 Found 176 hits with Last Name = 'fang' and Initial = 'e'
Found 176 hits with Last Name = 'fang' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM50188913
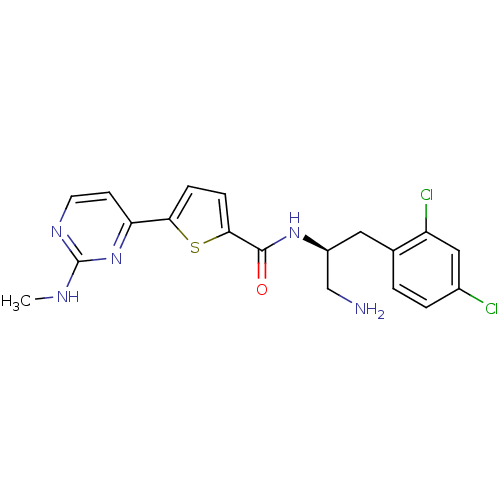
(CHEMBL213618 | N-((S)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PKA |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313013
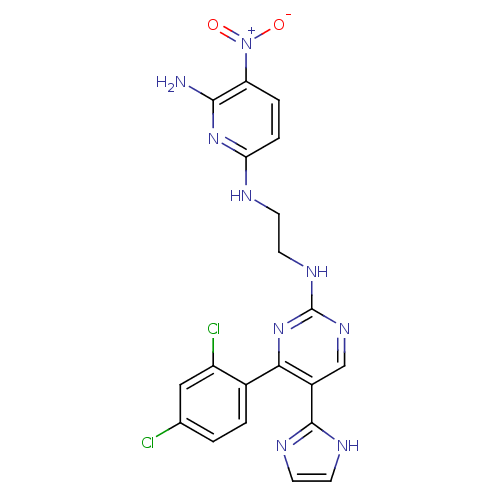
(CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...)Show SMILES Nc1nc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-11-1-2-12(14(22)9-11)17-13(19-25-6-7-26-19)10-28-20(30-17)27-8-5-24-16-4-3-15(31(32)33)18(23)29-16/h1-4,6-7,9-10H,5,8H2,(H,25,26)(H3,23,24,29)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50243389
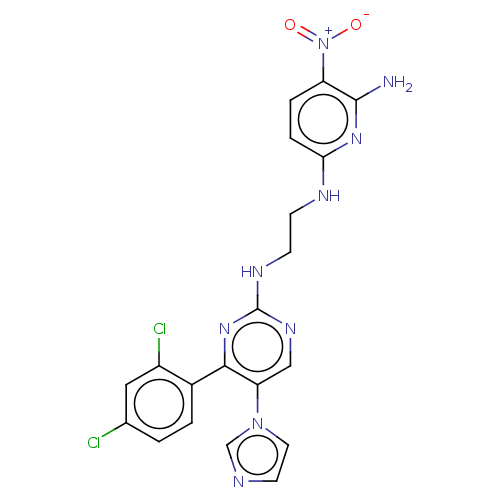
(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PKA |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcium/calmodulin-dependent protein kinase type II subunit alpha
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CamK2alpha |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Kit |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PKCa |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188913

(CHEMBL213618 | N-((S)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603975

(CHEMBL5201780) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188913

(CHEMBL213618 | N-((S)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188945
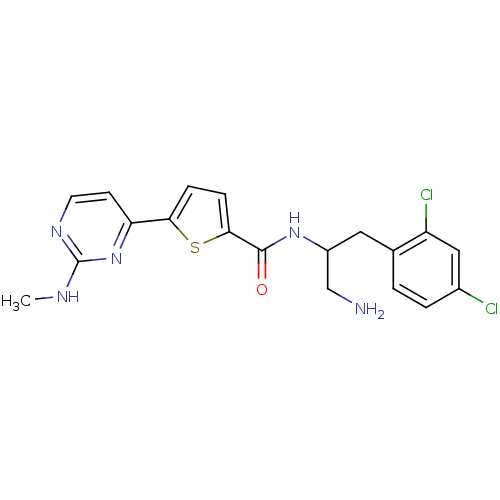
(CHEMBL379507 | N-(1-amino-3-(2,4-dichlorophenyl)pr...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)NC(CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603974

