 Found 3721 hits with Last Name = 'fan' and Initial = 'k'
Found 3721 hits with Last Name = 'fan' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50276642
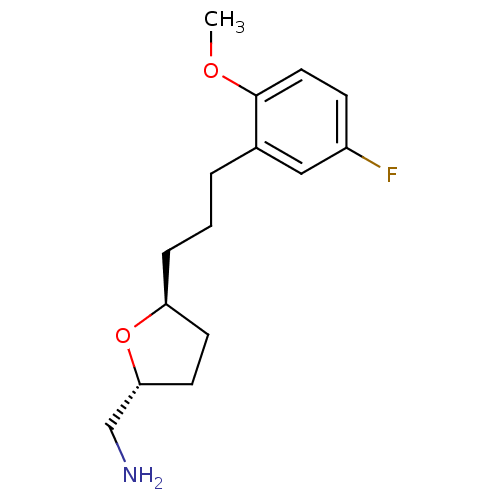
(CHEMBL460407 | trans-2-Aminomethyl-5-((2''-methoxy...)Show InChI InChI=1S/C15H22FNO2/c1-18-15-8-5-12(16)9-11(15)3-2-4-13-6-7-14(10-17)19-13/h5,8-9,13-14H,2-4,6-7,10,17H2,1H3/t13-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-Columbia
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from Sigma1-receptor in guinea pig brain membrane |
Bioorg Med Chem 19: 1852-9 (2011)
Article DOI: 10.1016/j.bmc.2011.02.006
BindingDB Entry DOI: 10.7270/Q2K64JC1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177012
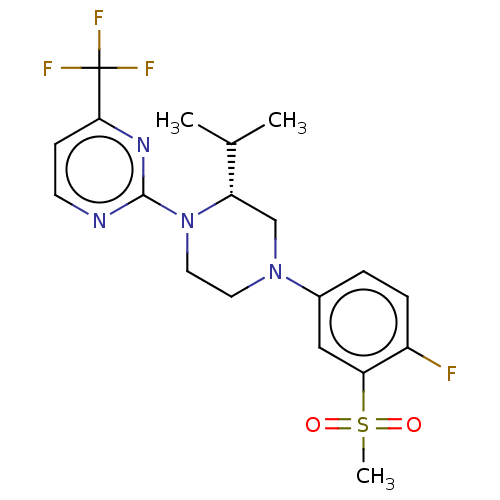
(CHEMBL3814153 | US10144715, Compound 7-32)Show SMILES CC(C)[C@@H]1CN(CCN1c1nccc(n1)C(F)(F)F)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C19H22F4N4O2S/c1-12(2)15-11-26(13-4-5-14(20)16(10-13)30(3,28)29)8-9-27(15)18-24-7-6-17(25-18)19(21,22)23/h4-7,10,12,15H,8-9,11H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177016
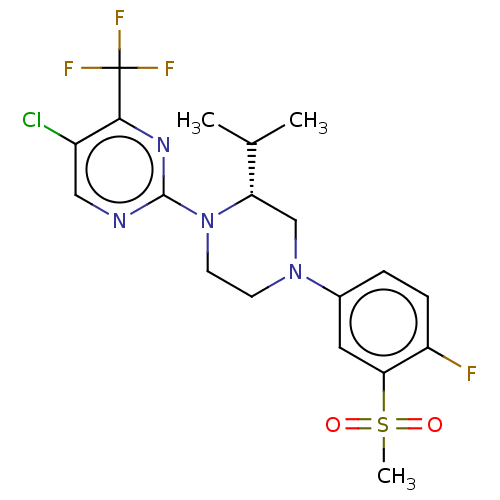
(CHEMBL3814501)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(Cl)c(n1)C(F)(F)F)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C19H21ClF4N4O2S/c1-11(2)15-10-27(12-4-5-14(21)16(8-12)31(3,29)30)6-7-28(15)18-25-9-13(20)17(26-18)19(22,23)24/h4-5,8-9,11,15H,6-7,10H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192752
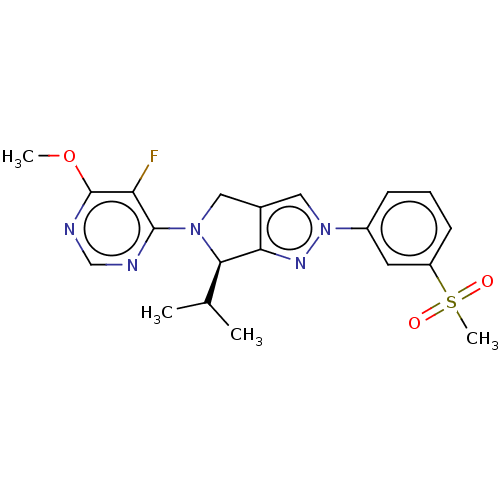
(CHEMBL3905741)Show SMILES COc1ncnc(N2Cc3cn(nc3[C@H]2C(C)C)-c2cccc(c2)S(C)(=O)=O)c1F |r| Show InChI InChI=1S/C20H22FN5O3S/c1-12(2)18-17-13(9-25(18)19-16(21)20(29-3)23-11-22-19)10-26(24-17)14-6-5-7-15(8-14)30(4,27)28/h5-8,10-12,18H,9H2,1-4H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50035738

((R)-3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2...)Show SMILES COC(=O)C1C2CCC(CC1c1ccc(Cl)cc1)N2C |TLB:11:10:18:6.7,THB:2:4:18:6.7| Show InChI InChI=1S/C16H20ClNO2/c1-18-12-7-8-14(18)15(16(19)20-2)13(9-12)10-3-5-11(17)6-4-10/h3-6,12-15H,7-9H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [125I]RTI-55 binding to dopamine transporter in HEK cells |
