10-9-18-5-1-2-6-20(18)21(22)17-30-25/h1-10,19,28H,11-17H2/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289405

(CHEMBL2371932 | Peptidic phenyl ketoether analogue)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C30H38N4O9/c1-17(2)27(34-29(41)24(32-19(4)35)14-20-10-12-21(36)13-11-20)30(42)31-18(3)28(40)33-23(15-26(38)39)25(37)16-43-22-8-6-5-7-9-22/h5-13,17-18,23-24,27,36H,14-16H2,1-4H3,(H,31,42)(H,32,35)(H,33,40)(H,34,41)(H,38,39)/t18-,23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082405

(1-phenoxy-3-spiro[1,3-dihydrobenzo[e]isobenzofuran...)Show SMILES O[C@H](COc1ccccc1)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C25H27NO3/c27-20(17-28-21-7-2-1-3-8-21)16-26-14-12-25(13-15-26)24-11-10-19-6-4-5-9-22(19)23(24)18-29-25/h1-11,20,27H,12-18H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130167
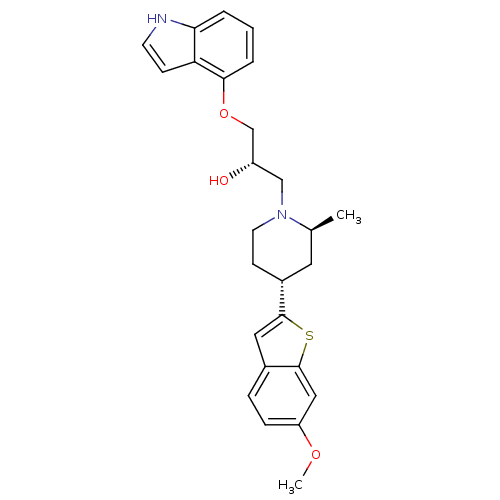
((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(6-methoxy-b...)Show SMILES COc1ccc2cc(sc2c1)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-12-19(25-13-18-6-7-21(30-2)14-26(18)32-25)9-11-28(17)15-20(29)16-31-24-5-3-4-23-22(24)8-10-27-23/h3-8,10,13-14,17,19-20,27,29H,9,11-12,15-16H2,1-2H3/t17-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Maryland Psychiatric Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 236: 483-6 (1993)
Article DOI: 10.1016/0014-2999(93)90488-4
BindingDB Entry DOI: 10.7270/Q25Q4TKG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130165
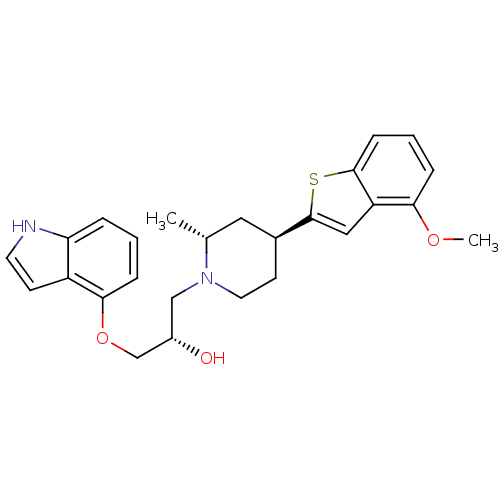
((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6S)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50068950