(CHEMBL5201904)Show SMILES COc1ccc(cn1)-c1cnc2sc(N[C@@H]3CC[C@H](N)CC3)nn12 |r,wU:15.15,18.19,(-7.26,4.65,;-5.72,4.65,;-4.95,3.32,;-5.72,1.98,;-4.96,.65,;-3.42,.65,;-2.64,1.99,;-3.42,3.32,;-2.65,-.68,;-3.1,-2.13,;-1.86,-3.05,;-.65,-2.17,;.9,-2.18,;1.42,-.69,;2.91,-.3,;4,-1.38,;3.6,-2.87,;4.69,-3.96,;6.17,-3.56,;7.26,-4.65,;6.57,-2.07,;5.48,-.99,;.2,.22,;-1.11,-.68,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188913

(CHEMBL213618 | N-((S)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188929
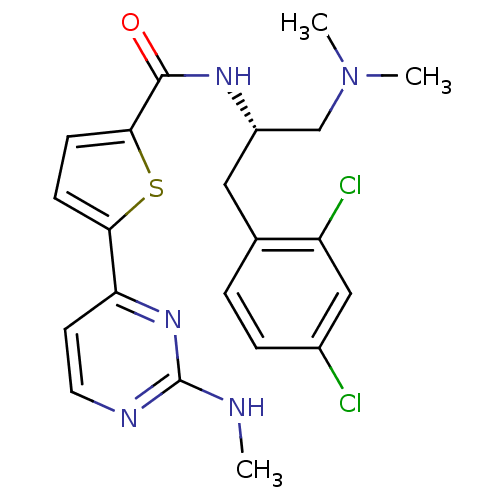
(CHEMBL384964 | N-((S)-1-(2,4-dichlorophenyl)-3-(di...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN(C)C)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C21H23Cl2N5OS/c1-24-21-25-9-8-17(27-21)18-6-7-19(30-18)20(29)26-15(12-28(2)3)10-13-4-5-14(22)11-16(13)23/h4-9,11,15H,10,12H2,1-3H3,(H,26,29)(H,24,25,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603992
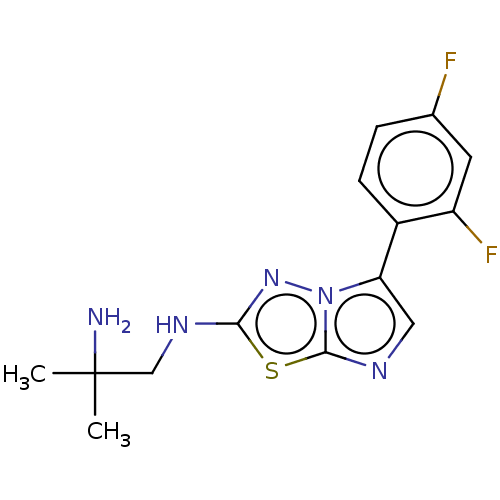
(CHEMBL5179988) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188913

(CHEMBL213618 | N-((S)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50313013

(CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...)Show SMILES Nc1nc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-11-1-2-12(14(22)9-11)17-13(19-25-6-7-26-19)10-28-20(30-17)27-8-5-24-16-4-3-15(31(32)33)18(23)29-16/h1-4,6-7,9-10H,5,8H2,(H,25,26)(H3,23,24,29)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3alpha using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188927
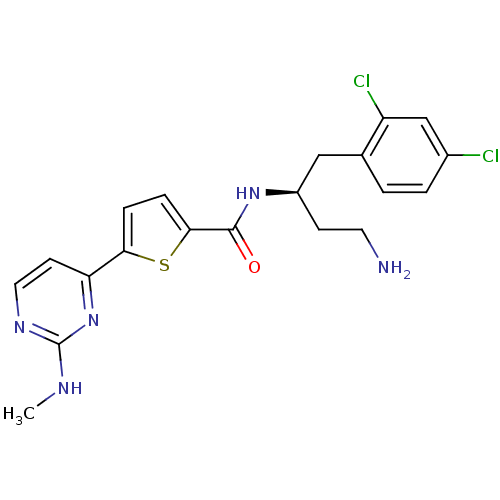
(CHEMBL385143 | N-((S)-4-amino-1-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CCN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C20H21Cl2N5OS/c1-24-20-25-9-7-16(27-20)17-4-5-18(29-17)19(28)26-14(6-8-23)10-12-2-3-13(21)11-15(12)22/h2-5,7,9,11,14H,6,8,10,23H2,1H3,(H,26,28)(H,24,25,27)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603978
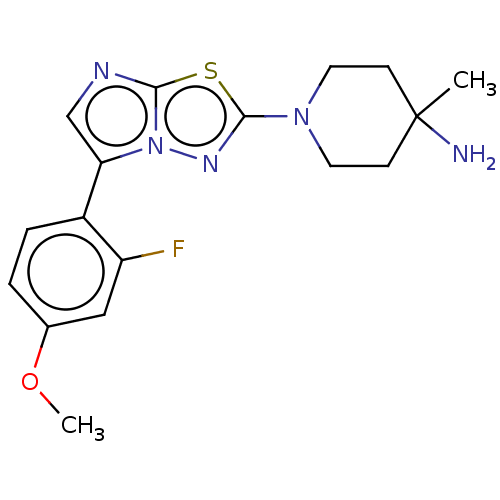
(CHEMBL5187817)Show SMILES COc1ccc(-c2cnc3sc(nn23)N2CCC(C)(N)CC2)c(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603989
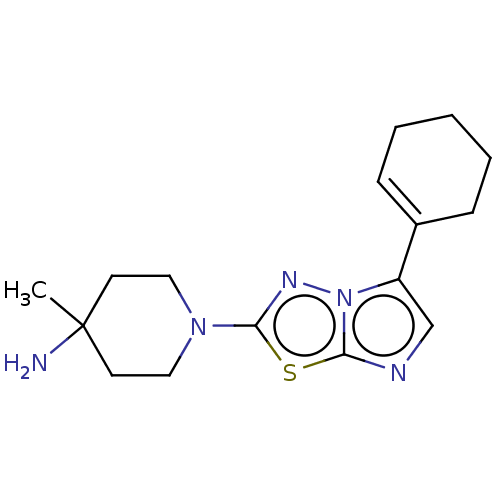
(CHEMBL5192443)Show SMILES CC1(N)CCN(CC1)c1nn2c(cnc2s1)C1=CCCCC1 |t:19| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50188913

(CHEMBL213618 | N-((S)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PKCa |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603986

(CHEMBL5187610) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50188913

(CHEMBL213618 | N-((S)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Kit |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Met |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603988
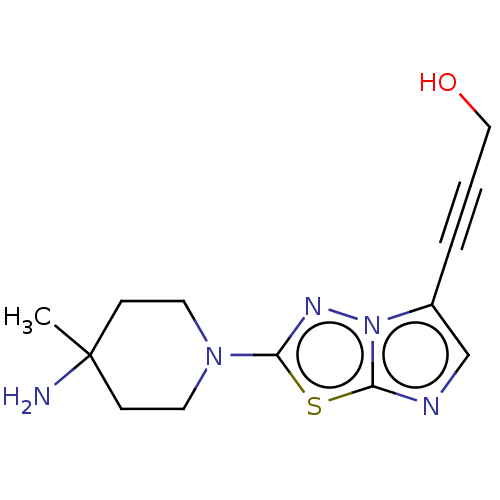
(CHEMBL5170360) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of RAF1 |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603984

(CHEMBL5195201)Show SMILES CC1(N)CCN(CC1)c1nn2c(cnc2s1)-c1cccc2cc[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM7266

