 Found 4216 hits with Last Name = 'fan' and Initial = 'h'
Found 4216 hits with Last Name = 'fan' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan Provinc
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2 (unknown origin) by TR-FRET assay |
Eur J Med Chem 146: 471-482 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.076
BindingDB Entry DOI: 10.7270/Q2ZG6VS2 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440788
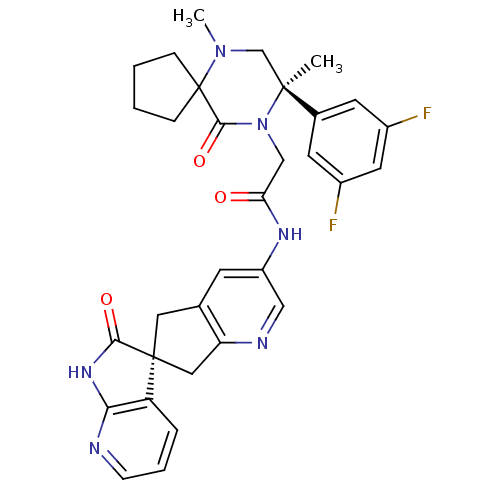
(CHEMBL2431249)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H32F2N6O3/c1-30(20-11-21(33)13-22(34)12-20)18-39(2)32(7-3-4-8-32)29(43)40(30)17-26(41)37-23-10-19-14-31(15-25(19)36-16-23)24-6-5-9-35-27(24)38-28(31)42/h5-6,9-13,16H,3-4,7-8,14-15,17-18H2,1-2H3,(H,37,41)(H,35,38,42)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440782
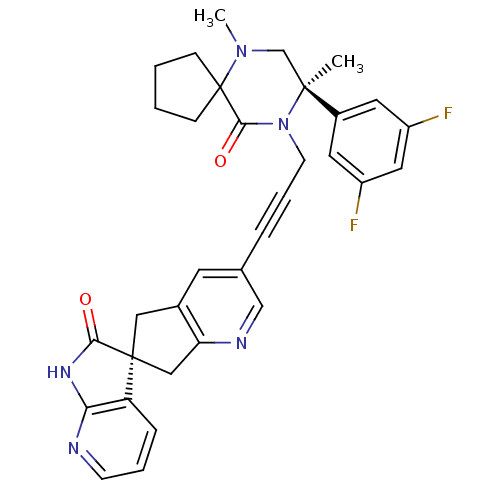
(CHEMBL2431246)Show SMILES CN1C[C@](C)(N(CC#Cc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H31F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50356282
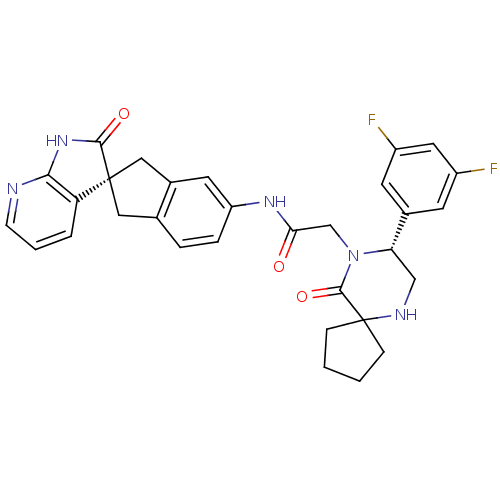
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440784
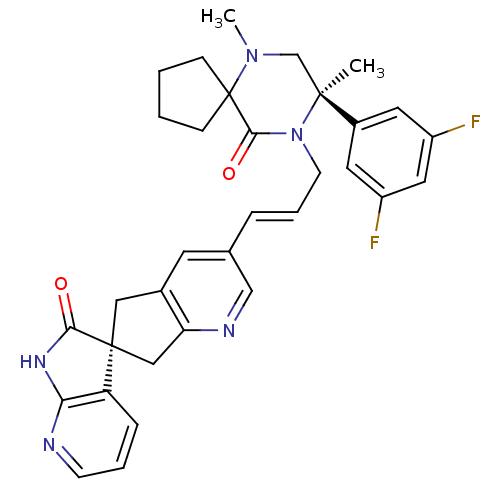
(CHEMBL2431253)Show SMILES CN1C[C@](C)(N(C\C=C\c2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5-8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/b7-6+/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440791
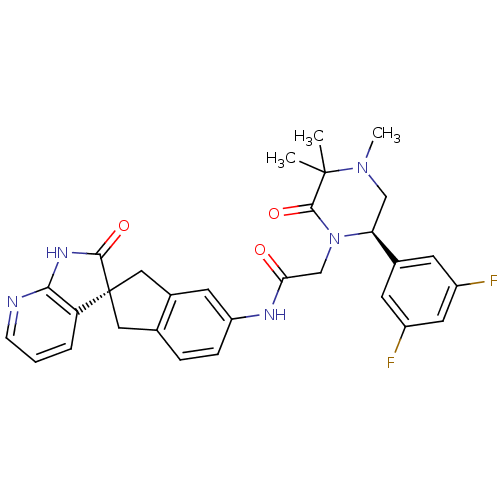
(CHEMBL2431256)Show SMILES CN1C[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-29(2)28(40)37(24(15-36(29)3)18-9-20(31)12-21(32)10-18)16-25(38)34-22-7-6-17-13-30(14-19(17)11-22)23-5-4-8-33-26(23)35-27(30)39/h4-12,24H,13-16H2,1-3H3,(H,34,38)(H,33,35,39)/t24-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135609