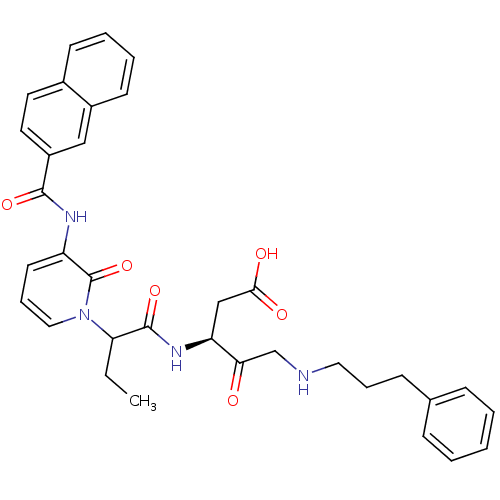
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES CCC(C(=O)N[C@@H](CC(O)=O)C(=O)CNCCCc1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C34H36N4O6/c1-2-29(33(43)37-28(21-31(40)41)30(39)22-35-18-8-12-23-10-4-3-5-11-23)38-19-9-15-27(34(38)44)36-32(42)26-17-16-24-13-6-7-14-25(24)20-26/h3-7,9-11,13-17,19-20,28-29,35H,2,8,12,18,21-22H2,1H3,(H,36,42)(H,37,43)(H,40,41)/t28-,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ferring Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IL-1 beta converting enzyme |
Bioorg Med Chem Lett 8: 959-64 (1999)
BindingDB Entry DOI: 10.7270/Q2ZP458C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Maryland Psychiatric Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 236: 483-6 (1993)
Article DOI: 10.1016/0014-2999(93)90488-4
BindingDB Entry DOI: 10.7270/Q25Q4TKG |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50084633
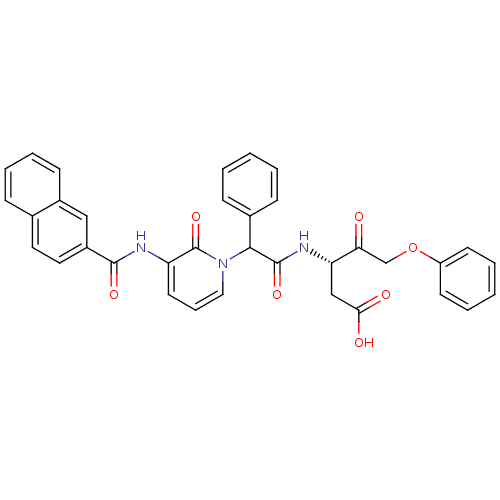
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)C(c1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C35H29N3O7/c39-30(22-45-27-14-5-2-6-15-27)29(21-31(40)41)37-34(43)32(24-11-3-1-4-12-24)38-19-9-16-28(35(38)44)36-33(42)26-18-17-23-10-7-8-13-25(23)20-26/h1-20,29,32H,21-22H2,(H,36,42)(H,37,43)(H,40,41)/t29-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289402

