 Found 189 hits with Last Name = 'faller' and Initial = 'b'
Found 189 hits with Last Name = 'faller' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771
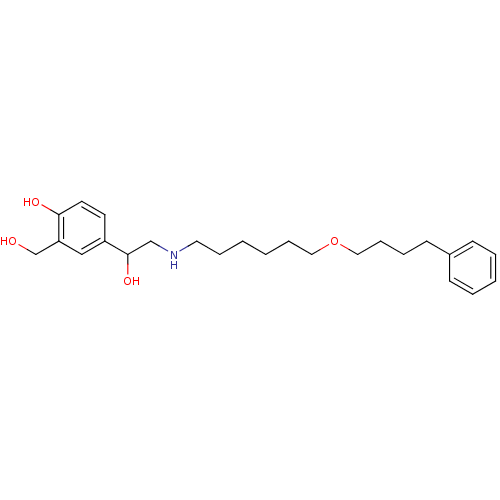
(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318156
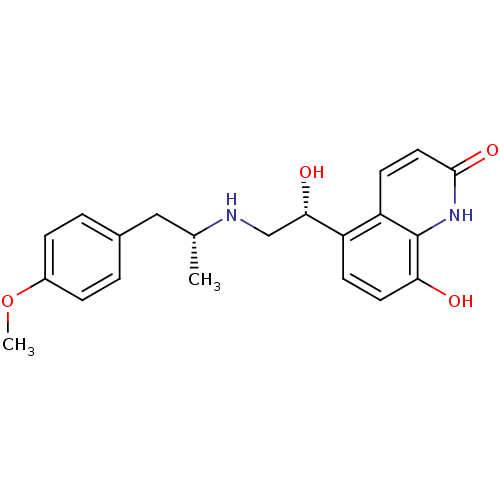
(CHEMBL1094785 | carmoterol)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C21H24N2O4/c1-13(11-14-3-5-15(27-2)6-4-14)22-12-19(25)16-7-9-18(24)21-17(16)8-10-20(26)23-21/h3-10,13,19,22,24-25H,11-12H2,1-2H3,(H,23,26)/t13-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151720
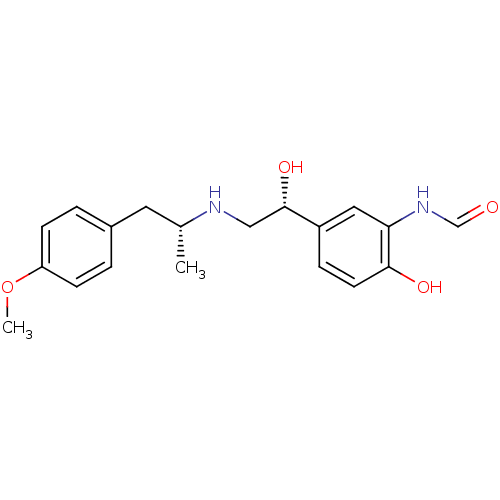
(ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c(NC=O)c2)cc1 |r| Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22)/t13-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318159
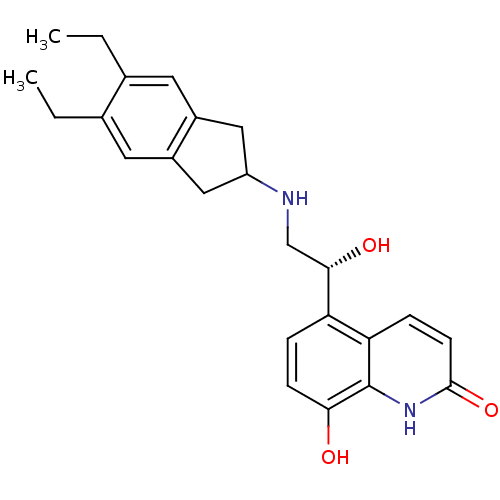
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318157
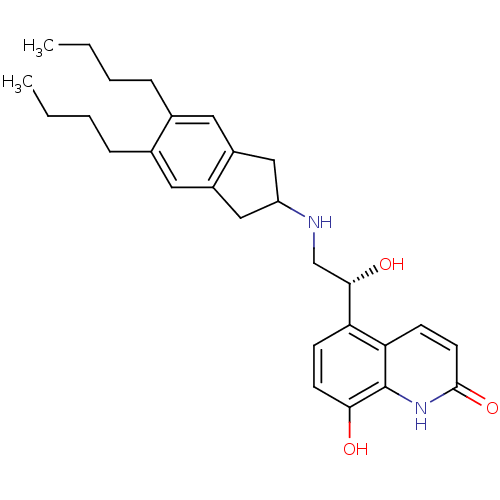
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-butylindan-...)Show SMILES CCCCc1cc2CC(Cc2cc1CCCC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C28H36N2O3/c1-3-5-7-18-13-20-15-22(16-21(20)14-19(18)8-6-4-2)29-17-26(32)23-9-11-25(31)28-24(23)10-12-27(33)30-28/h9-14,22,26,29,31-32H,3-8,15-17H2,1-2H3,(H,30,33)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318158
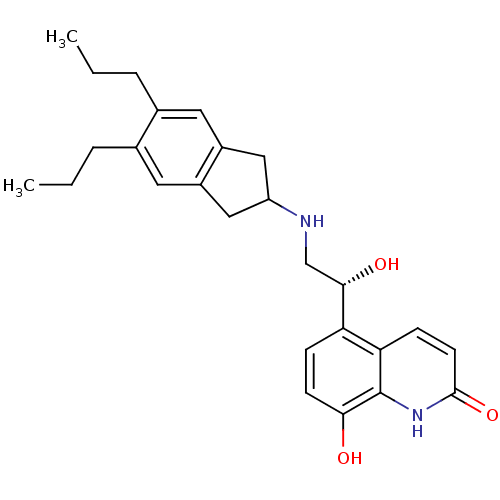