(US8846000, G-2)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4cc(C)ccc4c(=O)n3-c3ccc4cnn(C)c4c3)C(=O)c12 Show InChI InChI=1S/C30H27N5O4/c1-17(2)39-25-7-5-6-22-27(25)30(38)34(28(22)36)13-12-26-32-23-14-18(3)8-11-21(23)29(37)35(26)20-10-9-19-16-31-33(4)24(19)15-20/h5-11,14-17H,12-13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125967

(CHEMBL3627846)Show SMILES Cc1ccc2c(c1)nc(CCN1C(=O)c3ccccc3C1=O)n(-c1ccc3n(C)ncc3c1)c2=O Show InChI InChI=1S/C27H21N5O3/c1-16-7-9-21-22(13-16)29-24(11-12-31-25(33)19-5-3-4-6-20(19)26(31)34)32(27(21)35)18-8-10-23-17(14-18)15-28-30(23)2/h3-10,13-15H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440793
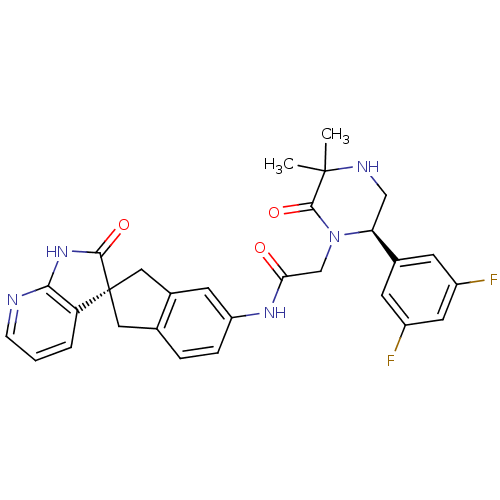
(CHEMBL2431254)Show SMILES CC1(C)NC[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C29H27F2N5O3/c1-28(2)27(39)36(23(14-33-28)17-8-19(30)11-20(31)9-17)15-24(37)34-21-6-5-16-12-29(13-18(16)10-21)22-4-3-7-32-25(22)35-26(29)38/h3-11,23,33H,12-15H2,1-2H3,(H,34,37)(H,32,35,38)/t23-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50385314
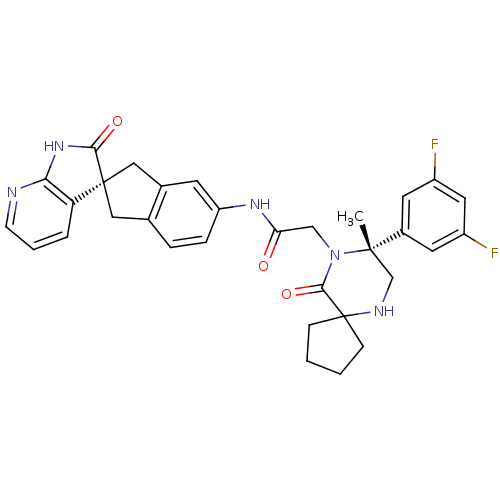
(CHEMBL2035981)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H31F2N5O3/c1-30(21-12-22(33)14-23(34)13-21)18-36-32(8-2-3-9-32)29(42)39(30)17-26(40)37-24-7-6-19-15-31(16-20(19)11-24)25-5-4-10-35-27(25)38-28(31)41/h4-7,10-14,36H,2-3,8-9,15-18H2,1H3,(H,37,40)(H,35,38,41)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440786
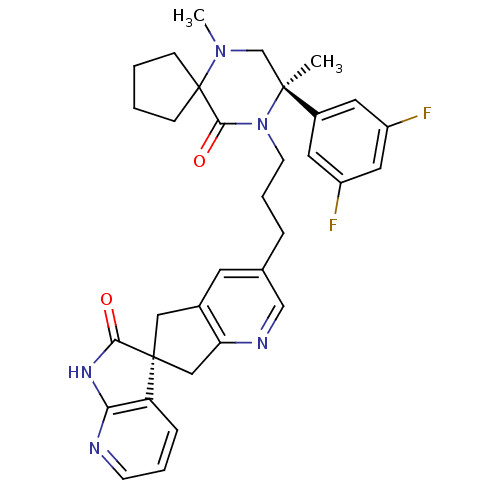
(CHEMBL2431251)Show SMILES CN1C[C@](C)(N(CCCc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H35F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,6-7,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440789

(CHEMBL2431248)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O3/c1-31(22-13-23(34)15-24(35)14-22)19-39(2)33(9-3-4-10-33)30(43)40(31)18-27(41)37-25-8-7-20-16-32(17-21(20)12-25)26-6-5-11-36-28(26)38-29(32)42/h5-8,11-15H,3-4,9-10,16-19H2,1-2H3,(H,37,41)(H,36,38,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135597