Bioorg Med Chem Lett 13: 2151-4 (2003)
BindingDB Entry DOI: 10.7270/Q2DN45K3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30132
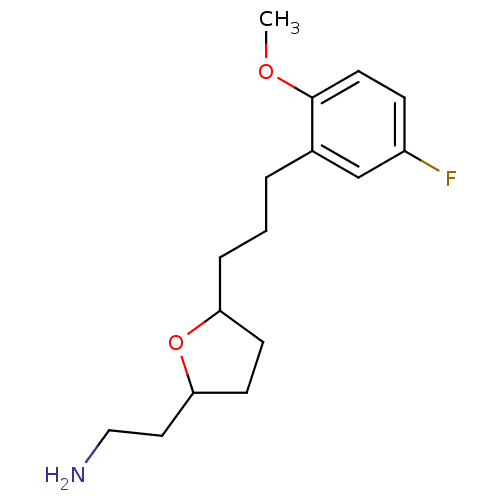
(CHEMBL450907 | tetrahydrofuranyl ethylamine, 15)Show InChI InChI=1S/C16H24FNO2/c1-19-16-8-5-13(17)11-12(16)3-2-4-14-6-7-15(20-14)9-10-18/h5,8,11,14-15H,2-4,6-7,9-10,18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30132

(CHEMBL450907 | tetrahydrofuranyl ethylamine, 15)Show InChI InChI=1S/C16H24FNO2/c1-19-16-8-5-13(17)11-12(16)3-2-4-14-6-7-15(20-14)9-10-18/h5,8,11,14-15H,2-4,6-7,9-10,18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177010
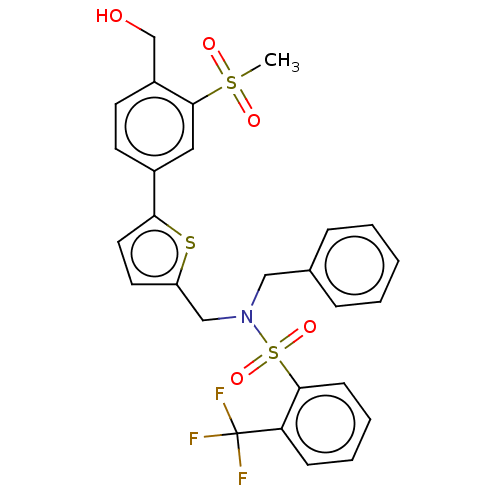
(CHEMBL3814006)Show SMILES CS(=O)(=O)c1cc(ccc1CO)-c1ccc(CN(Cc2ccccc2)S(=O)(=O)c2ccccc2C(F)(F)F)s1 Show InChI InChI=1S/C27H24F3NO5S3/c1-38(33,34)26-15-20(11-12-21(26)18-32)24-14-13-22(37-24)17-31(16-19-7-3-2-4-8-19)39(35,36)25-10-6-5-9-23(25)27(28,29)30/h2-15,32H,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372
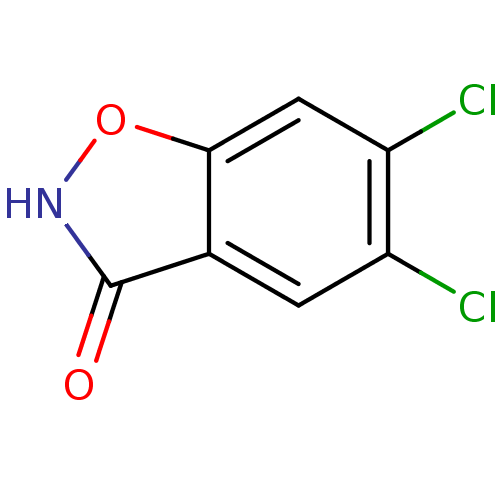
(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166849
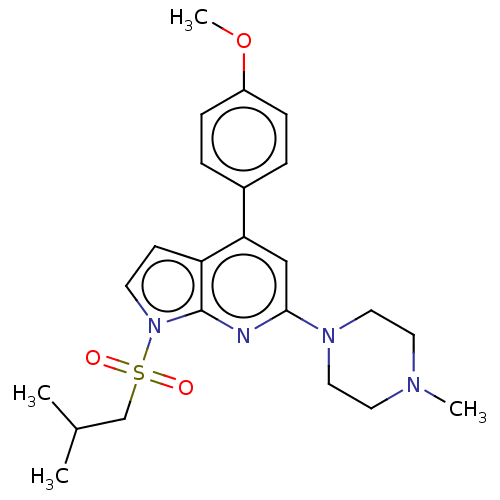
(CHEMBL3797651)Show SMILES COc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C23H30N4O3S/c1-17(2)16-31(28,29)27-10-9-20-21(18-5-7-19(30-4)8-6-18)15-22(24-23(20)27)26-13-11-25(3)12-14-26/h5-10,15,17H,11-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50276645
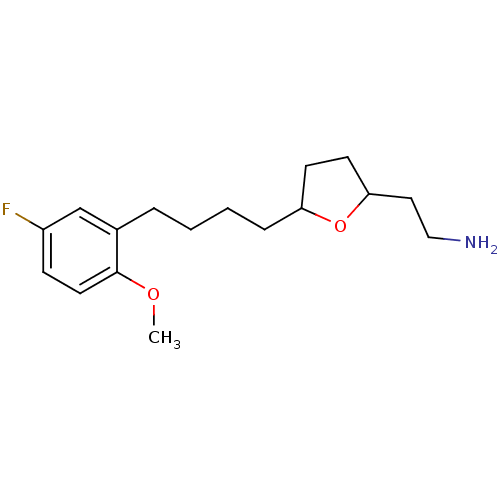
(2-Aminoethyl-5-(3'-(2''-methoxy-5''-fluorophenyl)-...)Show InChI InChI=1S/C17H26FNO2/c1-20-17-9-6-14(18)12-13(17)4-2-3-5-15-7-8-16(21-15)10-11-19/h6,9,12,15-16H,2-5,7-8,10-11,19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50276593
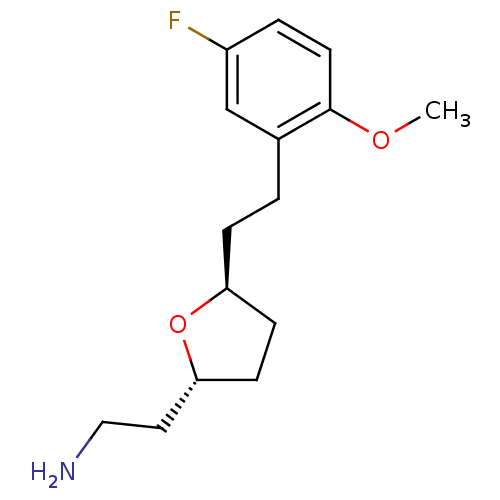
(CHEMBL462311 | trans-2-(Aminoethyl)-5-(2'-methoxy-...)Show InChI InChI=1S/C15H22FNO2/c1-18-15-7-3-12(16)10-11(15)2-4-13-5-6-14(19-13)8-9-17/h3,7,10,13-14H,2,4-6,8-9,17H2,1H3/t13-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50276643
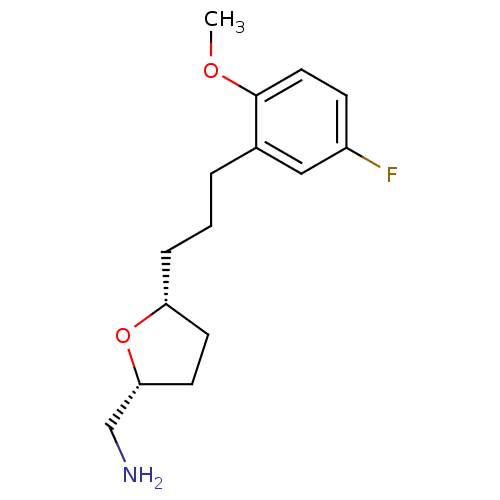
