 Found 3595 hits with Last Name = 'fan' and Initial = 'p'
Found 3595 hits with Last Name = 'fan' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50511450
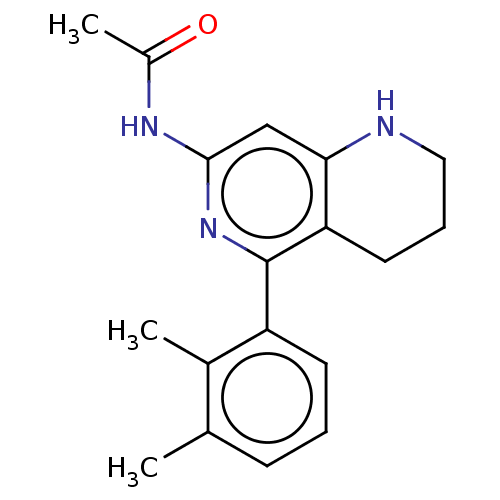
(CHEMBL4436749)Show InChI InChI=1S/C18H21N3O/c1-11-6-4-7-14(12(11)2)18-15-8-5-9-19-16(15)10-17(21-18)20-13(3)22/h4,6-7,10,19H,5,8-9H2,1-3H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... |
ACS Med Chem Lett 11: 358-364 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00420
BindingDB Entry DOI: 10.7270/Q2PV6PPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50511450