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-propylindan...)Show SMILES CCCc1cc2CC(Cc2cc1CCC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C26H32N2O3/c1-3-5-16-11-18-13-20(14-19(18)12-17(16)6-4-2)27-15-24(30)21-7-9-23(29)26-22(21)8-10-25(31)28-26/h7-12,20,24,27,29-30H,3-6,13-15H2,1-2H3,(H,28,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318161
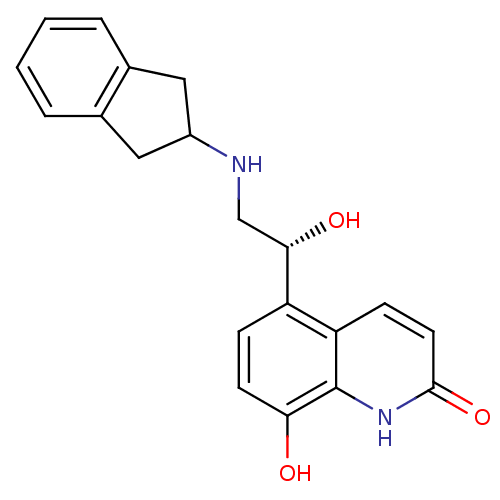
(8-Hydroxy-5-[(R)-1-hydroxy-2-(indan-2-ylamino)-eth...)Show SMILES O[C@@H](CNC1Cc2ccccc2C1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C20H20N2O3/c23-17-7-5-15(16-6-8-19(25)22-20(16)17)18(24)11-21-14-9-12-3-1-2-4-13(12)10-14/h1-8,14,18,21,23-24H,9-11H2,(H,22,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318155
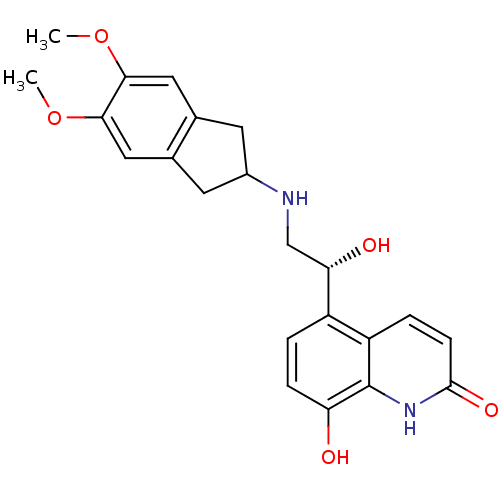
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-dimethoxyindan-2...)Show SMILES COc1cc2CC(Cc2cc1OC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C22H24N2O5/c1-28-19-9-12-7-14(8-13(12)10-20(19)29-2)23-11-18(26)15-3-5-17(25)22-16(15)4-6-21(27)24-22/h3-6,9-10,14,18,23,25-26H,7-8,11H2,1-2H3,(H,24,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318160
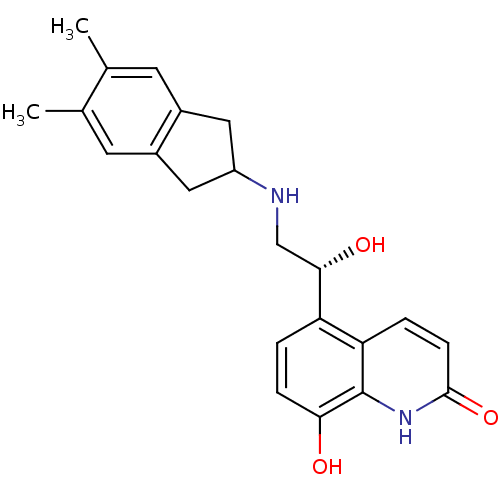