(CHEMBL459367 | cis-2-Aminomethyl-5-((2''-methoxy-5...)Show InChI InChI=1S/C15H22FNO2/c1-18-15-8-5-12(16)9-11(15)3-2-4-13-6-7-14(10-17)19-13/h5,8-9,13-14H,2-4,6-7,10,17H2,1H3/t13-,14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50177016

(CHEMBL3814501)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(Cl)c(n1)C(F)(F)F)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C19H21ClF4N4O2S/c1-11(2)15-10-27(12-4-5-14(21)16(8-12)31(3,29)30)6-7-28(15)18-25-9-13(20)17(26-18)19(22,23)24/h4-5,8-9,11,15H,6-7,10H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177015

(CHEMBL3814206 | US10144715, Compound 19-1)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(CO)c(n1)C(F)(F)F)c1ccc(CO)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H27F3N4O4S/c1-13(2)17-10-27(16-5-4-14(11-29)18(8-16)33(3,31)32)6-7-28(17)20-25-9-15(12-30)19(26-20)21(22,23)24/h4-5,8-9,13,17,29-30H,6-7,10-12H2,1-3H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166863

(CHEMBL3799529)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCNCC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H19NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177015

(CHEMBL3814206 | US10144715, Compound 19-1)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(CO)c(n1)C(F)(F)F)c1ccc(CO)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H27F3N4O4S/c1-13(2)17-10-27(16-5-4-14(11-29)18(8-16)33(3,31)32)6-7-28(17)20-25-9-15(12-30)19(26-20)21(22,23)24/h4-5,8-9,13,17,29-30H,6-7,10-12H2,1-3H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192753
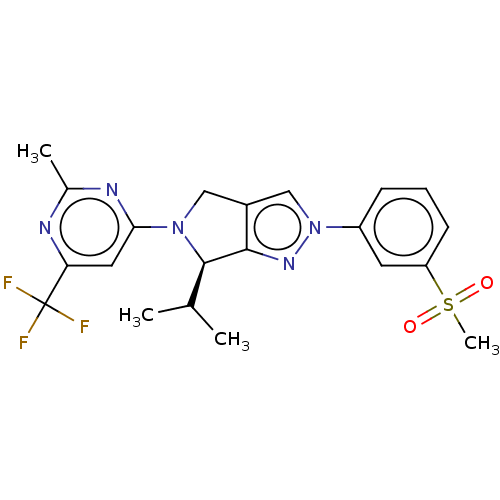
(CHEMBL3985591)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1cc(nc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N5O2S/c1-12(2)20-19-14(10-28(20)18-9-17(21(22,23)24)25-13(3)26-18)11-29(27-19)15-6-5-7-16(8-15)32(4,30)31/h5-9,11-12,20H,10H2,1-4H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50276591
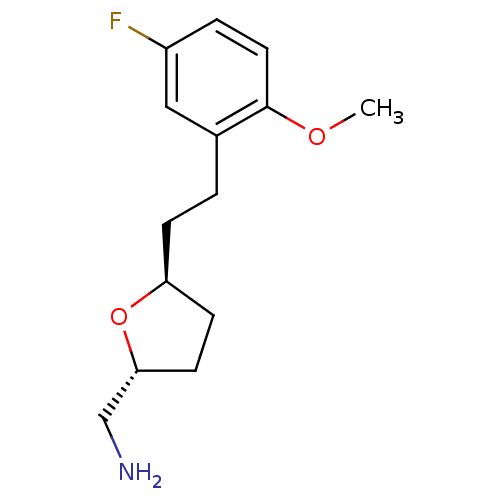
(CHEMBL511500 | trans-2-(Aminomethyl)-5-(2'-methoxy...)Show InChI InChI=1S/C14H20FNO2/c1-17-14-7-3-11(15)8-10(14)2-4-12-5-6-13(9-16)18-12/h3,7-8,12-13H,2,4-6,9,16H2,1H3/t12-,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725