(14-bromo-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show InChI InChI=1S/C16H11BrN2O/c17-9-5-6-14-11(7-9)12-8-15(20)18-13-4-2-1-3-10(13)16(12)19-14/h1-7,19H,8H2,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50166289
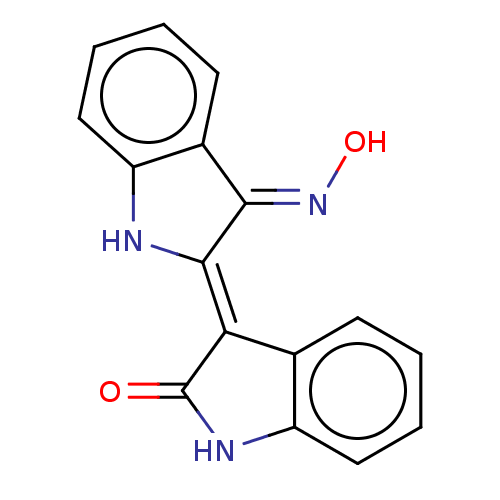
(CHEBI:43645 | CHEMBL216543)Show InChI InChI=1S/C16H11N3O2/c20-16-13(9-5-1-3-7-11(9)18-16)15-14(19-21)10-6-2-4-8-12(10)17-15/h1-8,17,21H,(H,18,20)/b15-13-,19-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188941
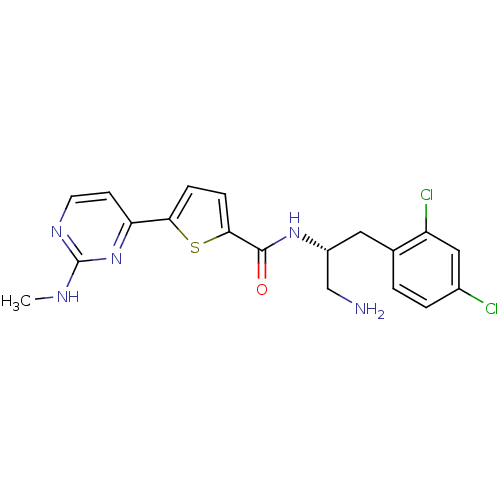
(CHEMBL380165 | N-((R)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188944
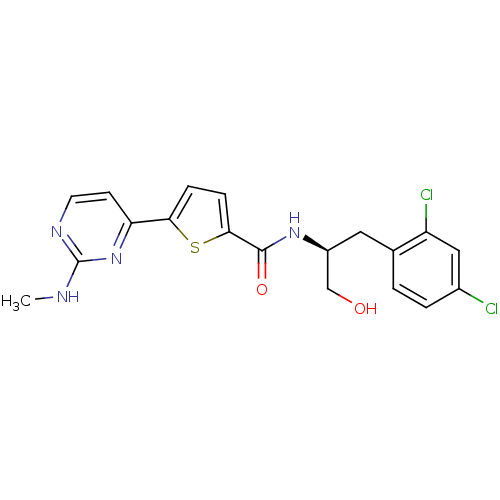
(CHEMBL211578 | N-((S)-3-(2,4-dichlorophenyl)-1-hyd...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CO)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H18Cl2N4O2S/c1-22-19-23-7-6-15(25-19)16-4-5-17(28-16)18(27)24-13(10-26)8-11-2-3-12(20)9-14(11)21/h2-7,9,13,26H,8,10H2,1H3,(H,24,27)(H,22,23,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50188913