((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES CCC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C31H29N3O7/c1-2-26(30(39)33-25(18-28(36)37)27(35)19-41-23-11-4-3-5-12-23)34-16-8-13-24(31(34)40)32-29(38)22-15-14-20-9-6-7-10-21(20)17-22/h3-17,25-26H,2,18-19H2,1H3,(H,32,38)(H,33,39)(H,36,37)/t25-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289412
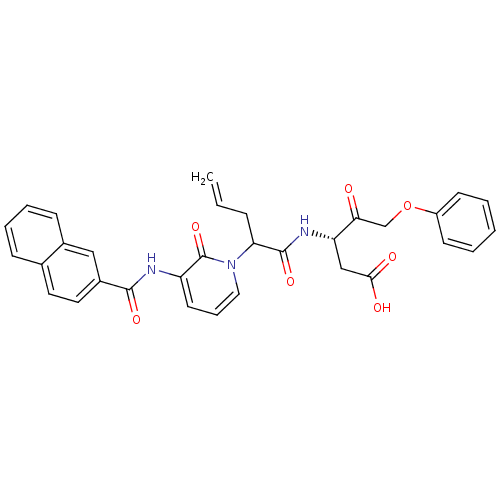
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)C(CC=C)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C32H29N3O7/c1-2-9-27(31(40)34-26(19-29(37)38)28(36)20-42-24-12-4-3-5-13-24)35-17-8-14-25(32(35)41)33-30(39)23-16-15-21-10-6-7-11-22(21)18-23/h2-8,10-18,26-27H,1,9,19-20H2,(H,33,39)(H,34,40)(H,37,38)/t26-,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082413
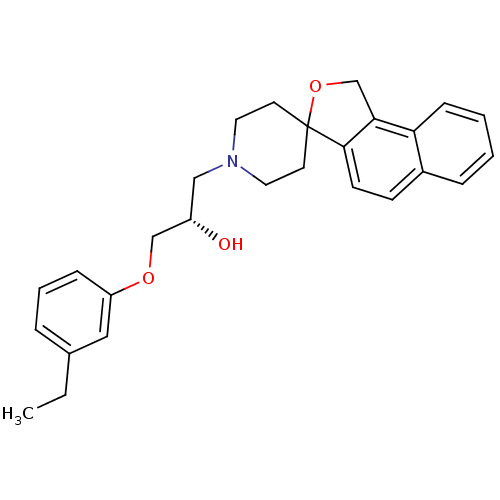
(1-(3-ethylphenoxy)-3-spiro[1,3-dihydrobenzo[e]isob...)Show SMILES CCc1cccc(OC[C@@H](O)CN2CCC3(CC2)OCc2c3ccc3ccccc23)c1 Show InChI InChI=1S/C27H31NO3/c1-2-20-6-5-8-23(16-20)30-18-22(29)17-28-14-12-27(13-15-28)26-11-10-21-7-3-4-9-24(21)25(26)19-31-27/h3-11,16,22,29H,2,12-15,17-19H2,1H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50091577
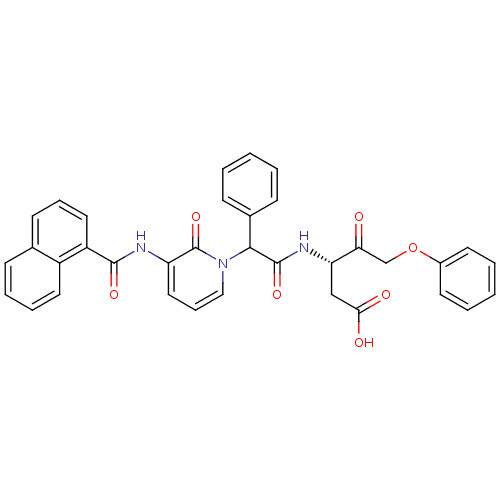
((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)C(c1ccccc1)n1cccc(NC(=O)c2cccc3ccccc23)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C35H29N3O7/c39-30(22-45-25-15-5-2-6-16-25)29(21-31(40)41)37-34(43)32(24-12-3-1-4-13-24)38-20-10-19-28(35(38)44)36-33(42)27-18-9-14-23-11-7-8-17-26(23)27/h1-20,29,32H,21-22H2,(H,36,42)(H,37,43)(H,40,41)/t29-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289393
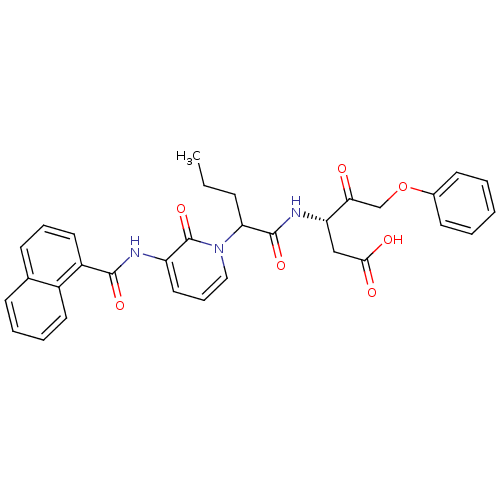