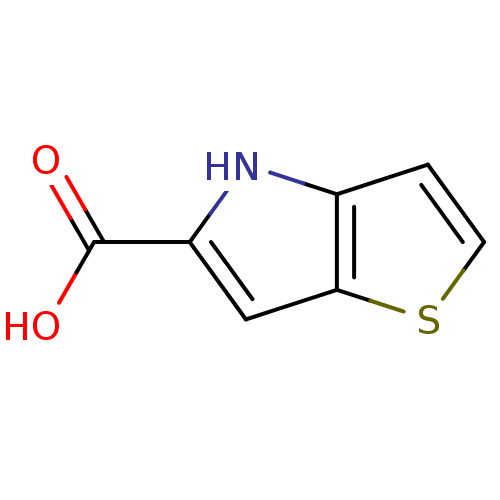
(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177011
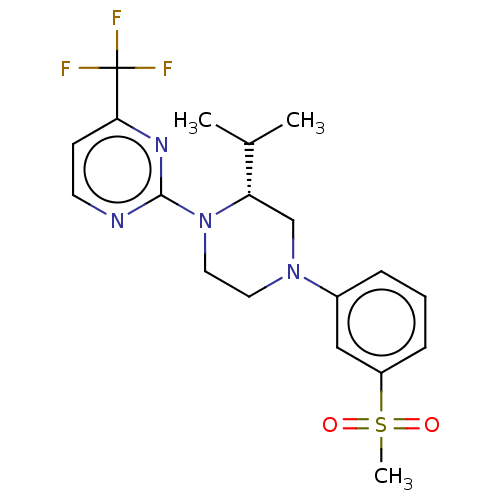
(CHEMBL3815014 | US10144715, Compound 7-13)Show SMILES CC(C)[C@@H]1CN(CCN1c1nccc(n1)C(F)(F)F)c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C19H23F3N4O2S/c1-13(2)16-12-25(14-5-4-6-15(11-14)29(3,27)28)9-10-26(16)18-23-8-7-17(24-18)19(20,21)22/h4-8,11,13,16H,9-10,12H2,1-3H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166852
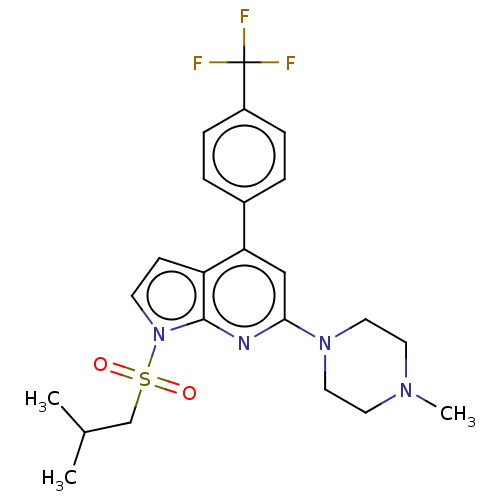
(CHEMBL3797717)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2S/c1-16(2)15-33(31,32)30-9-8-19-20(17-4-6-18(7-5-17)23(24,25)26)14-21(27-22(19)30)29-12-10-28(3)11-13-29/h4-9,14,16H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192757
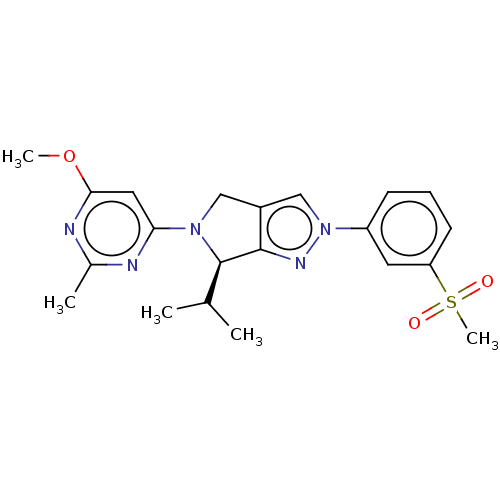
(CHEMBL3914727)Show SMILES COc1cc(nc(C)n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H25N5O3S/c1-13(2)21-20-15(11-25(21)18-10-19(29-4)23-14(3)22-18)12-26(24-20)16-7-6-8-17(9-16)30(5,27)28/h6-10,12-13,21H,11H2,1-5H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50276644
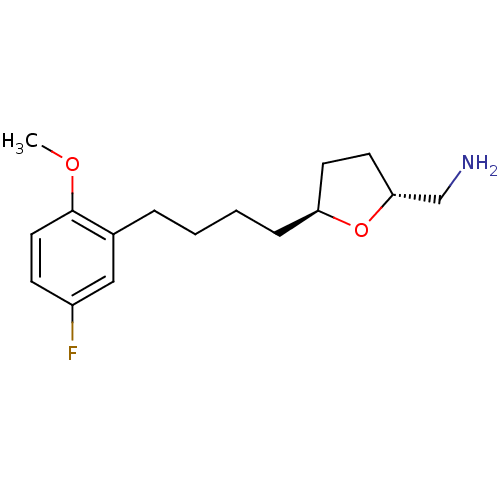
(CHEMBL455221 | trans-2-Aminomethyl-5-(5''-fluoro-2...)Show InChI InChI=1S/C16H24FNO2/c1-19-16-9-6-13(17)10-12(16)4-2-3-5-14-7-8-15(11-18)20-14/h6,9-10,14-15H,2-5,7-8,11,18H2,1H3/t14-,15+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50338990
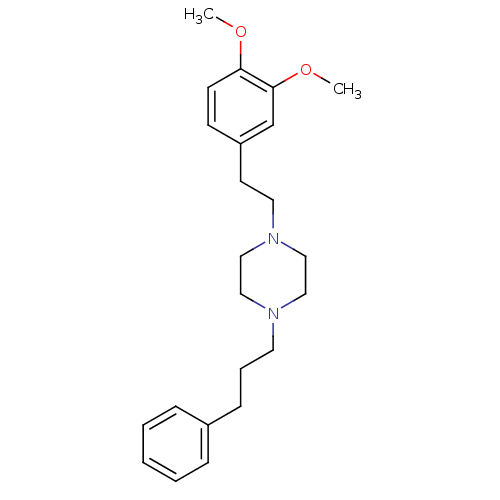
(1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piper...)Show InChI InChI=1S/C23H32N2O2/c1-26-22-11-10-21(19-23(22)27-2)12-14-25-17-15-24(16-18-25)13-6-9-20-7-4-3-5-8-20/h3-5,7-8,10-11,19H,6,9,12-18H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-Columbia
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from Sigma1-receptor in guinea pig brain membrane |
Bioorg Med Chem 19: 1852-9 (2011)
Article DOI: 10.1016/j.bmc.2011.02.006
BindingDB Entry DOI: 10.7270/Q2K64JC1 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192750
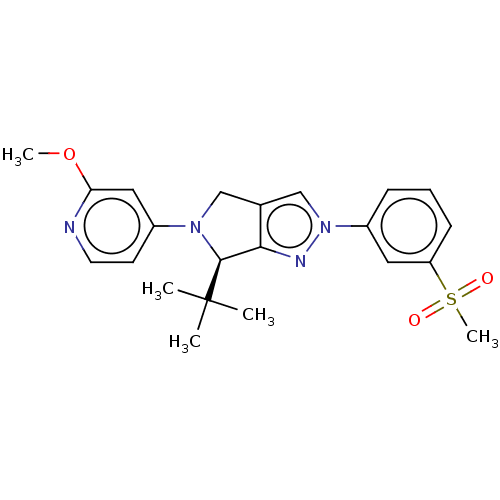