(US8846000, 1-13)Show SMILES COc1ccc2c(c1)nc(CCN1C(=O)c3cccc(OC(C)C)c3C1=O)n(-c1ccc3cc[nH]c3c1)c2=O Show InChI InChI=1S/C30H26N4O5/c1-17(2)39-25-6-4-5-22-27(25)30(37)33(28(22)35)14-12-26-32-24-16-20(38-3)9-10-21(24)29(36)34(26)19-8-7-18-11-13-31-23(18)15-19/h4-11,13,15-17,31H,12,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440790
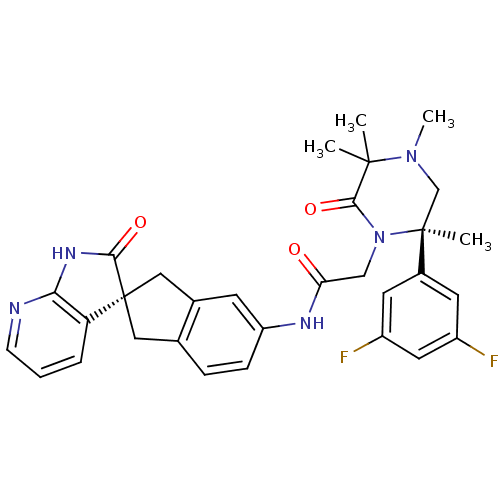
(CHEMBL2431247)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H31F2N5O3/c1-29(2)28(41)38(30(3,17-37(29)4)20-11-21(32)13-22(33)12-20)16-25(39)35-23-8-7-18-14-31(15-19(18)10-23)24-6-5-9-34-26(24)36-27(31)40/h5-13H,14-17H2,1-4H3,(H,35,39)(H,34,36,40)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Escherichia coli) | BDBM50140062
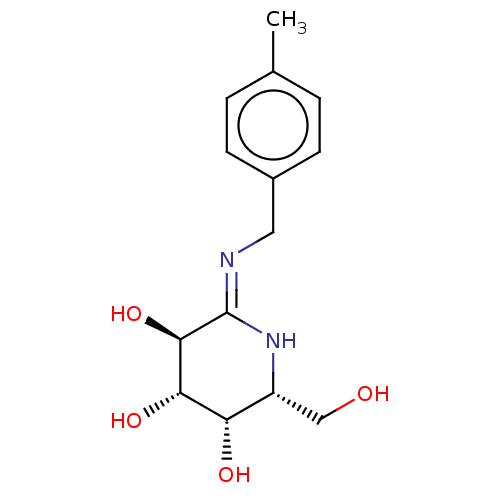
(CHEMBL3359672)Show SMILES Cc1ccc(C\N=C2/N[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C14H20N2O4/c1-8-2-4-9(5-3-8)6-15-14-13(20)12(19)11(18)10(7-17)16-14/h2-5,10-13,17-20H,6-7H2,1H3,(H,15,16)/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas
Curated by ChEMBL
| Assay Description
Competitive inhibition of Escherichia coli beta-galactosidase assessed as p-nitrophenyl-beta-D-galactopyranoside substrate hydrolysis by UV/Vis spect... |
Bioorg Med Chem 24: 661-71 (2016)
Article DOI: 10.1016/j.bmc.2015.12.034
BindingDB Entry DOI: 10.7270/Q20P11WZ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600
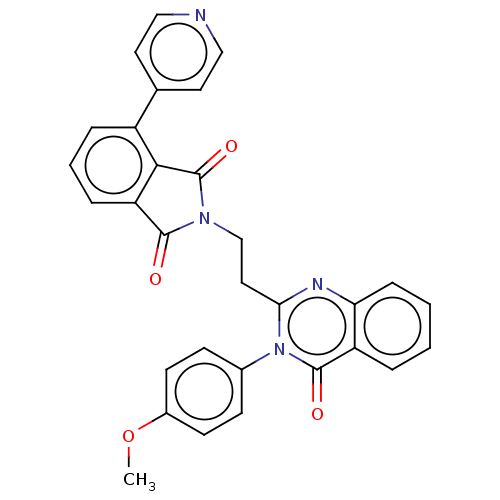
(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600

(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135598

(US8846000, 1-14)Show SMILES COc1ccc2c(c1)nc(CCN1C(=O)c3cccc(OC(C)C)c3C1=O)n(-c1cccc(C)c1)c2=O Show InChI InChI=1S/C29H27N3O5/c1-17(2)37-24-10-6-9-22-26(24)29(35)31(27(22)33)14-13-25-30-23-16-20(36-4)11-12-21(23)28(34)32(25)19-8-5-7-18(3)15-19/h5-12,15-17H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440785
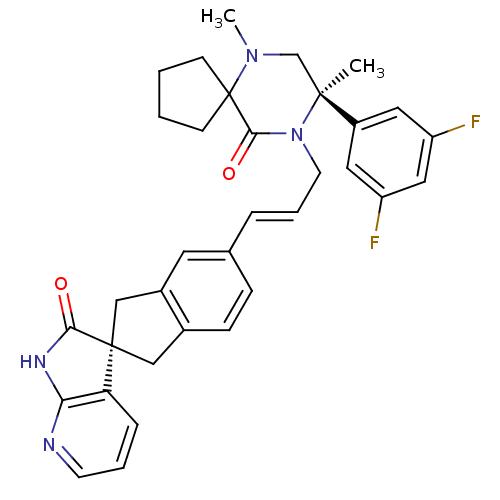
(CHEMBL2431252)Show SMILES CN1C[C@](C)(N(C\C=C\c2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H34F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/b7-6+/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135606