(CHEMBL4436749)Show InChI InChI=1S/C18H21N3O/c1-11-6-4-7-14(12(11)2)18-15-8-5-9-19-16(15)10-17(21-18)20-13(3)22/h4,6-7,10,19H,5,8-9H2,1-3H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... |
ACS Med Chem Lett 11: 358-364 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00420
BindingDB Entry DOI: 10.7270/Q2PV6PPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | CHEMBL5271774
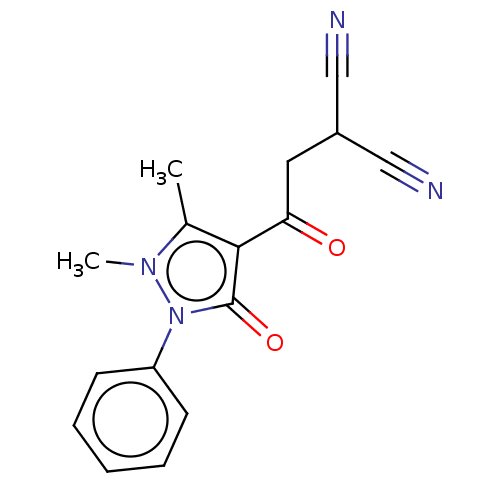
| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM312809
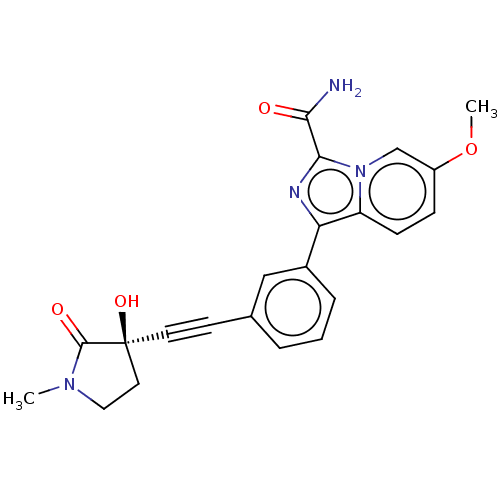
(1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...)Show SMILES COc1ccc2c(nc(C(N)=O)n2c1)-c1cccc(c1)C#C[C@]1(O)CCN(C)C1=O |r| Show InChI InChI=1S/C22H20N4O4/c1-25-11-10-22(29,21(25)28)9-8-14-4-3-5-15(12-14)18-17-7-6-16(30-2)13-26(17)20(24-18)19(23)27/h3-7,12-13,29H,10-11H2,1-2H3,(H2,23,27)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM50457820
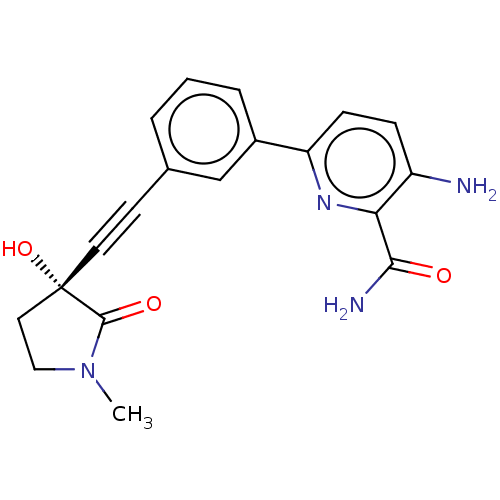
(CHEMBL4216289)Show SMILES CN1CC[C@@](O)(C#Cc2cccc(c2)-c2ccc(N)c(n2)C(N)=O)C1=O |r| Show InChI InChI=1S/C19H18N4O3/c1-23-10-9-19(26,18(23)25)8-7-12-3-2-4-13(11-12)15-6-5-14(20)16(22-15)17(21)24/h2-6,11,26H,9-10,20H2,1H3,(H2,21,24)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM50457816
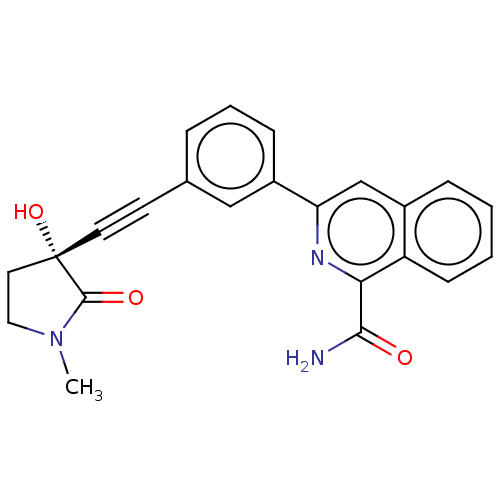
(CHEMBL4215425)Show SMILES CN1CC[C@@](O)(C#Cc2cccc(c2)-c2cc3ccccc3c(n2)C(N)=O)C1=O |r| Show InChI InChI=1S/C23H19N3O3/c1-26-12-11-23(29,22(26)28)10-9-15-5-4-7-17(13-15)19-14-16-6-2-3-8-18(16)20(25-19)21(24)27/h2-8,13-14,29H,11-12H2,1H3,(H2,24,27)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM312763
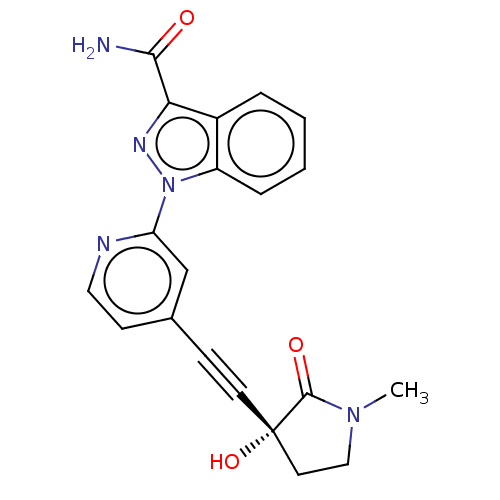
(1-[4-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...)Show SMILES CN1CC[C@@](O)(C#Cc2ccnc(c2)-n2nc(C(N)=O)c3ccccc23)C1=O |r| Show InChI InChI=1S/C20H17N5O3/c1-24-11-9-20(28,19(24)27)8-6-13-7-10-22-16(12-13)25-15-5-3-2-4-14(15)17(23-25)18(21)26/h2-5,7,10,12,28H,9,11H2,1H3,(H2,21,26)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM312785
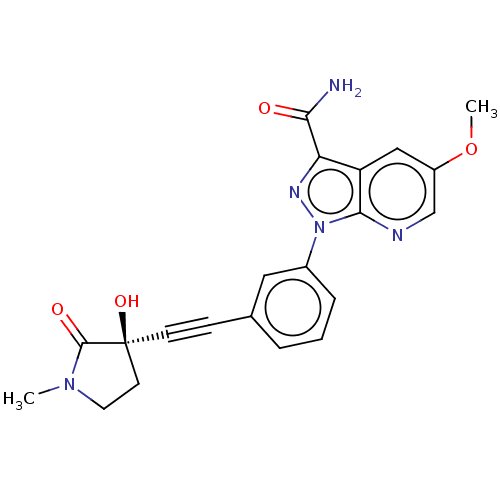
(1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...)Show SMILES COc1cnc2n(nc(C(N)=O)c2c1)-c1cccc(c1)C#C[C@]1(O)CCN(C)C1=O |r| Show InChI InChI=1S/C21H19N5O4/c1-25-9-8-21(29,20(25)28)7-6-13-4-3-5-14(10-13)26-19-16(17(24-26)18(22)27)11-15(30-2)12-23-19/h3-5,10-12,29H,8-9H2,1-2H3,(H2,22,27)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM312765
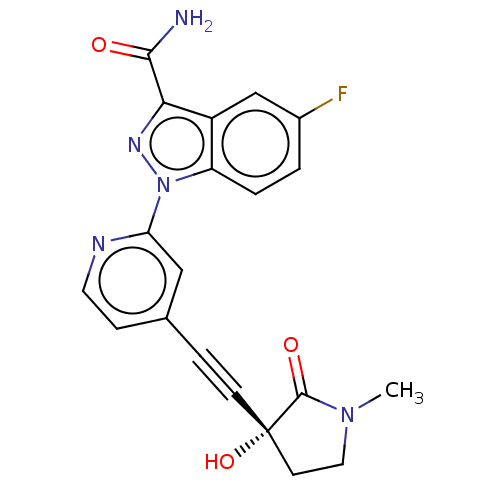
(5-fluoro-1-[4-[2-[(3R)-3-hy- droxy-1-methyl-2-oxo-...)Show SMILES CN1CC[C@@](O)(C#Cc2ccnc(c2)-n2nc(C(N)=O)c3cc(F)ccc23)C1=O |r| Show InChI InChI=1S/C20H16FN5O3/c1-25-9-7-20(29,19(25)28)6-4-12-5-8-23-16(10-12)26-15-3-2-13(21)11-14(15)17(24-26)18(22)27/h2-3,5,8,10-11,29H,7,9H2,1H3,(H2,22,27)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM312788