(CHEMBL3940521)Show SMILES COc1cc(ccn1)N1Cc2cn(nc2[C@H]1C(C)(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H26N4O3S/c1-22(2,3)21-20-15(13-25(21)16-9-10-23-19(12-16)29-4)14-26(24-20)17-7-6-8-18(11-17)30(5,27)28/h6-12,14,21H,13H2,1-5H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166844
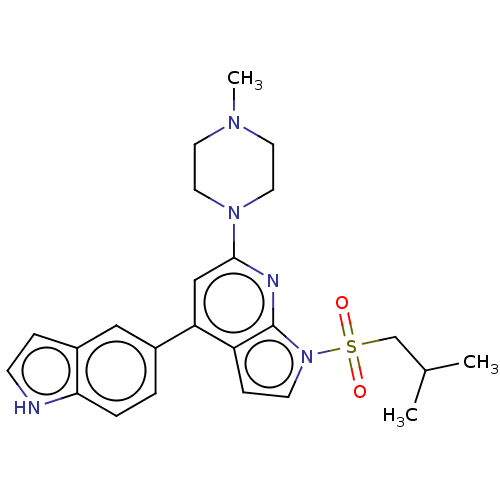
(CHEMBL3798478)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C24H29N5O2S/c1-17(2)16-32(30,31)29-9-7-20-21(18-4-5-22-19(14-18)6-8-25-22)15-23(26-24(20)29)28-12-10-27(3)11-13-28/h4-9,14-15,17,25H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166859
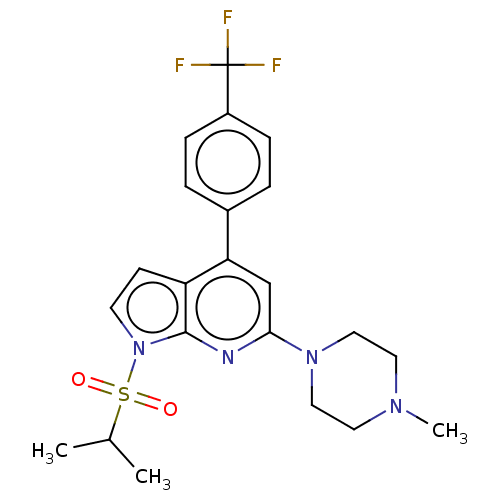
(CHEMBL3799120)Show SMILES CC(C)S(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N4O2S/c1-15(2)32(30,31)29-9-8-18-19(16-4-6-17(7-5-16)22(23,24)25)14-20(26-21(18)29)28-12-10-27(3)11-13-28/h4-9,14-15H,10-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192756
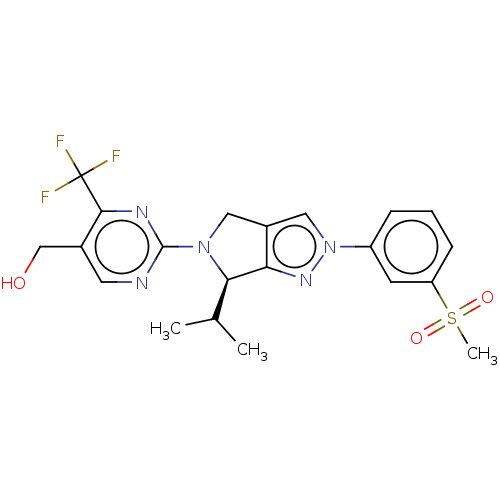
(CHEMBL3932169)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N5O3S/c1-12(2)18-17-14(10-29(27-17)15-5-4-6-16(7-15)33(3,31)32)9-28(18)20-25-8-13(11-30)19(26-20)21(22,23)24/h4-8,10,12,18,30H,9,11H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192758
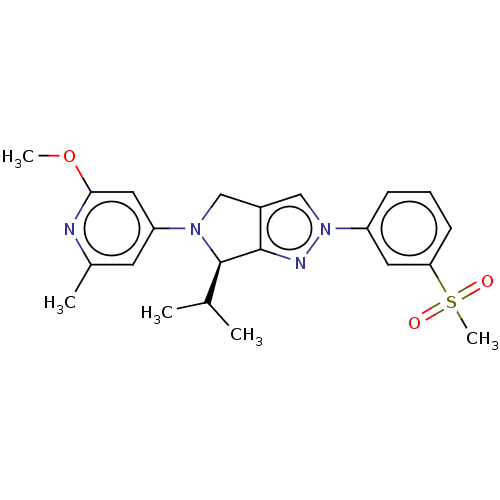
(CHEMBL3976470)Show SMILES COc1cc(cc(C)n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H26N4O3S/c1-14(2)22-21-16(12-25(22)18-9-15(3)23-20(11-18)29-4)13-26(24-21)17-7-6-8-19(10-17)30(5,27)28/h6-11,13-14,22H,12H2,1-5H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192761
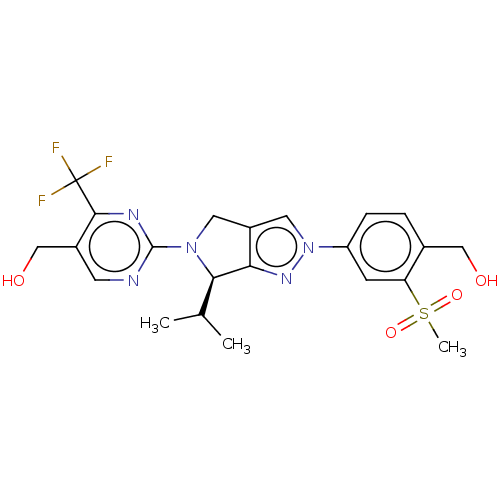
(CHEMBL3978980)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1ccc(CO)c(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C22H24F3N5O4S/c1-12(2)19-18-15(8-29(19)21-26-7-14(11-32)20(27-21)22(23,24)25)9-30(28-18)16-5-4-13(10-31)17(6-16)35(3,33)34/h4-7,9,12,19,31-32H,8,10-11H2,1-3H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50129369