(US8846000, 1-24)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2cc(C)ccc2c1=O Show InChI InChI=1S/C29H27N3O5/c1-17(2)37-24-7-5-6-22-26(24)29(35)31(27(22)33)15-14-25-30-23-16-18(3)8-13-21(23)28(34)32(25)19-9-11-20(36-4)12-10-19/h5-13,16-17H,14-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135608

(US8846000, G-1)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4cc(C)ccc4c(=O)n3-c3ccc4cn[nH]c4c3)C(=O)c12 Show InChI InChI=1S/C29H25N5O4/c1-16(2)38-24-6-4-5-21-26(24)29(37)33(27(21)35)12-11-25-31-23-13-17(3)7-10-20(23)28(36)34(25)19-9-8-18-15-30-32-22(18)14-19/h4-10,13-16H,11-12H2,1-3H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440783
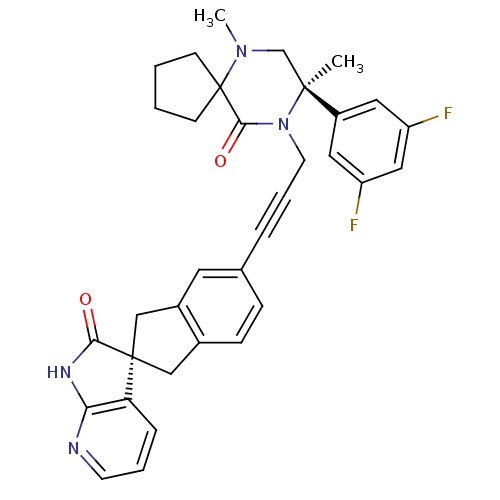
(CHEMBL2429882)Show SMILES CN1C[C@](C)(N(CC#Cc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H32F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5,8-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440792

(CHEMBL2431255)Show SMILES CC1(C)NC[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-28(2)27(40)37(29(3,16-34-28)19-10-20(31)12-21(32)11-19)15-24(38)35-22-7-6-17-13-30(14-18(17)9-22)23-5-4-8-33-25(23)36-26(30)39/h4-12,34H,13-16H2,1-3H3,(H,35,38)(H,33,36,39)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135614

(US8846000, D-7)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H25N3O5/c1-17(2)36-23-10-6-8-21-25(23)28(34)30(26(21)32)16-15-24-29-22-9-5-4-7-20(22)27(33)31(24)18-11-13-19(35-3)14-12-18/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135614

(US8846000, D-7)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H25N3O5/c1-17(2)36-23-10-6-8-21-25(23)28(34)30(26(21)32)16-15-24-29-22-9-5-4-7-20(22)27(33)31(24)18-11-13-19(35-3)14-12-18/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135596

(US8846000, 1-12)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(OCCF)cc3)C(=O)c12 Show InChI InChI=1S/C29H26FN3O5/c1-18(2)38-24-9-5-7-22-26(24)29(36)32(27(22)34)16-14-25-31-23-8-4-3-6-21(23)28(35)33(25)19-10-12-20(13-11-19)37-17-15-30/h3-13,18H,14-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135613
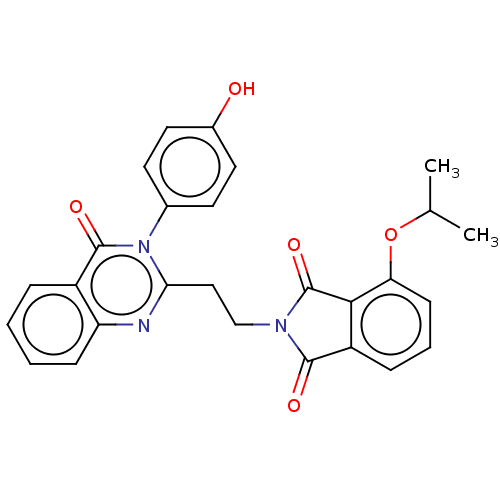
(US8846000, D-6)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(O)cc3)C(=O)c12 Show InChI InChI=1S/C27H23N3O5/c1-16(2)35-22-9-5-7-20-24(22)27(34)29(25(20)32)15-14-23-28-21-8-4-3-6-19(21)26(33)30(23)17-10-12-18(31)13-11-17/h3-13,16,31H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135595
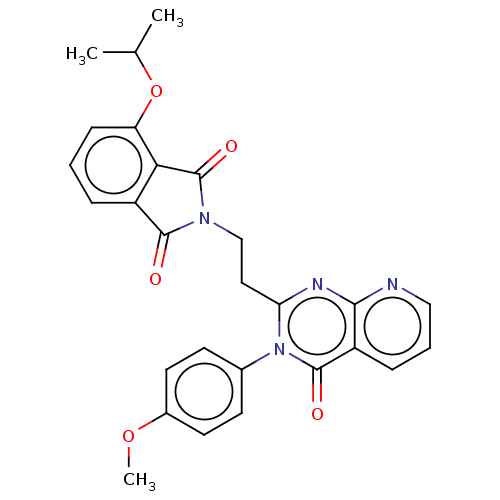
(US8846000, 1-11)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ncccc2c1=O Show InChI InChI=1S/C27H24N4O5/c1-16(2)36-21-8-4-6-19-23(21)27(34)30(25(19)32)15-13-22-29-24-20(7-5-14-28-24)26(33)31(22)17-9-11-18(35-3)12-10-17/h4-12,14,16H,13,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135599
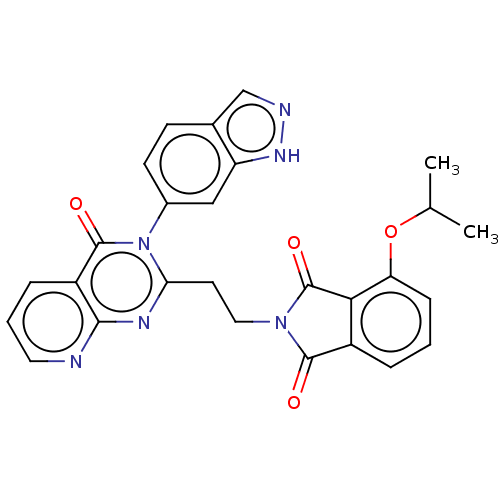
(US8846000, 1-15)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ncccc4c(=O)n3-c3ccc4cn[nH]c4c3)C(=O)c12 Show InChI InChI=1S/C27H22N6O4/c1-15(2)37-21-7-3-5-18-23(21)27(36)32(25(18)34)12-10-22-30-24-19(6-4-11-28-24)26(35)33(22)17-9-8-16-14-29-31-20(16)13-17/h3-9,11,13-15H,10,12H2,1-2H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440787
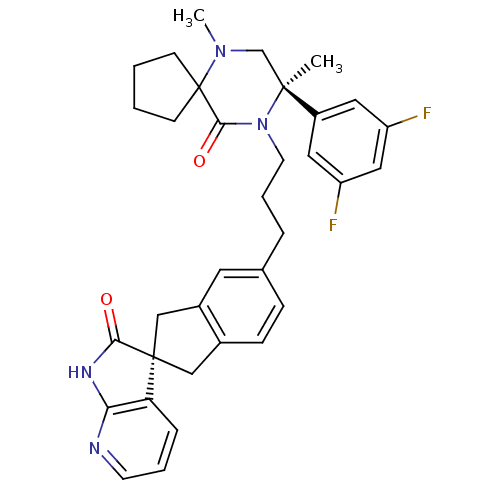
(CHEMBL2431250)Show SMILES CN1C[C@](C)(N(CCCc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H36F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5,8-10,13,15-18H,3-4,6-7,11-12,14,19-21H2,1-2H3,(H,37,38,41)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135595