(1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...)Show SMILES CN1CC[C@@](O)(C#Cc2cccc(c2)-c2nc(C(N)=O)n3ccccc23)C1=O |r| Show InChI InChI=1S/C21H18N4O3/c1-24-12-10-21(28,20(24)27)9-8-14-5-4-6-15(13-14)17-16-7-2-3-11-25(16)19(23-17)18(22)26/h2-7,11,13,28H,10,12H2,1H3,(H2,22,26)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636
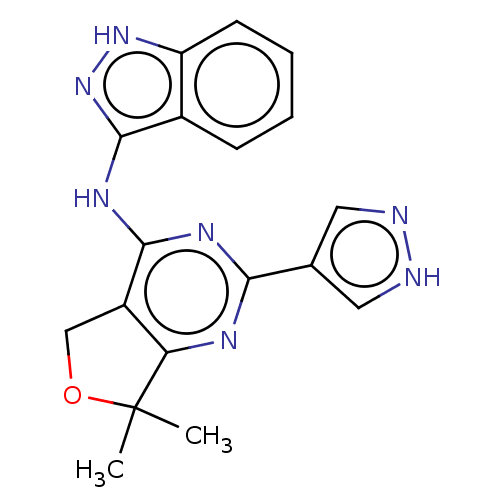
(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50172545
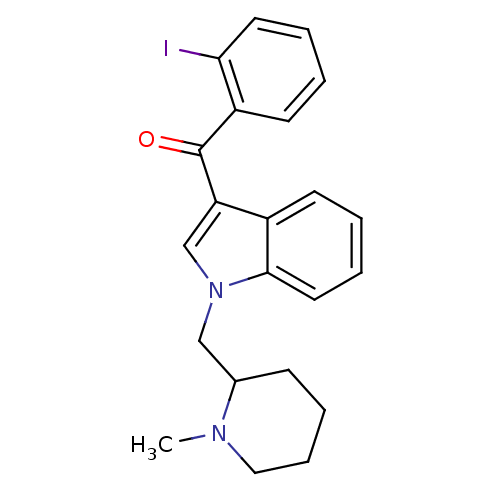
((2-Iodo-phenyl)-[1-((S)-1-methyl-piperidin-2-ylmet...)Show InChI InChI=1S/C22H23IN2O/c1-24-13-7-6-8-16(24)14-25-15-19(17-9-3-5-12-21(17)25)22(26)18-10-2-4-11-20(18)23/h2-5,9-12,15-16H,6-8,13-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse hippocampal membranes cannabinoid receptor 1 using [131I]-(R)-8 |
J Med Chem 48: 6386-92 (2005)
Article DOI: 10.1021/jm050135l
BindingDB Entry DOI: 10.7270/Q21V5FQ7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM312711
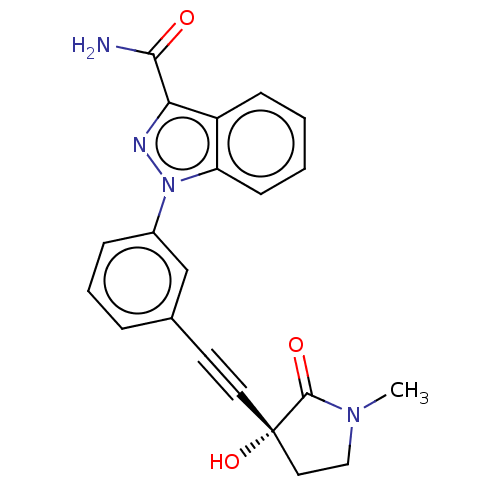
(1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...)Show SMILES CN1CC[C@@](O)(C#Cc2cccc(c2)-n2nc(C(N)=O)c3ccccc23)C1=O |r| Show InChI InChI=1S/C21H18N4O3/c1-24-12-11-21(28,20(24)27)10-9-14-5-4-6-15(13-14)25-17-8-3-2-7-16(17)18(23-25)19(22)26/h2-8,13,28H,11-12H2,1H3,(H2,22,26)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM50457815
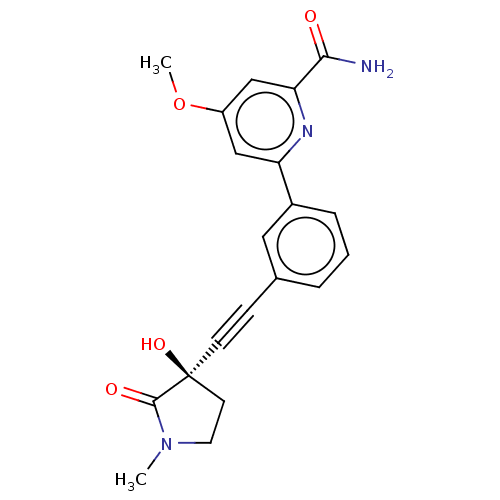
(CHEMBL4211840)Show SMILES COc1cc(nc(c1)-c1cccc(c1)C#C[C@]1(O)CCN(C)C1=O)C(N)=O |r| Show InChI InChI=1S/C20H19N3O4/c1-23-9-8-20(26,19(23)25)7-6-13-4-3-5-14(10-13)16-11-15(27-2)12-17(22-16)18(21)24/h3-5,10-12,26H,8-9H2,1-2H3,(H2,21,24)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM312764
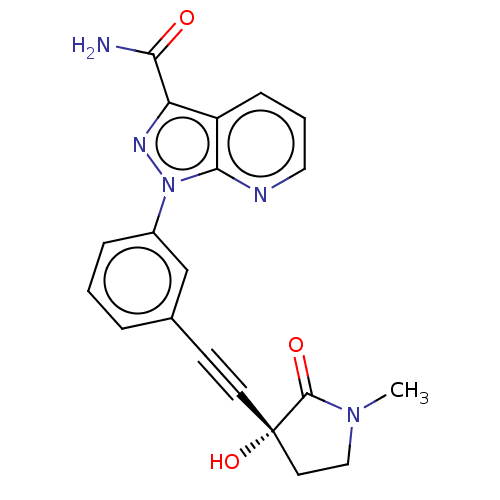
(1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...)Show SMILES CN1CC[C@@](O)(C#Cc2cccc(c2)-n2nc(C(N)=O)c3cccnc23)C1=O |r| Show InChI InChI=1S/C20H17N5O3/c1-24-11-9-20(28,19(24)27)8-7-13-4-2-5-14(12-13)25-18-15(6-3-10-22-18)16(23-25)17(21)26/h2-6,10,12,28H,9,11H2,1H3,(H2,21,26)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM312723