(CHEMBL213618 | N-((S)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188947
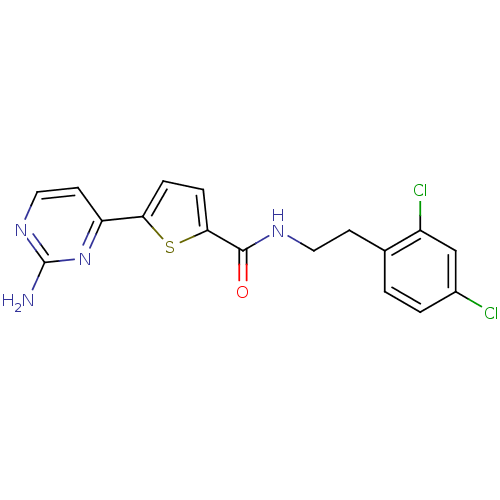
(CHEMBL377568 | N-(2,4-dichlorophenethyl)-5-(2-amin...)Show SMILES Nc1nccc(n1)-c1ccc(s1)C(=O)NCCc1ccc(Cl)cc1Cl Show InChI InChI=1S/C17H14Cl2N4OS/c18-11-2-1-10(12(19)9-11)5-7-21-16(24)15-4-3-14(25-15)13-6-8-22-17(20)23-13/h1-4,6,8-9H,5,7H2,(H,21,24)(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188942
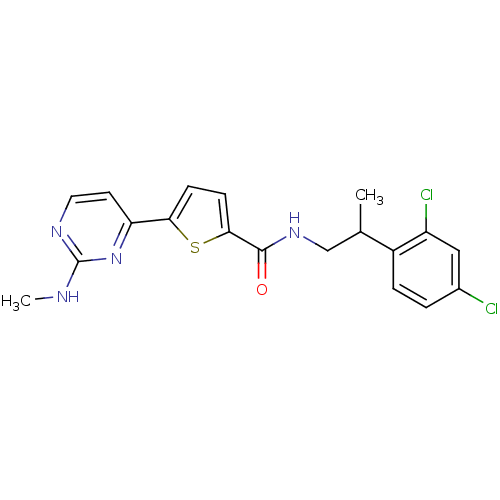
(CHEMBL212450 | N-(2-(2,4-dichlorophenyl)propyl)-5-...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)NCC(C)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H18Cl2N4OS/c1-11(13-4-3-12(20)9-14(13)21)10-24-18(26)17-6-5-16(27-17)15-7-8-23-19(22-2)25-15/h3-9,11H,10H2,1-2H3,(H,24,26)(H,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603985
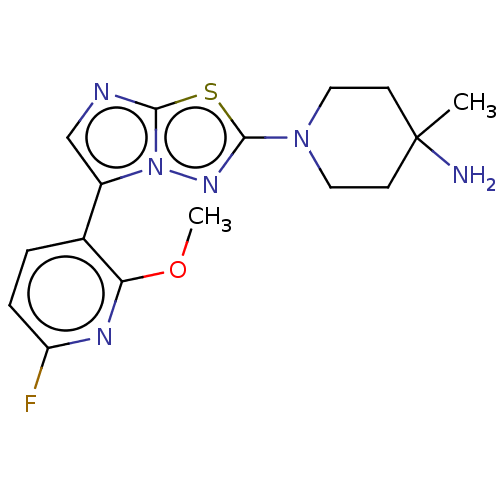
(CHEMBL5207627) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603979
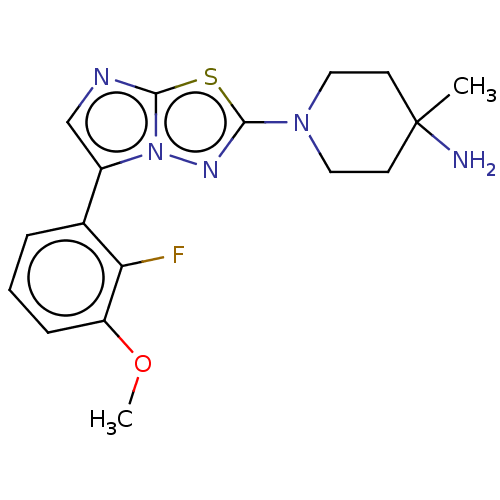
(CHEMBL5204715) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
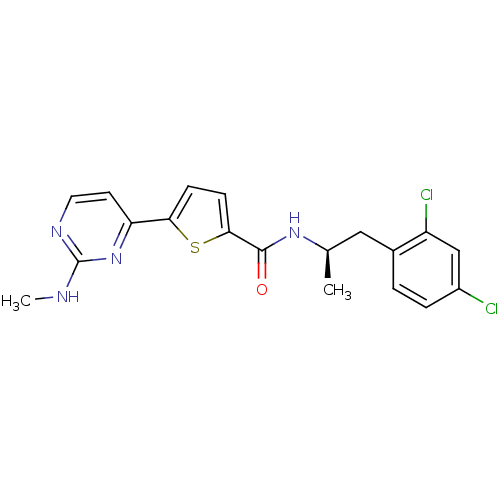
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188946
(CHEMBL379263 | N-((R)-1-(2,4-dichlorophenyl)propan...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](C)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H18Cl2N4OS/c1-11(9-12-3-4-13(20)10-14(12)21)24-18(26)17-6-5-16(27-17)15-7-8-23-19(22-2)25-15/h3-8,10-11H,9H2,1-2H3,(H,24,26)(H,22,23,25)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