(US8846000, 1-11)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ncccc2c1=O Show InChI InChI=1S/C27H24N4O5/c1-16(2)36-21-8-4-6-19-23(21)27(34)30(25(19)32)15-13-22-29-24-20(7-5-14-28-24)26(33)31(22)17-9-11-18(35-3)12-10-17/h4-12,14,16H,13,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135591

(US8846000, 1-7)Show SMILES COc1ccc2c(c1)nc(CCN1C(=O)c3cccc(OC)c3C1=O)n(-c1cccc(C)c1)c2=O Show InChI InChI=1S/C27H23N3O5/c1-16-6-4-7-17(14-16)30-23(28-21-15-18(34-2)10-11-19(21)26(30)32)12-13-29-25(31)20-8-5-9-22(35-3)24(20)27(29)33/h4-11,14-15H,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224431
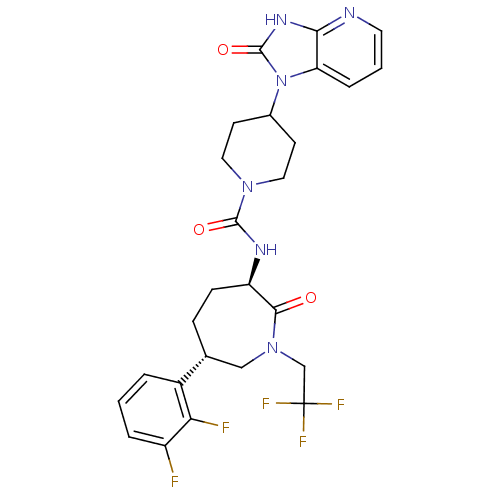
(CHEMBL236593 | MK-0974 | N-[(3R,6S)-6-(2,3-difluor...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C(=O)N(CC(F)(F)F)C2)c1F Show InChI InChI=1S/C26H27F5N6O3/c27-18-4-1-3-17(21(18)28)15-6-7-19(23(38)36(13-15)14-26(29,30)31)33-24(39)35-11-8-16(9-12-35)37-20-5-2-10-32-22(20)34-25(37)40/h1-5,10,15-16,19H,6-9,11-14H2,(H,33,39)(H,32,34,40)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135602