(5-fluoro-1-[3-[2-[(3R)-3-hy- droxy-1-methyl-2-oxo-...)Show SMILES CN1CC[C@@](O)(C#Cc2cccc(c2)-n2nc(C(N)=O)c3cc(F)ccc23)C1=O |r| Show InChI InChI=1S/C21H17FN4O3/c1-25-10-9-21(29,20(25)28)8-7-13-3-2-4-15(11-13)26-17-6-5-14(22)12-16(17)18(24-26)19(23)27/h2-6,11-12,29H,9-10H2,1H3,(H2,23,27)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | CHEMBL5270286

| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 0.346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50080030

((6aR,10aR)-3-Heptyl-6,6,9-trimethyl-6a,7,10,10a-te...)Show SMILES CCCCCCCc1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:15| Show InChI InChI=1S/C23H34O2/c1-5-6-7-8-9-10-17-14-20(24)22-18-13-16(2)11-12-19(18)23(3,4)25-21(22)15-17/h11,14-15,18-19,24H,5-10,12-13H2,1-4H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity towards Cannabinoid receptor 2 in mouse spleen membranes |
Bioorg Med Chem Lett 9: 2119-24 (1999)
BindingDB Entry DOI: 10.7270/Q2N015QC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141902

(CHEMBL3758602)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2c(Cl)cccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H16ClN7O/c1-18(2)14-11(8-27-18)16(23-15(22-14)9-6-20-21-7-9)24-17-10-4-3-5-12(19)13(10)25-26-17/h3-7H,8H2,1-2H3,(H,20,21)(H2,22,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM50457818

(CHEMBL4211560)Show SMILES CN1CC[C@@](O)(C#Cc2cccc(c2)-c2cccc(n2)C(N)=O)C1=O |r| Show InChI InChI=1S/C19H17N3O3/c1-22-11-10-19(25,18(22)24)9-8-13-4-2-5-14(12-13)15-6-3-7-16(21-15)17(20)23/h2-7,12,25H,10-11H2,1H3,(H2,20,23)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50080030

((6aR,10aR)-3-Heptyl-6,6,9-trimethyl-6a,7,10,10a-te...)Show SMILES CCCCCCCc1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:15| Show InChI InChI=1S/C23H34O2/c1-5-6-7-8-9-10-17-14-20(24)22-18-13-16(2)11-12-19(18)23(3,4)25-21(22)15-17/h11,14-15,18-19,24H,5-10,12-13H2,1-4H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity towards cannabinoid receptor 1 in rat forebrain membranes. |
Bioorg Med Chem Lett 9: 2119-24 (1999)
BindingDB Entry DOI: 10.7270/Q2N015QC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50219076

((6aR-trans)-3-(1-heptylcyclopropyl)-6a,7,10,10a-te...)Show SMILES CCCCCCCC1(CC1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:19| Show InChI InChI=1S/C26H38O2/c1-5-6-7-8-9-12-26(13-14-26)19-16-22(27)24-20-15-18(2)10-11-21(20)25(3,4)28-23(24)17-19/h10,16-17,20-21,27H,5-9,11-15H2,1-4H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in rat brain synaptosomal membrane |
J Med Chem 50: 4048-60 (2007)
Article DOI: 10.1021/jm070121a
BindingDB Entry DOI: 10.7270/Q21N80VQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141610
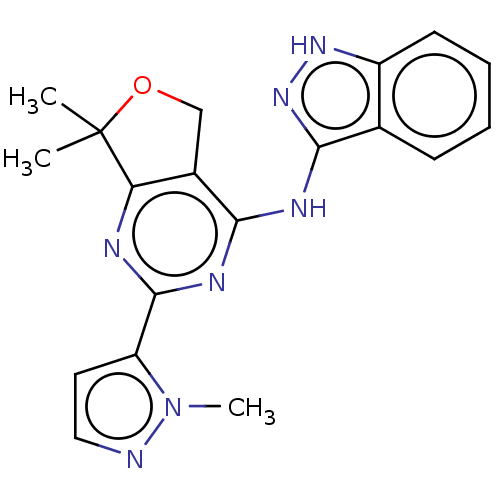
(CHEMBL3759096)Show SMILES Cn1nccc1-c1nc2c(COC2(C)C)c(Nc2n[nH]c3ccccc23)n1 Show InChI InChI=1S/C19H19N7O/c1-19(2)15-12(10-27-19)16(23-18(21-15)14-8-9-20-26(14)3)22-17-11-6-4-5-7-13(11)24-25-17/h4-9H,10H2,1-3H3,(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141610