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-dimethylindan-2-...)Show SMILES Cc1cc2CC(Cc2cc1C)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C22H24N2O3/c1-12-7-14-9-16(10-15(14)8-13(12)2)23-11-20(26)17-3-5-19(25)22-18(17)4-6-21(27)24-22/h3-8,16,20,23,25-26H,9-11H2,1-2H3,(H,24,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318154
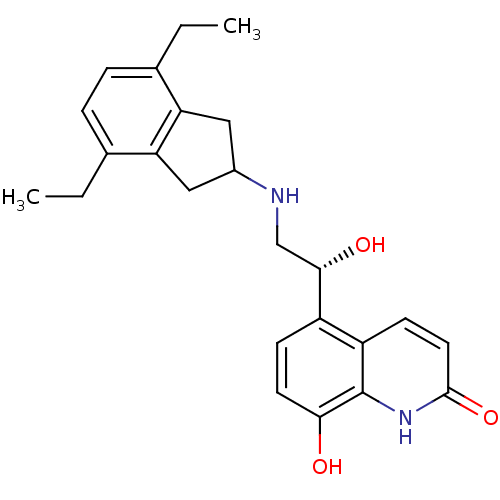
(8-Hydroxy-5-[(R)-1-hydroxy-2-(4,7-diethylindan-2-y...)Show SMILES CCc1ccc(CC)c2CC(Cc12)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-3-14-5-6-15(4-2)20-12-16(11-19(14)20)25-13-22(28)17-7-9-21(27)24-18(17)8-10-23(29)26-24/h5-10,16,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25769
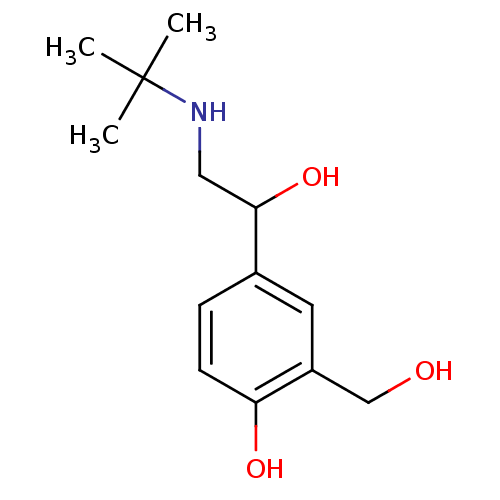
(4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxym...)Show InChI InChI=1S/C13H21NO3/c1-13(2,3)14-7-12(17)9-4-5-11(16)10(6-9)8-15/h4-6,12,14-17H,7-8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4-catalyzed midazolam 1'-hydroxylation in human liver microsomes |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318153
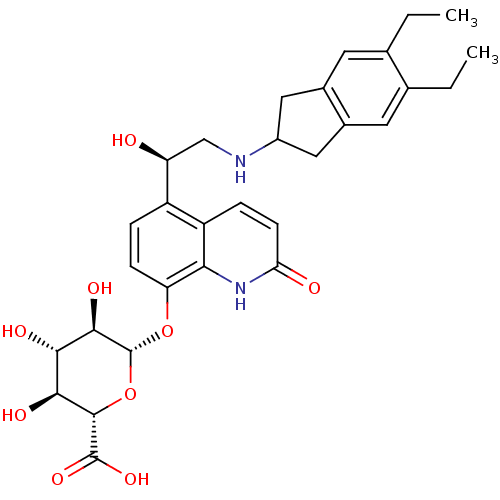
((2S,3S,4S,5R,6S)-6-(5-((R)-2-(5,6-diethyl-2,3-dihy...)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C30H36N2O9/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)31-13-21(33)19-5-7-22(24-20(19)6-8-23(34)32-24)40-30-27(37)25(35)26(36)28(41-30)29(38)39/h5-10,18,21,25-28,30-31,33,35-37H,3-4,11-13H2,1-2H3,(H,32,34)(H,38,39)/t21-,25-,26-,27+,28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50393214