(US8846000, 1-19)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OCC(F)(F)F)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C27H20F3N3O5/c1-37-17-11-9-16(10-12-17)33-22(31-20-7-3-2-5-18(20)25(33)35)13-14-32-24(34)19-6-4-8-21(23(19)26(32)36)38-15-27(28,29)30/h2-12H,13-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-Columbia
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from Sigma1-receptor in guinea pig brain membrane |
Bioorg Med Chem 19: 1852-9 (2011)
Article DOI: 10.1016/j.bmc.2011.02.006
BindingDB Entry DOI: 10.7270/Q2K64JC1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135612
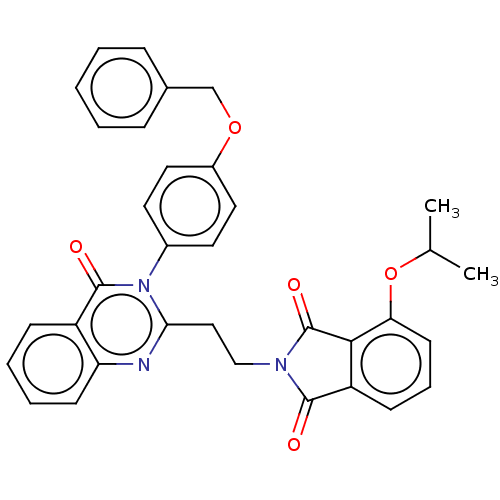
(US8846000, D-5)Show SMILES CC(C)Oc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(OCc4ccccc4)cc3)C(=O)c12 Show InChI InChI=1S/C34H29N3O5/c1-22(2)42-29-14-8-12-27-31(29)34(40)36(32(27)38)20-19-30-35-28-13-7-6-11-26(28)33(39)37(30)24-15-17-25(18-16-24)41-21-23-9-4-3-5-10-23/h3-18,22H,19-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398004
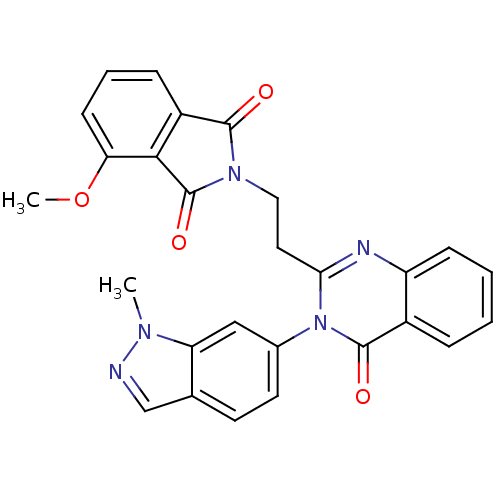
(CHEMBL2180401)Show SMILES COc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc4cnn(C)c4c3)C(=O)c12 Show InChI InChI=1S/C27H21N5O4/c1-30-21-14-17(11-10-16(21)15-28-30)32-23(29-20-8-4-3-6-18(20)26(32)34)12-13-31-25(33)19-7-5-9-22(36-2)24(19)27(31)35/h3-11,14-15H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135604