(CHEMBL3759096)Show SMILES Cn1nccc1-c1nc2c(COC2(C)C)c(Nc2n[nH]c3ccccc23)n1 Show InChI InChI=1S/C19H19N7O/c1-19(2)15-12(10-27-19)16(23-18(21-15)14-8-9-20-26(14)3)22-17-11-6-4-5-7-13(11)24-25-17/h4-9H,10H2,1-3H3,(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636

(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM50457817
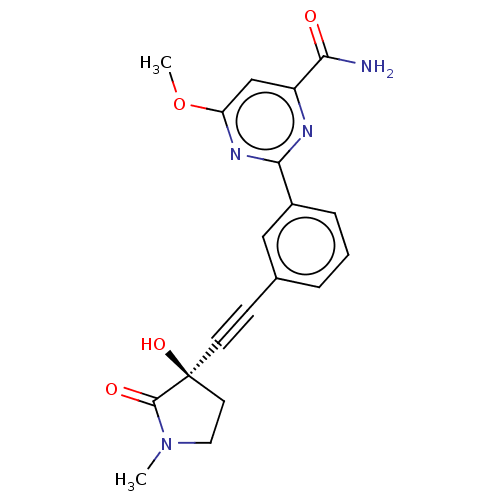
(CHEMBL4214032)Show SMILES COc1cc(nc(n1)-c1cccc(c1)C#C[C@]1(O)CCN(C)C1=O)C(N)=O |r| Show InChI InChI=1S/C19H18N4O4/c1-23-9-8-19(26,18(23)25)7-6-12-4-3-5-13(10-12)17-21-14(16(20)24)11-15(22-17)27-2/h3-5,10-11,26H,8-9H2,1-2H3,(H2,20,24)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383522
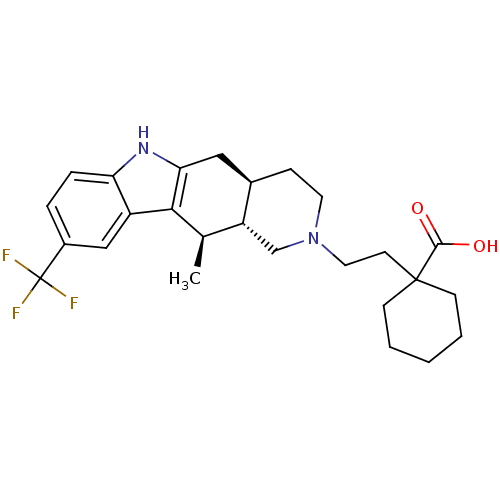
(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to human MCHR1 |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636

(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50063882
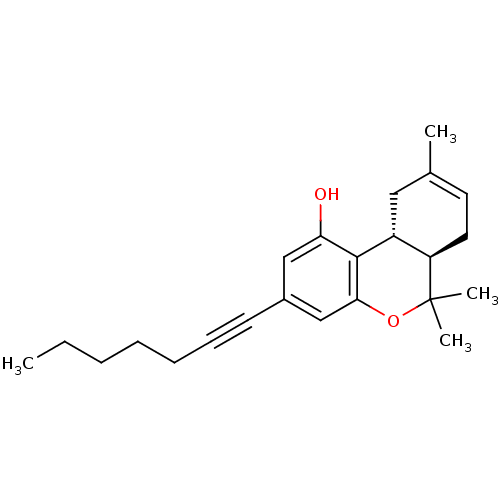
((6aR,10aR)-3-(hept-1-ynyl)-6,6,9-trimethyl-6a,7,10...)Show SMILES CCCCCC#Cc1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:15| Show InChI InChI=1S/C23H30O2/c1-5-6-7-8-9-10-17-14-20(24)22-18-13-16(2)11-12-19(18)23(3,4)25-21(22)15-17/h11,14-15,18-19,24H,5-8,12-13H2,1-4H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from rat brain CB1 receptor |
Bioorg Med Chem Lett 16: 1616-20 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.026
BindingDB Entry DOI: 10.7270/Q2833RMF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50219078

((6aR-trans)-3-[1-[(1Z)-1-hexenyl]cyclopentyl]-6a,7...)Show SMILES CCCC\C=C/C1(CCCC1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:20| Show InChI InChI=1S/C27H38O2/c1-5-6-7-8-13-27(14-9-10-15-27)20-17-23(28)25-21-16-19(2)11-12-22(21)26(3,4)29-24(25)18-20/h8,11,13,17-18,21-22,28H,5-7,9-10,12,14-16H2,1-4H3/b13-8-/t21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in rat brain synaptosomal membrane |
J Med Chem 50: 4048-60 (2007)
Article DOI: 10.1021/jm070121a
BindingDB Entry DOI: 10.7270/Q21N80VQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50172553