(CHEMBL2151411)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3c(C)cccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C26H24N6O2/c1-15-6-5-8-16-18(14-27-22(15)16)20-21(25(34)30-24(20)33)23-17-7-3-4-9-19(17)28-26(29-23)32-12-10-31(2)11-13-32/h3-9,14,27H,10-13H2,1-2H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50393214

(CHEMBL2151411)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3c(C)cccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C26H24N6O2/c1-15-6-5-8-16-18(14-27-22(15)16)20-21(25(34)30-24(20)33)23-17-7-3-4-9-19(17)28-26(29-23)32-12-10-31(2)11-13-32/h3-9,14,27H,10-13H2,1-2H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393214

(CHEMBL2151411)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3c(C)cccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C26H24N6O2/c1-15-6-5-8-16-18(14-27-22(15)16)20-21(25(34)30-24(20)33)23-17-7-3-4-9-19(17)28-26(29-23)32-12-10-31(2)11-13-32/h3-9,14,27H,10-13H2,1-2H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50318156

(CHEMBL1094785 | carmoterol)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C21H24N2O4/c1-13(11-14-3-5-15(27-2)6-4-14)22-12-19(25)16-7-9-18(24)21-17(16)8-10-20(26)23-21/h3-10,13,19,22,24-25H,11-12H2,1-2H3,(H,23,26)/t13-,19+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50151720

(ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c(NC=O)c2)cc1 |r| Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22)/t13-,19+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50393218
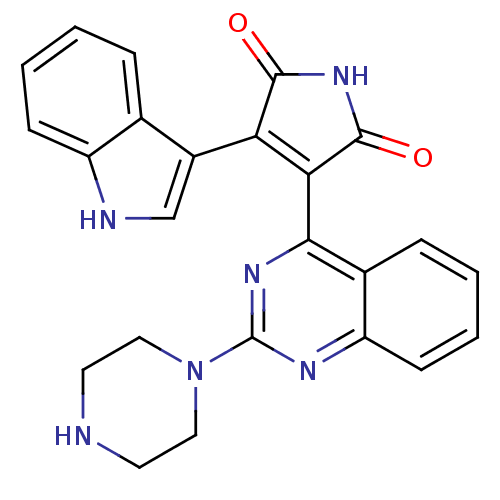
(CHEMBL1996510)Show SMILES O=C1NC(=O)C(=C1c1c[nH]c2ccccc12)c1nc(nc2ccccc12)N1CCNCC1 |c:5| Show InChI InChI=1S/C24H20N6O2/c31-22-19(16-13-26-17-7-3-1-5-14(16)17)20(23(32)29-22)21-15-6-2-4-8-18(15)27-24(28-21)30-11-9-25-10-12-30/h1-8,13,25-26H,9-12H2,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50393214

(CHEMBL2151411)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3c(C)cccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C26H24N6O2/c1-15-6-5-8-16-18(14-27-22(15)16)20-21(25(34)30-24(20)33)23-17-7-3-4-9-19(17)28-26(29-23)32-12-10-31(2)11-13-32/h3-9,14,27H,10-13H2,1-2H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50393218

(CHEMBL1996510)Show SMILES O=C1NC(=O)C(=C1c1c[nH]c2ccccc12)c1nc(nc2ccccc12)N1CCNCC1 |c:5| Show InChI InChI=1S/C24H20N6O2/c31-22-19(16-13-26-17-7-3-1-5-14(16)17)20(23(32)29-22)21-15-6-2-4-8-18(15)27-24(28-21)30-11-9-25-10-12-30/h1-8,13,25-26H,9-12H2,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50393218