((S)-3-(2-{3-[(Naphthalene-1-carbonyl)-amino]-2-oxo...)Show SMILES CCCC(C(=O)N[C@@H](CC(O)=O)C(=O)COc1ccccc1)n1cccc(NC(=O)c2cccc3ccccc23)c1=O Show InChI InChI=1S/C32H31N3O7/c1-2-10-27(31(40)34-26(19-29(37)38)28(36)20-42-22-13-4-3-5-14-22)35-18-9-17-25(32(35)41)33-30(39)24-16-8-12-21-11-6-7-15-23(21)24/h3-9,11-18,26-27H,2,10,19-20H2,1H3,(H,33,39)(H,34,40)(H,37,38)/t26-,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50068945
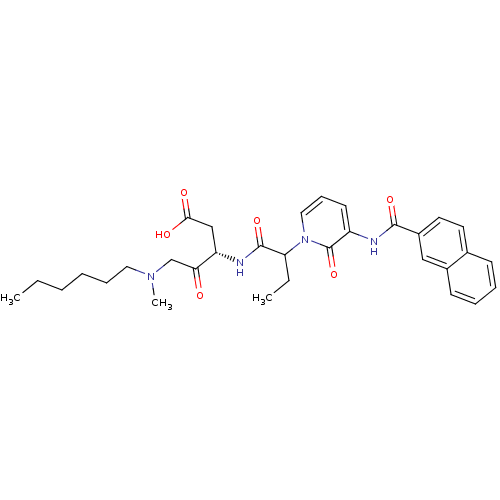
((S)-5-(Hexyl-methyl-amino)-3-(2-{3-[(naphthalene-2...)Show SMILES CCCCCCN(C)CC(=O)[C@H](CC(O)=O)NC(=O)C(CC)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C32H40N4O6/c1-4-6-7-10-17-35(3)21-28(37)26(20-29(38)39)34-31(41)27(5-2)36-18-11-14-25(32(36)42)33-30(40)24-16-15-22-12-8-9-13-23(22)19-24/h8-9,11-16,18-19,26-27H,4-7,10,17,20-21H2,1-3H3,(H,33,40)(H,34,41)(H,38,39)/t26-,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ferring Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IL-1 beta converting enzyme |
Bioorg Med Chem Lett 8: 959-64 (1999)
BindingDB Entry DOI: 10.7270/Q2ZP458C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50128368
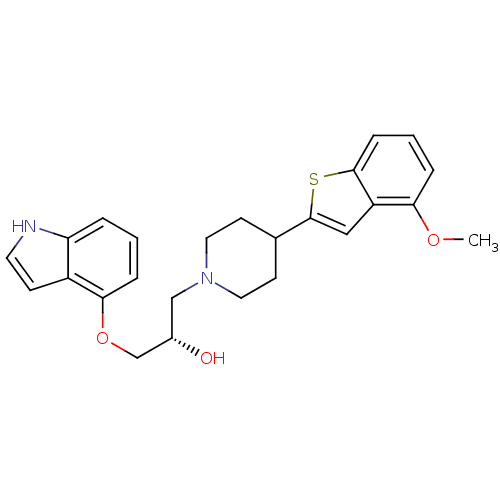
((S)-1-(1H-Indol-4-yloxy)-3-[4-(4-methoxy-benzo[b]t...)Show SMILES COc1cccc2sc(cc12)C1CCN(C[C@H](O)COc2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C25H28N2O3S/c1-29-22-5-3-7-24-20(22)14-25(31-24)17-9-12-27(13-10-17)15-18(28)16-30-23-6-2-4-21-19(23)8-11-26-21/h2-8,11,14,17-18,26,28H,9-10,12-13,15-16H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM528094