(3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2.1]o...)Show SMILES CN1C2CCC1C(C(C2)c1ccc(Cl)cc1)C(=O)NCCCCCCCCNC(=O)C1C2CCC(CC1c1ccc(Cl)cc1)N2C |TLB:16:6:1:4.3,THB:28:30:44:32.33| Show InChI InChI=1S/C38H52Cl2N4O2/c1-43-29-17-19-33(43)35(31(23-29)25-9-13-27(39)14-10-25)37(45)41-21-7-5-3-4-6-8-22-42-38(46)36-32(26-11-15-28(40)16-12-26)24-30-18-20-34(36)44(30)2/h9-16,29-36H,3-8,17-24H2,1-2H3,(H,41,45)(H,42,46) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [125I]RTI-55 binding to dopamine transporter in HEK cells |
Bioorg Med Chem Lett 13: 2151-4 (2003)
BindingDB Entry DOI: 10.7270/Q2DN45K3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166846
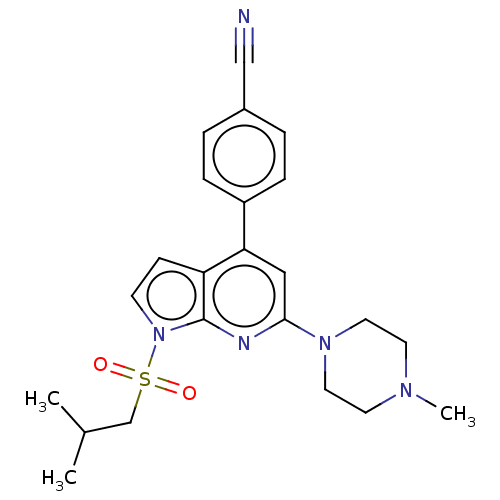
(CHEMBL3797435)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2S/c1-17(2)16-31(29,30)28-9-8-20-21(19-6-4-18(15-24)5-7-19)14-22(25-23(20)28)27-12-10-26(3)11-13-27/h4-9,14,17H,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166861
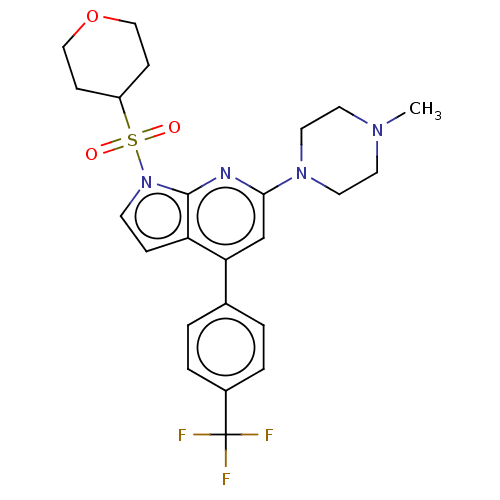
(CHEMBL3799448)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(=O)(=O)C1CCOCC1 Show InChI InChI=1S/C26H23NO5/c1-14-3-4-17(11-15(14)2)21(28)13-32-26(31)16-7-9-20(10-8-16)27-24(29)22-18-5-6-19(12-18)23(22)25(27)30/h3-11,18-19,29-30H,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192752

(CHEMBL3905741)Show SMILES COc1ncnc(N2Cc3cn(nc3[C@H]2C(C)C)-c2cccc(c2)S(C)(=O)=O)c1F |r| Show InChI InChI=1S/C20H22FN5O3S/c1-12(2)18-17-13(9-25(18)19-16(21)20(29-3)23-11-22-19)10-26(24-17)14-6-5-7-15(8-14)30(4,27)28/h5-8,10-12,18H,9H2,1-4H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192762
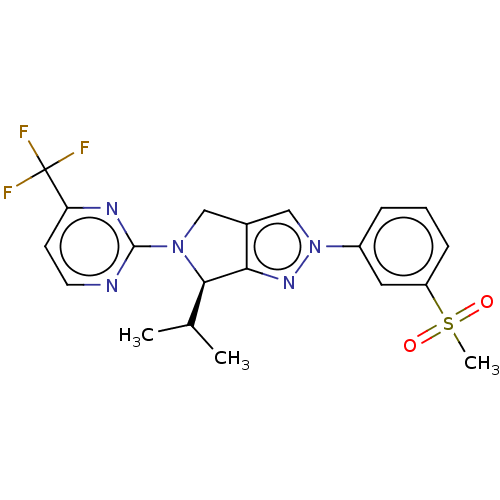
(CHEMBL3959681)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1nccc(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2S/c1-12(2)18-17-13(10-27(18)19-24-8-7-16(25-19)20(21,22)23)11-28(26-17)14-5-4-6-15(9-14)31(3,29)30/h4-9,11-12,18H,10H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192759

(CHEMBL3960195)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1cc(ncn1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2S/c1-12(2)19-18-13(9-27(19)17-8-16(20(21,22)23)24-11-25-17)10-28(26-18)14-5-4-6-15(7-14)31(3,29)30/h4-8,10-12,19H,9H2,1-3H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260722
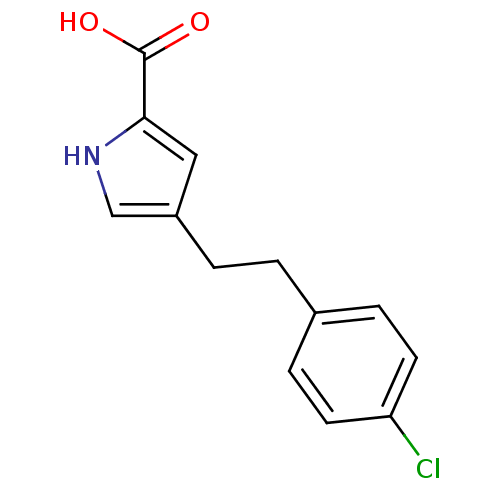