(CHEMBL1996510)Show SMILES O=C1NC(=O)C(=C1c1c[nH]c2ccccc12)c1nc(nc2ccccc12)N1CCNCC1 |c:5| Show InChI InChI=1S/C24H20N6O2/c31-22-19(16-13-26-17-7-3-1-5-14(16)17)20(23(32)29-22)21-15-6-2-4-8-18(15)27-24(28-21)30-11-9-25-10-12-30/h1-8,13,25-26H,9-12H2,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393218

(CHEMBL1996510)Show SMILES O=C1NC(=O)C(=C1c1c[nH]c2ccccc12)c1nc(nc2ccccc12)N1CCNCC1 |c:5| Show InChI InChI=1S/C24H20N6O2/c31-22-19(16-13-26-17-7-3-1-5-14(16)17)20(23(32)29-22)21-15-6-2-4-8-18(15)27-24(28-21)30-11-9-25-10-12-30/h1-8,13,25-26H,9-12H2,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50393228
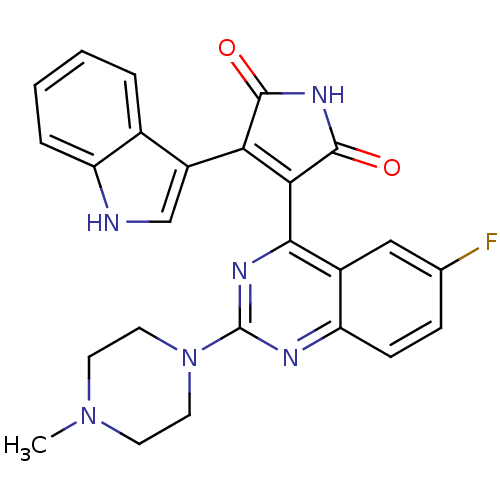
(CHEMBL2153750)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(F)ccc2n1 |t:11| Show InChI InChI=1S/C25H21FN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50393228

(CHEMBL2153750)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(F)ccc2n1 |t:11| Show InChI InChI=1S/C25H21FN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50393214

(CHEMBL2151411)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3c(C)cccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C26H24N6O2/c1-15-6-5-8-16-18(14-27-22(15)16)20-21(25(34)30-24(20)33)23-17-7-3-4-9-19(17)28-26(29-23)32-12-10-31(2)11-13-32/h3-9,14,27H,10-13H2,1-2H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCepsilon by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50318161

(8-Hydroxy-5-[(R)-1-hydroxy-2-(indan-2-ylamino)-eth...)Show SMILES O[C@@H](CNC1Cc2ccccc2C1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C20H20N2O3/c23-17-7-5-15(16-6-8-19(25)22-20(16)17)18(24)11-21-14-9-12-3-1-2-4-13(12)10-14/h1-8,14,18,21,23-24H,9-11H2,(H,22,25)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM33970
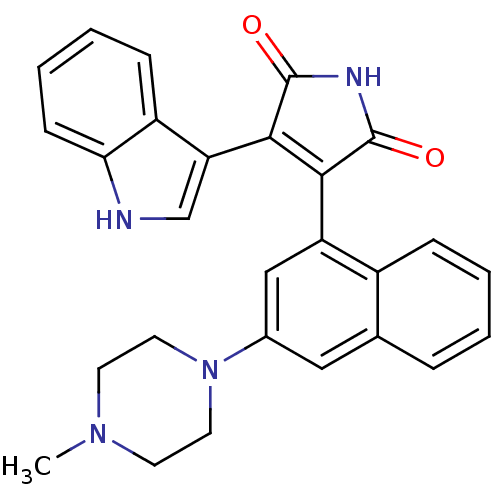
(maleimide derivative, 12)Show SMILES CN1CCN(CC1)c1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:11| Show InChI InChI=1S/C27H24N4O2/c1-30-10-12-31(13-11-30)18-14-17-6-2-3-7-19(17)21(15-18)24-25(27(33)29-26(24)32)22-16-28-23-9-5-4-8-20(22)23/h2-9,14-16,28H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50393218