(US8846000, 1-21)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC4CCC4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C29H25N3O5/c1-36-19-14-12-18(13-15-19)32-25(30-23-10-3-2-8-21(23)28(32)34)16-17-31-27(33)22-9-5-11-24(26(22)29(31)35)37-20-6-4-7-20/h2-3,5,8-15,20H,4,6-7,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50270877
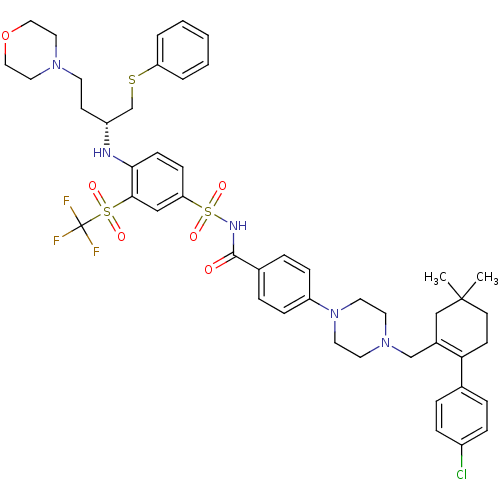
((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan Provinc
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-2 (unknown origin) by fluorescence polarization assay |
Eur J Med Chem 146: 471-482 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.076
BindingDB Entry DOI: 10.7270/Q2ZG6VS2 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126108
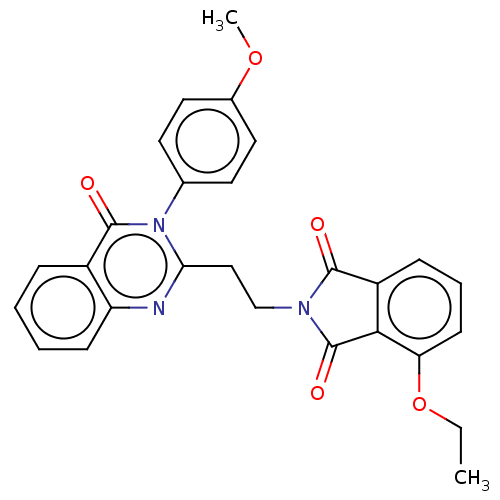
(CHEMBL3627840)Show SMILES CCOc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(OC)cc3)C(=O)c12 Show InChI InChI=1S/C27H23N3O5/c1-3-35-22-10-6-8-20-24(22)27(33)29(25(20)31)16-15-23-28-21-9-5-4-7-19(21)26(32)30(23)17-11-13-18(34-2)14-12-17/h4-14H,3,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50304179
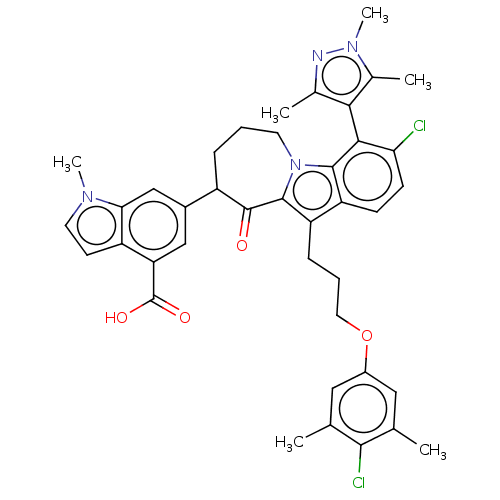
(CHEMBL4159388)Show SMILES Cc1nn(C)c(C)c1-c1c(Cl)ccc2c(CCCOc3cc(C)c(Cl)c(C)c3)c3C(=O)C(CCCn3c12)c1cc(C(O)=O)c2ccn(C)c2c1 |(.79,-3.59,;2.26,-3.11,;2.73,-1.65,;4.27,-1.65,;5.18,-.4,;4.75,-3.11,;6.22,-3.59,;3.51,-4.02,;3.51,-5.56,;2.19,-6.34,;.85,-5.57,;2.19,-7.88,;3.51,-8.64,;4.85,-7.87,;6.31,-8.34,;7.07,-9.67,;6.3,-11,;7.06,-12.34,;6.28,-13.67,;7.04,-15.01,;8.58,-15.01,;9.34,-16.34,;10.88,-16.34,;8.57,-17.68,;9.33,-19.02,;7.02,-17.67,;6.24,-19,;6.26,-16.33,;7.21,-7.09,;8.75,-7.15,;9.36,-8.57,;9.76,-5.98,;9.46,-4.47,;8.11,-3.75,;6.7,-4.37,;6.3,-5.86,;4.84,-6.34,;11.24,-6.38,;12.32,-5.29,;13.81,-5.69,;14.9,-4.6,;16.39,-5.01,;14.51,-3.12,;14.21,-7.18,;15.58,-7.88,;15.34,-9.4,;13.82,-9.64,;13.13,-11.01,;13.12,-8.27,;11.64,-7.87,)| Show InChI InChI=1S/C40H40Cl2N4O4/c1-21-17-26(18-22(2)36(21)42)50-16-8-10-29-30-11-12-32(41)35(34-23(3)43-45(6)24(34)4)37(30)46-14-7-9-27(39(47)38(29)46)25-19-31(40(48)49)28-13-15-44(5)33(28)20-25/h11-13,15,17-20,27H,7-10,14,16H2,1-6H3,(H,48,49) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan Provinc
Curated by ChEMBL
| Assay Description
Inhibition of His6 tagged MBP fused Mcl-1 (unknown origin) after 0.5 hrs by fluorescence polarization assay |
Eur J Med Chem 146: 471-482 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.076
BindingDB Entry DOI: 10.7270/Q2ZG6VS2 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50270877

((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan Provinc
Curated by ChEMBL
| Assay Description
Inhibition of Bcl-xL (unknown origin) by fluorescence polarization assay |
Eur J Med Chem 146: 471-482 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.076
BindingDB Entry DOI: 10.7270/Q2ZG6VS2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135592

(US8846000, 1-8)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC)c3C2=O)nc2cc(C)ccc2c1=O Show InChI InChI=1S/C27H23N3O5/c1-16-7-12-19-21(15-16)28-23(30(26(19)32)17-8-10-18(34-2)11-9-17)13-14-29-25(31)20-5-4-6-22(35-3)24(20)27(29)33/h4-12,15H,13-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Escherichia coli) | BDBM50140061
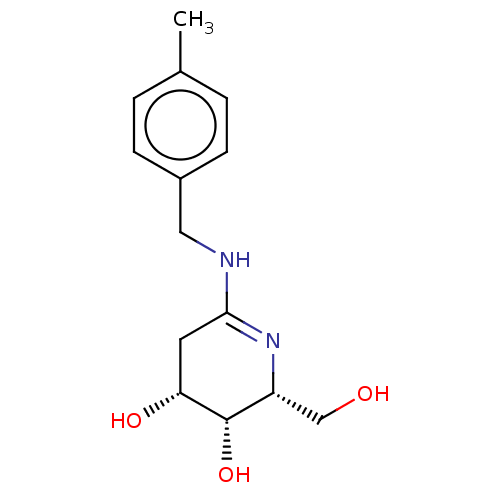
(CHEMBL3752112)Show SMILES Cc1ccc(CNC2=N[C@H](CO)[C@H](O)[C@H](O)C2)cc1 |r,t:7| Show InChI InChI=1S/C14H20N2O3/c1-9-2-4-10(5-3-9)7-15-13-6-12(18)14(19)11(8-17)16-13/h2-5,11-12,14,17-19H,6-8H2,1H3,(H,15,16)/t11-,12-,14+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas
Curated by ChEMBL
| Assay Description
Competitive inhibition of Escherichia coli beta-galactosidase assessed as p-nitrophenyl-beta-D-galactopyranoside substrate hydrolysis by UV/Vis spect... |
Bioorg Med Chem 24: 661-71 (2016)
Article DOI: 10.1016/j.bmc.2015.12.034
BindingDB Entry DOI: 10.7270/Q20P11WZ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50206243