(4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...)Show InChI InChI=1S/C13H12ClNO2/c14-11-5-3-9(4-6-11)1-2-10-7-12(13(16)17)15-8-10/h3-8,15H,1-2H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50129369

(3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2.1]o...)Show SMILES CN1C2CCC1C(C(C2)c1ccc(Cl)cc1)C(=O)NCCCCCCCCNC(=O)C1C2CCC(CC1c1ccc(Cl)cc1)N2C |TLB:16:6:1:4.3,THB:28:30:44:32.33| Show InChI InChI=1S/C38H52Cl2N4O2/c1-43-29-17-19-33(43)35(31(23-29)25-9-13-27(39)14-10-25)37(45)41-21-7-5-3-4-6-8-22-42-38(46)36-32(26-11-15-28(40)16-12-26)24-30-18-20-34(36)44(30)2/h9-16,29-36H,3-8,17-24H2,1-2H3,(H,41,45)(H,42,46) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [125I]RTI-55 binding to Serotonin transporter in HEK cells |
Bioorg Med Chem Lett 13: 2151-4 (2003)
BindingDB Entry DOI: 10.7270/Q2DN45K3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166862
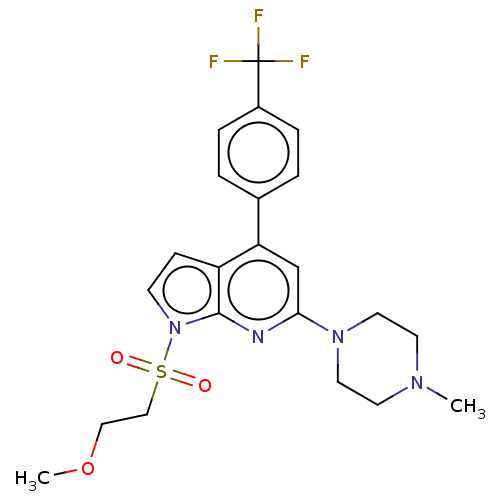
(CHEMBL3798490)Show SMILES COCCS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H21NO5/c1-13-4-5-17(10-14(13)2)19(24)12-28-22(27)16-6-8-18(9-7-16)23-20(25)11-15(3)21(23)26/h4-11,25-26H,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177018
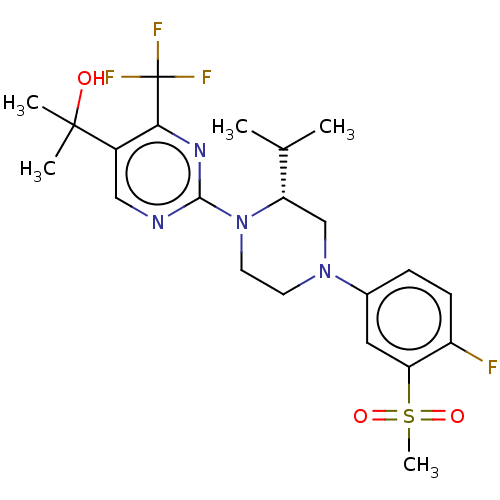
(CHEMBL3814478 | US10144715, Compound 14-1)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(c(n1)C(F)(F)F)C(C)(C)O)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28F4N4O3S/c1-13(2)17-12-29(14-6-7-16(23)18(10-14)34(5,32)33)8-9-30(17)20-27-11-15(21(3,4)31)19(28-20)22(24,25)26/h6-7,10-11,13,17,31H,8-9,12H2,1-5H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177017
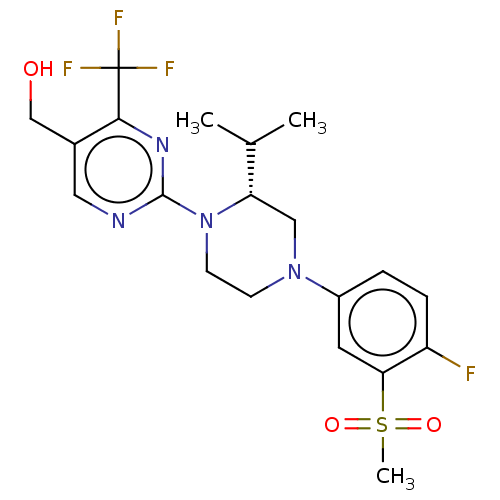
(CHEMBL3815001 | US10144715, Compound 11-1)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(CO)c(n1)C(F)(F)F)c1ccc(F)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H24F4N4O3S/c1-12(2)16-10-27(14-4-5-15(21)17(8-14)32(3,30)31)6-7-28(16)19-25-9-13(11-29)18(26-19)20(22,23)24/h4-5,8-9,12,16,29H,6-7,10-11H2,1-3H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting |
J Med Chem 59: 3264-71 (2016)
Article DOI: 10.1021/acs.jmedchem.5b02029
BindingDB Entry DOI: 10.7270/Q2XP76V7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166853