(CHEMBL1996510)Show SMILES O=C1NC(=O)C(=C1c1c[nH]c2ccccc12)c1nc(nc2ccccc12)N1CCNCC1 |c:5| Show InChI InChI=1S/C24H20N6O2/c31-22-19(16-13-26-17-7-3-1-5-14(16)17)20(23(32)29-22)21-15-6-2-4-8-18(15)27-24(28-21)30-11-9-25-10-12-30/h1-8,13,25-26H,9-12H2,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCepsilon by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50393228

(CHEMBL2153750)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(F)ccc2n1 |t:11| Show InChI InChI=1S/C25H21FN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50393218

(CHEMBL1996510)Show SMILES O=C1NC(=O)C(=C1c1c[nH]c2ccccc12)c1nc(nc2ccccc12)N1CCNCC1 |c:5| Show InChI InChI=1S/C24H20N6O2/c31-22-19(16-13-26-17-7-3-1-5-14(16)17)20(23(32)29-22)21-15-6-2-4-8-18(15)27-24(28-21)30-11-9-25-10-12-30/h1-8,13,25-26H,9-12H2,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCeta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50393219
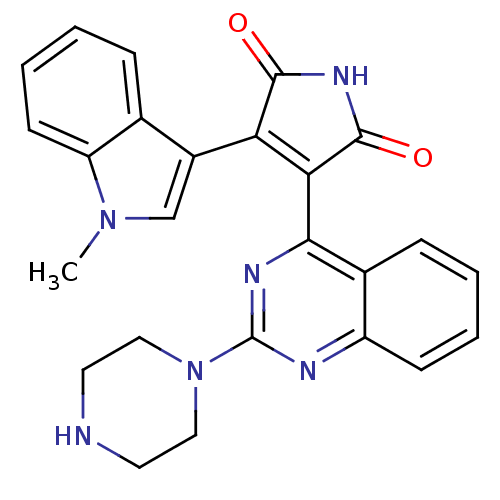
(CHEMBL2151415)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2nc(nc3ccccc23)N2CCNCC2)c2ccccc12 |t:4| Show InChI InChI=1S/C25H22N6O2/c1-30-14-17(15-6-3-5-9-19(15)30)20-21(24(33)29-23(20)32)22-16-7-2-4-8-18(16)27-25(28-22)31-12-10-26-11-13-31/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50393229
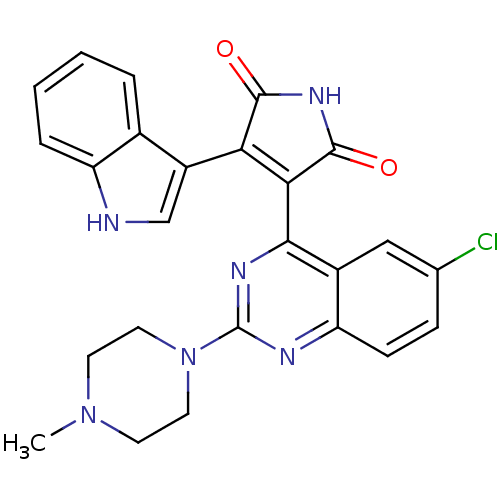
(CHEMBL2153751)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(Cl)ccc2n1 |t:11| Show InChI InChI=1S/C25H21ClN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50393229

(CHEMBL2153751)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(Cl)ccc2n1 |t:11| Show InChI InChI=1S/C25H21ClN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393228

(CHEMBL2153750)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(F)ccc2n1 |t:11| Show InChI InChI=1S/C25H21FN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50393219

(CHEMBL2151415)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2nc(nc3ccccc23)N2CCNCC2)c2ccccc12 |t:4| Show InChI InChI=1S/C25H22N6O2/c1-30-14-17(15-6-3-5-9-19(15)30)20-21(24(33)29-23(20)32)22-16-7-2-4-8-18(16)27-25(28-22)31-12-10-26-11-13-31/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50393226
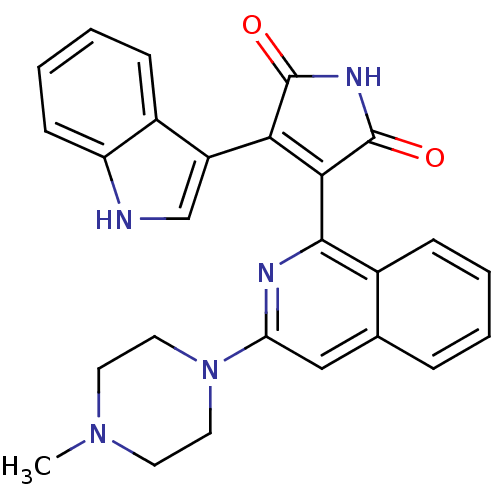
(CHEMBL2153748)Show SMILES CN1CCN(CC1)c1cc2ccccc2c(n1)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:20| Show InChI InChI=1S/C26H23N5O2/c1-30-10-12-31(13-11-30)21-14-16-6-2-3-7-17(16)24(28-21)23-22(25(32)29-26(23)33)19-15-27-20-9-5-4-8-18(19)20/h2-9,14-15,27H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50393219