(CHEMBL68641 | [1-(1-Methyl-piperidin-2-ylmethyl)-1...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cccc3ccccc23)c2ccccc12 Show InChI InChI=1S/C26H26N2O/c1-27-16-7-6-11-20(27)17-28-18-24(22-13-4-5-15-25(22)28)26(29)23-14-8-10-19-9-2-3-12-21(19)23/h2-5,8-10,12-15,18,20H,6-7,11,16-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Binding affinity towards rat forebrain cannabinoid receptor 1 using [3H]CP-55940 |
J Med Chem 48: 6386-92 (2005)
Article DOI: 10.1021/jm050135l
BindingDB Entry DOI: 10.7270/Q21V5FQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50053265

((6aR,9R,10aR)-3-((Z)-Hept-1-enyl)-9-hydroxymethyl-...)Show SMILES CCCCC\C=C/c1cc(O)c2[C@@H]3C[C@H](CO)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C23H34O3/c1-4-5-6-7-8-9-16-13-20(25)22-18-12-17(15-24)10-11-19(18)23(2,3)26-21(22)14-16/h8-9,13-14,17-19,24-25H,4-7,10-12,15H2,1-3H3/b9-8-/t17-,18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 1 enriched rat brain microsome prepara... |
J Med Chem 39: 3790-6 (1996)
Article DOI: 10.1021/jm950934b
BindingDB Entry DOI: 10.7270/Q2TT4RM1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM312762
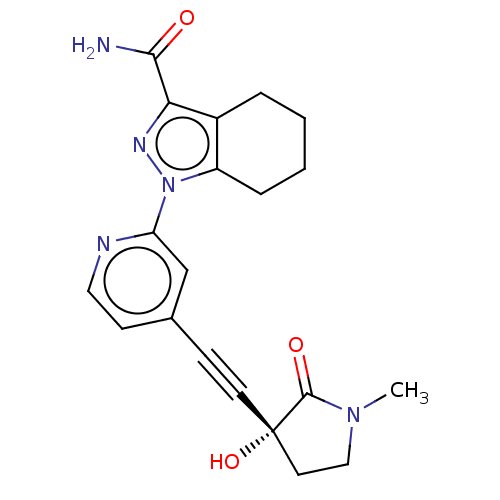
(1-[4-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...)Show SMILES CN1CC[C@@](O)(C#Cc2ccnc(c2)-n2nc(C(N)=O)c3CCCCc23)C1=O |r| Show InChI InChI=1S/C20H21N5O3/c1-24-11-9-20(28,19(24)27)8-6-13-7-10-22-16(12-13)25-15-5-3-2-4-14(15)17(23-25)18(21)26/h7,10,12,28H,2-5,9,11H2,1H3,(H2,21,26)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50475505
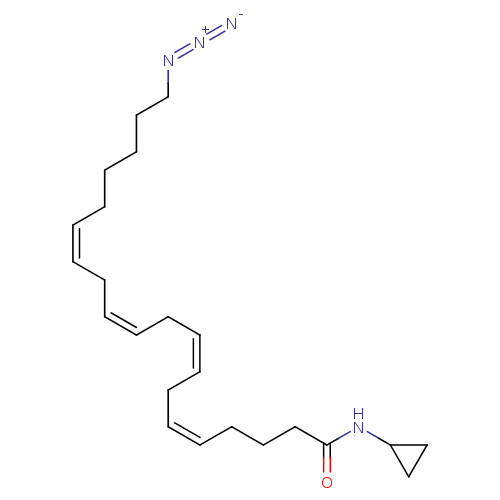
(CHEMBL2113102)Show SMILES [N-]=[N+]=NCCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NC1CC1 Show InChI InChI=1S/C23H36N4O/c24-27-25-21-17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16-18-23(28)26-22-19-20-22/h1,3-4,6-7,9-10,12,22H,2,5,8,11,13-21H2,(H,26,28)/b3-1-,6-4-,9-7-,12-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Binding affinity towards rat brain cannabinoid receptor 1 in the presence of PMSF upon incubation for 15 min at 4 degree C in pH 7.4 using [3H]CP-559... |
J Med Chem 48: 6423-9 (2005)
Article DOI: 10.1021/jm050272i
BindingDB Entry DOI: 10.7270/Q2G44T17 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50064786
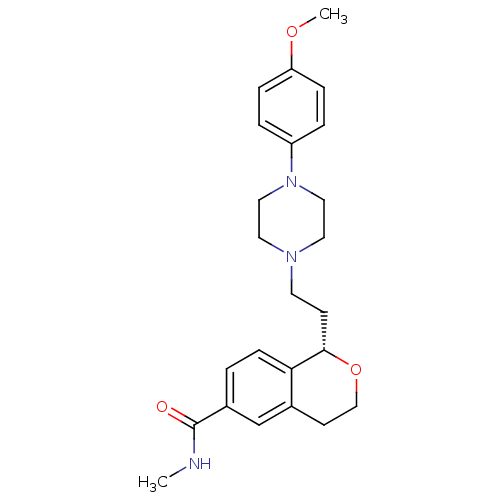
((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...)Show SMILES CNC(=O)c1ccc2[C@H](CCN3CCN(CC3)c3ccc(OC)cc3)OCCc2c1 |r| Show InChI InChI=1S/C24H31N3O3/c1-25-24(28)19-3-8-22-18(17-19)10-16-30-23(22)9-11-26-12-14-27(15-13-26)20-4-6-21(29-2)7-5-20/h3-8,17,23H,9-16H2,1-2H3,(H,25,28)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50219072
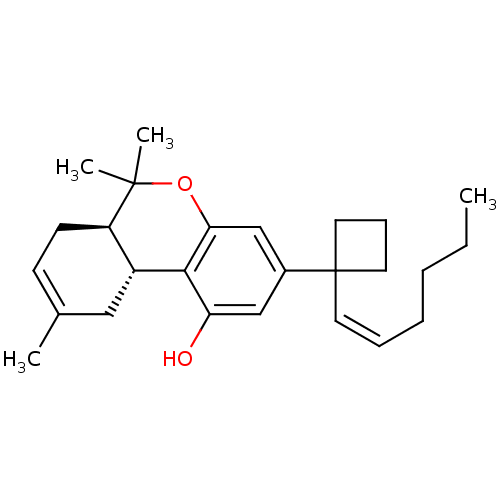
((6aR-trans)-3-[1-[(1Z)-1-hexenyl]cyclobutyl]-6a,7,...)Show SMILES CCCC\C=C/C1(CCC1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:19| Show InChI InChI=1S/C26H36O2/c1-5-6-7-8-12-26(13-9-14-26)19-16-22(27)24-20-15-18(2)10-11-21(20)25(3,4)28-23(24)17-19/h8,10,12,16-17,20-21,27H,5-7,9,11,13-15H2,1-4H3/b12-8-/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor in rat brain synaptosomal membrane |
J Med Chem 50: 4048-60 (2007)
Article DOI: 10.1021/jm070121a
BindingDB Entry DOI: 10.7270/Q21N80VQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141610