(CHEMBL3798953)Show SMILES CCc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C24H32N4O2S/c1-5-19-6-8-20(9-7-19)22-16-23(27-14-12-26(4)13-15-27)25-24-21(22)10-11-28(24)31(29,30)17-18(2)3/h6-11,16,18H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166856
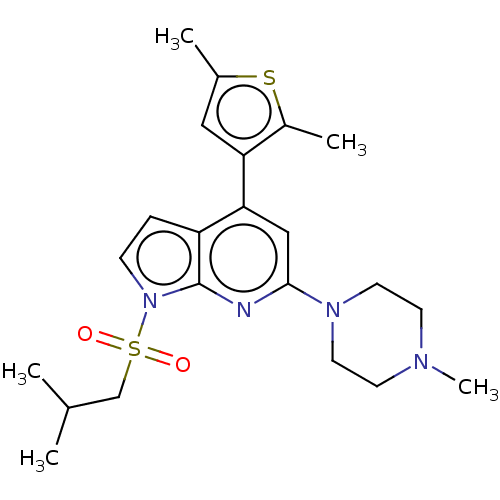
(CHEMBL3800251)Show SMILES CC(C)CS(=O)(=O)n1ccc2c(cc(nc12)N1CCN(C)CC1)-c1cc(C)sc1C Show InChI InChI=1S/C22H30N4O2S2/c1-15(2)14-30(27,28)26-7-6-18-20(19-12-16(3)29-17(19)4)13-21(23-22(18)26)25-10-8-24(5)9-11-25/h6-7,12-13,15H,8-11,14H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192760
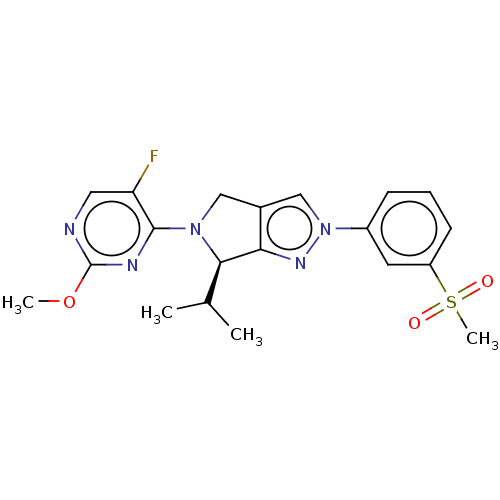
(CHEMBL3972392)Show SMILES COc1ncc(F)c(n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H22FN5O3S/c1-12(2)18-17-13(10-25(18)19-16(21)9-22-20(23-19)29-3)11-26(24-17)14-6-5-7-15(8-14)30(4,27)28/h5-9,11-12,18H,10H2,1-4H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50206007
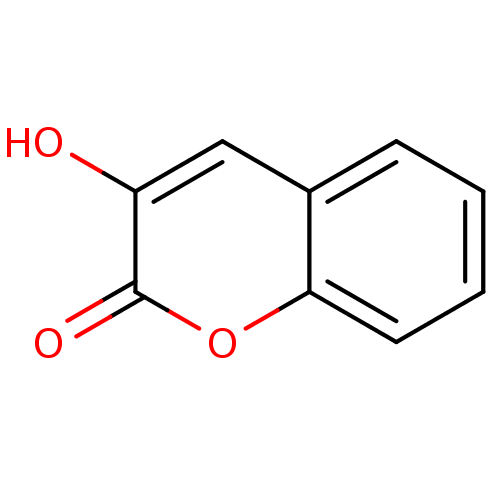
(3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...)Show InChI InChI=1S/C9H6O3/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192763
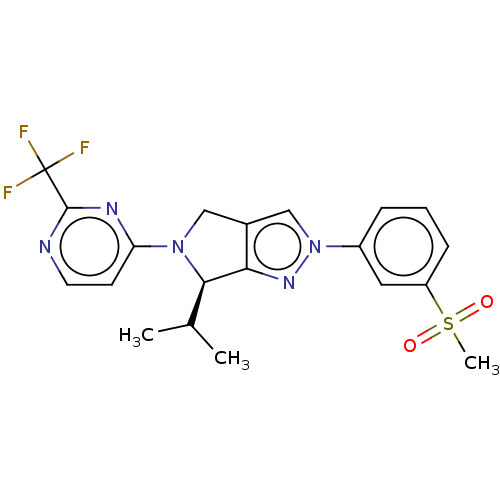
(CHEMBL3933543)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1ccnc(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2S/c1-12(2)18-17-13(10-27(18)16-7-8-24-19(25-16)20(21,22)23)11-28(26-17)14-5-4-6-15(9-14)31(3,29)30/h4-9,11-12,18H,10H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192754
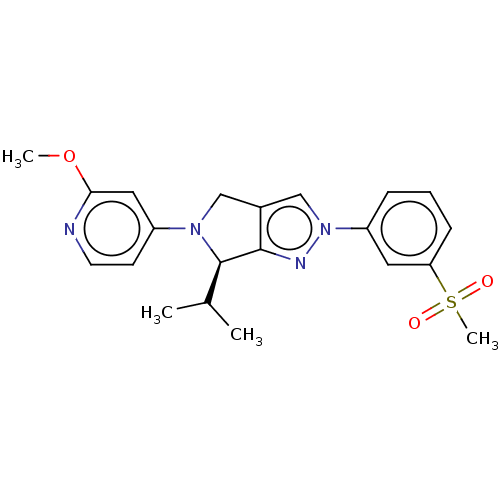
(CHEMBL3913348)Show SMILES COc1cc(ccn1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H24N4O3S/c1-14(2)21-20-15(12-24(21)16-8-9-22-19(11-16)28-3)13-25(23-20)17-6-5-7-18(10-17)29(4,26)27/h5-11,13-14,21H,12H2,1-4H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166860
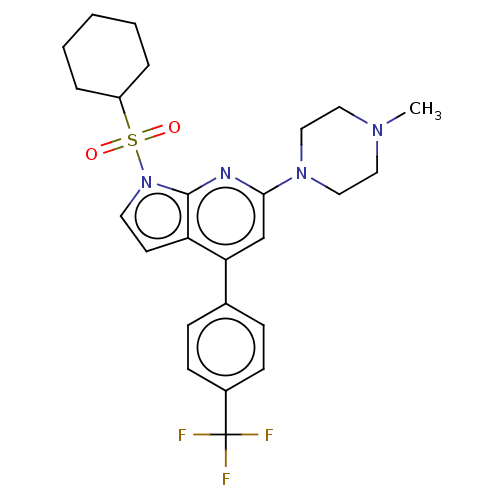
(CHEMBL3799330)Show SMILES CN1CCN(CC1)c1cc(-c2ccc(cc2)C(F)(F)F)c2ccn(c2n1)S(=O)(=O)C1CCCCC1 Show InChI InChI=1S/C25H29F3N4O2S/c1-30-13-15-31(16-14-30)23-17-22(18-7-9-19(10-8-18)25(26,27)28)21-11-12-32(24(21)29-23)35(33,34)20-5-3-2-4-6-20/h7-12,17,20H,2-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data