(CHEMBL2151415)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2nc(nc3ccccc23)N2CCNCC2)c2ccccc12 |t:4| Show InChI InChI=1S/C25H22N6O2/c1-30-14-17(15-6-3-5-9-19(15)30)20-21(24(33)29-23(20)32)22-16-7-2-4-8-18(16)27-25(28-22)31-12-10-26-11-13-31/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393219

(CHEMBL2151415)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2nc(nc3ccccc23)N2CCNCC2)c2ccccc12 |t:4| Show InChI InChI=1S/C25H22N6O2/c1-30-14-17(15-6-3-5-9-19(15)30)20-21(24(33)29-23(20)32)22-16-7-2-4-8-18(16)27-25(28-22)31-12-10-26-11-13-31/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50393214

(CHEMBL2151411)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3c(C)cccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C26H24N6O2/c1-15-6-5-8-16-18(14-27-22(15)16)20-21(25(34)30-24(20)33)23-17-7-3-4-9-19(17)28-26(29-23)32-12-10-31(2)11-13-32/h3-9,14,27H,10-13H2,1-2H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCeta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50393229

(CHEMBL2153751)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(Cl)ccc2n1 |t:11| Show InChI InChI=1S/C25H21ClN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50393226

(CHEMBL2153748)Show SMILES CN1CCN(CC1)c1cc2ccccc2c(n1)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:20| Show InChI InChI=1S/C26H23N5O2/c1-30-10-12-31(13-11-30)21-14-16-6-2-3-7-17(16)24(28-21)23-22(25(32)29-26(23)33)19-15-27-20-9-5-4-8-18(19)20/h2-9,14-15,27H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33970

(maleimide derivative, 12)Show SMILES CN1CCN(CC1)c1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:11| Show InChI InChI=1S/C27H24N4O2/c1-30-10-12-31(13-11-30)18-14-17-6-2-3-7-19(17)21(15-18)24-25(27(33)29-26(24)32)22-16-28-23-9-5-4-8-20(22)23/h2-9,14-16,28H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50393228

(CHEMBL2153750)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(F)ccc2n1 |t:11| Show InChI InChI=1S/C25H21FN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCeta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50393226

(CHEMBL2153748)Show SMILES CN1CCN(CC1)c1cc2ccccc2c(n1)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:20| Show InChI InChI=1S/C26H23N5O2/c1-30-10-12-31(13-11-30)21-14-16-6-2-3-7-17(16)24(28-21)23-22(25(32)29-26(23)33)19-15-27-20-9-5-4-8-18(19)20/h2-9,14-15,27H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta-1 by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50393228

(CHEMBL2153750)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2cc(F)ccc2n1 |t:11| Show InChI InChI=1S/C25H21FN6O2/c1-31-8-10-32(11-9-31)25-28-19-7-6-14(26)12-16(19)22(29-25)21-20(23(33)30-24(21)34)17-13-27-18-5-3-2-4-15(17)18/h2-7,12-13,27H,8-11H2,1H3,(H,30,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCepsilon by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50393226

(CHEMBL2153748)Show SMILES CN1CCN(CC1)c1cc2ccccc2c(n1)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:20| Show InChI InChI=1S/C26H23N5O2/c1-30-10-12-31(13-11-30)21-14-16-6-2-3-7-17(16)24(28-21)23-22(25(32)29-26(23)33)19-15-27-20-9-5-4-8-18(19)20/h2-9,14-15,27H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by scintillation proximity assay |
J Med Chem 54: 6028-39 (2011)
Article DOI: 10.1021/jm200469u
BindingDB Entry DOI: 10.7270/Q2K35VR1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data