(CHEMBL3759096)Show SMILES Cn1nccc1-c1nc2c(COC2(C)C)c(Nc2n[nH]c3ccccc23)n1 Show InChI InChI=1S/C19H19N7O/c1-19(2)15-12(10-27-19)16(23-18(21-15)14-8-9-20-26(14)3)22-17-11-6-4-5-7-13(11)24-25-17/h4-9H,10H2,1-3H3,(H2,21,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50108659
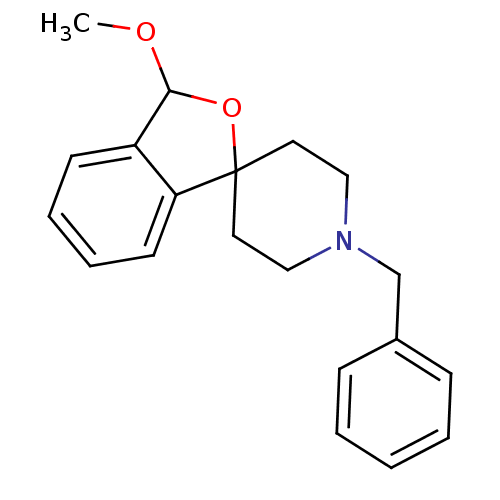
(1'-benzyl-3-methoxy-3H-spiro[2-benzofuran-1,4'-pip...)Show InChI InChI=1S/C20H23NO2/c1-22-19-17-9-5-6-10-18(17)20(23-19)11-13-21(14-12-20)15-16-7-3-2-4-8-16/h2-10,19H,11-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50219072

((6aR-trans)-3-[1-[(1Z)-1-hexenyl]cyclobutyl]-6a,7,...)Show SMILES CCCC\C=C/C1(CCC1)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:19| Show InChI InChI=1S/C26H36O2/c1-5-6-7-8-12-26(13-9-14-26)19-16-22(27)24-20-15-18(2)10-11-21(20)25(3,4)28-23(24)17-19/h8,10,12,16-17,20-21,27H,5-7,9,11,13-15H2,1-4H3/b12-8-/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor in mouse spleen membrane |
J Med Chem 50: 4048-60 (2007)
Article DOI: 10.1021/jm070121a
BindingDB Entry DOI: 10.7270/Q21N80VQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50053266
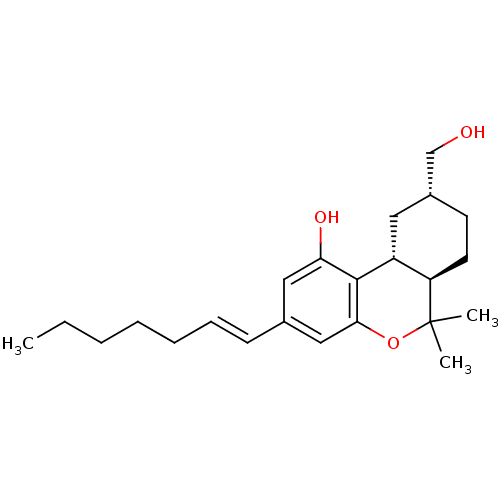
((6aR,9R,10aR)-3-((E)-Hept-1-enyl)-9-hydroxymethyl-...)Show SMILES CCCCC\C=C\c1cc(O)c2[C@@H]3C[C@H](CO)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C23H34O3/c1-4-5-6-7-8-9-16-13-20(25)22-18-12-17(15-24)10-11-19(18)23(2,3)26-21(22)14-16/h8-9,13-14,17-19,24-25H,4-7,10-12,15H2,1-3H3/b9-8+/t17-,18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 1 enriched rat brain microsome prepara... |
J Med Chem 39: 3790-6 (1996)
Article DOI: 10.1021/jm950934b
BindingDB Entry DOI: 10.7270/Q2TT4RM1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141902