(CHEMBL3918431)Show SMILES C1N=C(Nc2cc3ccccc3cn2)O[C@]11CN2CCC1CC2 |r,wU:15.16,t:1,TLB:14:15:18.19:22.21,THB:0:15:18.19:22.21,(17.63,-8.02,;19,-7.33,;18.76,-5.81,;19.85,-4.72,;21.34,-5.11,;22.41,-4.02,;23.9,-4.41,;24.98,-3.32,;26.47,-3.71,;26.88,-5.2,;25.79,-6.3,;24.3,-5.9,;23.22,-7,;21.73,-6.6,;17.24,-5.56,;16.54,-6.93,;15.8,-8.21,;14.52,-7.62,;14.52,-5.71,;15.36,-4.63,;15.36,-6.25,;13.79,-6.93,;13,-8.11,)| Show InChI InChI=1S/C18H20N4O/c1-2-4-14-10-19-16(9-13(14)3-1)21-17-20-11-18(23-17)12-22-7-5-15(18)6-8-22/h1-4,9-10,15H,5-8,11-12H2,(H,19,20,21)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [125I]alpha-bungarotoxin from human alpha7 nAChR expressed in HEK293 cell membranes incubated for 2 hrs and measured by gamma countin... |
ACS Med Chem Lett 8: 366-371 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00032
BindingDB Entry DOI: 10.7270/Q2S46V8B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135603

(US8846000, 1-20)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OCC4CC4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C29H25N3O5/c1-36-20-13-11-19(12-14-20)32-25(30-23-7-3-2-5-21(23)28(32)34)15-16-31-27(33)22-6-4-8-24(26(22)29(31)35)37-17-18-9-10-18/h2-8,11-14,18H,9-10,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting |
Eur J Med Chem 44: 593-608 (2009)
Article DOI: 10.1016/j.ejmech.2008.03.040
BindingDB Entry DOI: 10.7270/Q2K35TFK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135611
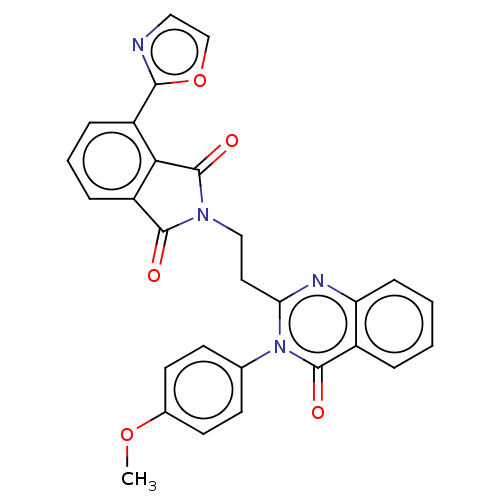
(US8846000, C-2)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(-c4ncco4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H20N4O5/c1-36-18-11-9-17(10-12-18)32-23(30-22-8-3-2-5-19(22)27(32)34)13-15-31-26(33)21-7-4-6-20(24(21)28(31)35)25-29-14-16-37-25/h2-12,14,16H,13,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8846000 (2014)
BindingDB Entry DOI: 10.7270/Q2X63KM9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126110
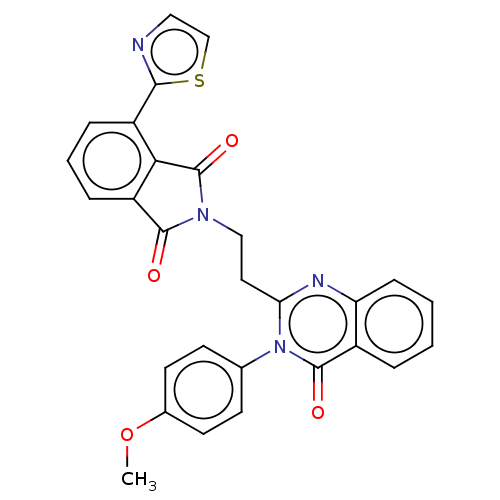
(CHEMBL3627843)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(-c4nccs4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H20N4O4S/c1-36-18-11-9-17(10-12-18)32-23(30-22-8-3-2-5-19(22)27(32)34)13-15-31-26(33)21-7-4-6-20(24(21)28(31)35)25-29-14-16-37-25/h2-12,14,16H,13,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398008
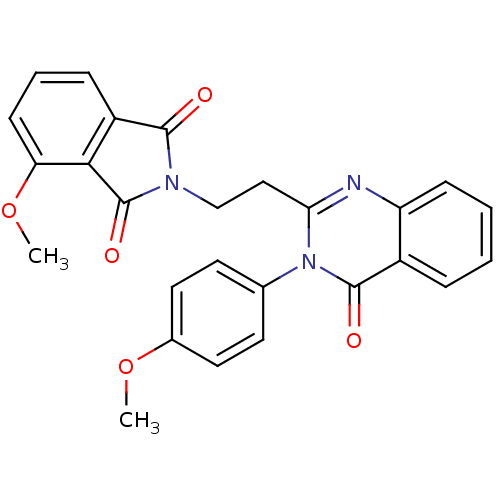
(CHEMBL2180426)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O5/c1-33-17-12-10-16(11-13-17)29-22(27-20-8-4-3-6-18(20)25(29)31)14-15-28-24(30)19-7-5-9-21(34-2)23(19)26(28)32/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data