(N-[(4-methoxy-3-methylpyridin-2-yl)methyl]-3-(meth...)Show SMILES COCc1nn(Cc2ccc(Cn3ccccc3=O)cc2)cc1C(=O)NCc1nccc(OC)c1C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 18... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2MG7SP5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50128367
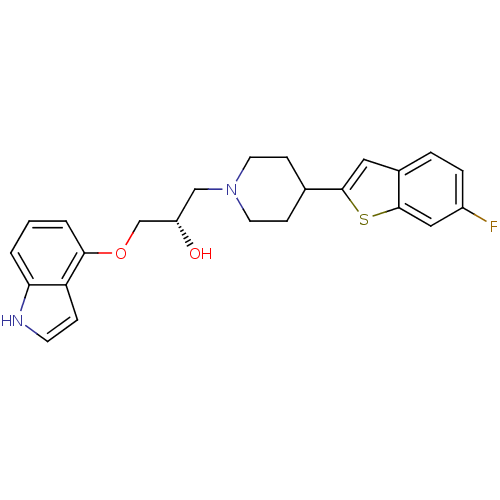
((S)-1-(1H-indol-4-yloxy)-3-(4-(6-fluorobenzo[b]thi...)Show SMILES O[C@H](COc1cccc2[nH]ccc12)CN1CCC(CC1)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C24H25FN2O2S/c25-18-5-4-17-12-23(30-24(17)13-18)16-7-10-27(11-8-16)14-19(28)15-29-22-3-1-2-21-20(22)6-9-26-21/h1-6,9,12-13,16,19,26,28H,7-8,10-11,14-15H2/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50005118
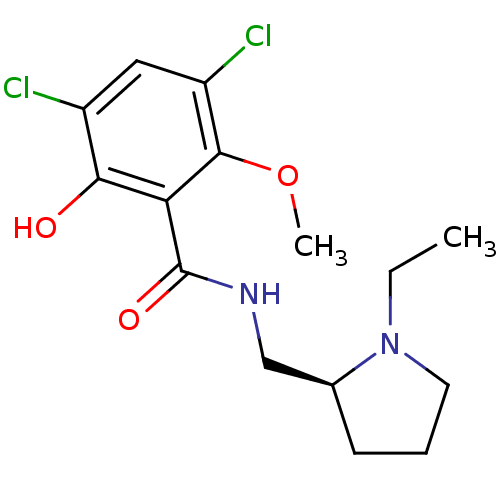
((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...)Show InChI InChI=1S/C15H20Cl2N2O3/c1-3-19-6-4-5-9(19)8-18-15(21)12-13(20)10(16)7-11(17)14(12)22-2/h7,9,20H,3-6,8H2,1-2H3,(H,18,21)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Maryland Psychiatric Research Center
Curated by PDSP Ki Database
| |
Eur J Pharmacol 236: 483-6 (1993)
Article DOI: 10.1016/0014-2999(93)90488-4
BindingDB Entry DOI: 10.7270/Q25Q4TKG |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289407
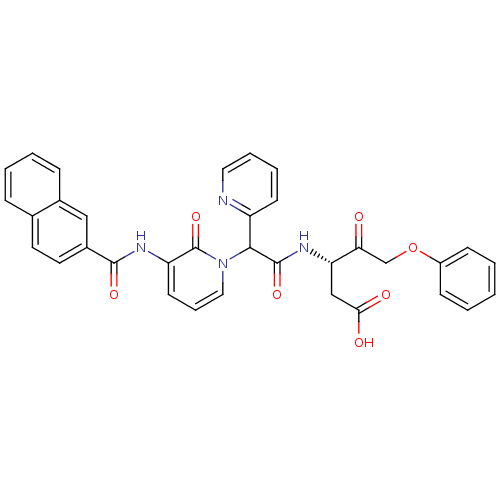
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES OC(=O)C[C@H](NC(=O)C(c1ccccn1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C34H28N4O7/c39-29(21-45-25-11-2-1-3-12-25)28(20-30(40)41)37-33(43)31(26-13-6-7-17-35-26)38-18-8-14-27(34(38)44)36-32(42)24-16-15-22-9-4-5-10-23(22)19-24/h1-19,28,31H,20-21H2,(H,36,42)(H,37,43)(H,40,41)/t28-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data