(CHEMBL3758602)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2c(Cl)cccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H16ClN7O/c1-18(2)14-11(8-27-18)16(23-15(22-14)9-6-20-21-7-9)24-17-10-4-3-5-12(19)13(10)25-26-17/h3-7H,8H2,1-2H3,(H,20,21)(H2,22,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM50172545

((2-Iodo-phenyl)-[1-((S)-1-methyl-piperidin-2-ylmet...)Show InChI InChI=1S/C22H23IN2O/c1-24-13-7-6-8-16(24)14-25-15-19(17-9-3-5-12-21(17)25)22(26)18-10-2-4-11-20(18)23/h2-5,9-12,15-16H,6-8,13-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse hippocampal membranes cannabinoid receptor 1 using [3H]SR-141,716A |
J Med Chem 48: 6386-92 (2005)
Article DOI: 10.1021/jm050135l
BindingDB Entry DOI: 10.7270/Q21V5FQ7 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50108653
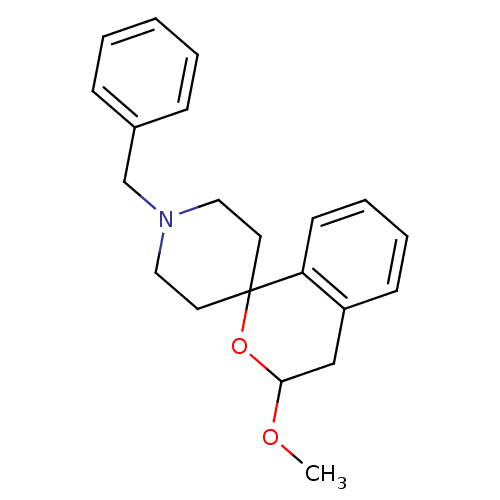
(1'-benzyl-3-methoxyspiro[3,4-dihydro-1H-isochromen...)Show InChI InChI=1S/C21H25NO2/c1-23-20-15-18-9-5-6-10-19(18)21(24-20)11-13-22(14-12-21)16-17-7-3-2-4-8-17/h2-10,20H,11-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50475506

(CHEMBL2113101)Show SMILES O=C(CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCCN=C=S)NC1CC1 Show InChI InChI=1S/C24H36N2OS/c27-24(26-23-19-20-23)18-16-14-12-10-8-6-4-2-1-3-5-7-9-11-13-15-17-21-25-22-28/h1,3-4,6-7,9-10,12,23H,2,5,8,11,13-21H2,(H,26,27)/b3-1-,6-4-,9-7-,12-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Binding affinity towards rat brain cannabinoid receptor 1 in the presence of PMSF upon incubation for 15 min at 4 degree C in pH 7.4 using [3H]CP-559... |
J Med Chem 48: 6423-9 (2005)
Article DOI: 10.1021/jm050272i
BindingDB Entry DOI: 10.7270/Q2G44T17 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 14
(Homo sapiens (Human)) | BDBM50457812

(CHEMBL4217923)Show SMILES CN1CC[C@@](O)(C#Cc2cccc(c2)-c2cncc(n2)C(N)=O)C1=O |r| Show InChI InChI=1S/C18H16N4O3/c1-22-8-7-18(25,17(22)24)6-5-12-3-2-4-13(9-12)14-10-20-11-15(21-14)16(19)23/h2-4,9-11,25H,7-8H2,1H3,(H2,19,23)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... |
J Med Chem 61: 6801-6813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00678
BindingDB Entry DOI: 10.7270/Q2PV6P00 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141902

(CHEMBL3758602)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2c(Cl)cccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H16ClN7O/c1-18(2)14-11(8-27-18)16(23-15(22-14)9-6-20-21-7-9)24-17-10-4-3-5-12(19)13(10)25-26-17/h3-7H,8H2,1-2H3,(H,20,21)(H2,22,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(MOUSE) | BDBM50066705
((6R,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-6,9-bis-h...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3C[C@H](CO)CC[C@H]3[C@](C)(CO)Oc2c1 Show InChI InChI=1S/C25H40O4/c1-5-6-7-8-11-24(2,3)18-13-21(28)23-19-12-17(15-26)9-10-20(19)25(4,16-27)29-22(23)14-18/h13-14,17,19-20,26-28H,5-12,15-16H2,1-4H3/t17-,19-,20-,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii
Curated by ChEMBL
| Assay Description
Binding affinity of the compound to Cannabinoid receptor 2 from mouse spleen was measured using [3H]CP-55,940 as radioligand |
J Med Chem 41: 3596-608 (1998)
Article DOI: 10.1021/jm960677q
BindingDB Entry DOI: 10.7270/Q26H4GHP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data