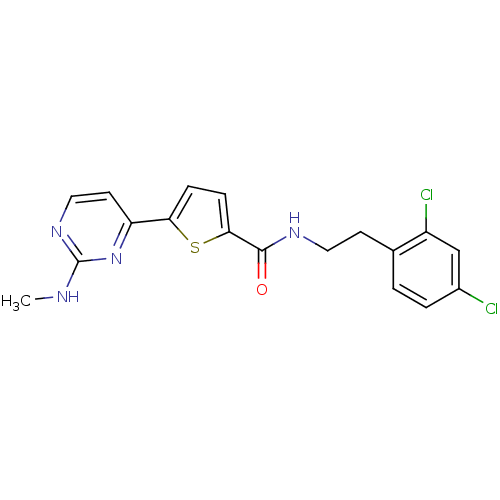
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188912
(CHEMBL209838 | N-(2,4-dichlorophenethyl)-5-(2-(met...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)NCCc1ccc(Cl)cc1Cl Show InChI InChI=1S/C18H16Cl2N4OS/c1-21-18-23-9-7-14(24-18)15-4-5-16(26-15)17(25)22-8-6-11-2-3-12(19)10-13(11)20/h2-5,7,9-10H,6,8H2,1H3,(H,22,25)(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
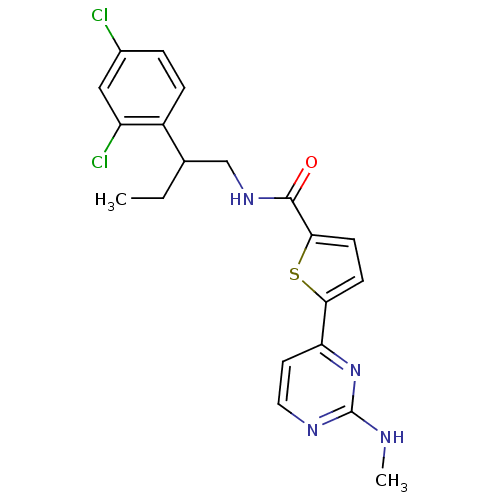
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188918
(CHEMBL211570 | N-(2-(2,4-dichlorophenyl)butyl)-5-(...)Show SMILES CCC(CNC(=O)c1ccc(s1)-c1ccnc(NC)n1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C20H20Cl2N4OS/c1-3-12(14-5-4-13(21)10-15(14)22)11-25-19(27)18-7-6-17(28-18)16-8-9-24-20(23-2)26-16/h4-10,12H,3,11H2,1-2H3,(H,25,27)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50243389

(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human bFGF receptor tyrosine kinase using KDRY1175 [B91616] biotin-GGGGQDGKDYIVLPI-NH2 peptide substrate after 1 hr by scintillation co... |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50188913

(CHEMBL213618 | N-((S)-1-amino-3-(2,4-dichloropheny...)Show SMILES CNc1nccc(n1)-c1ccc(s1)C(=O)N[C@H](CN)Cc1ccc(Cl)cc1Cl Show InChI InChI=1S/C19H19Cl2N5OS/c1-23-19-24-7-6-15(26-19)16-4-5-17(28-16)18(27)25-13(10-22)8-11-2-3-12(20)9-14(11)21/h2-7,9,13H,8,10,22H2,1H3,(H,25,27)(H,23,24,26)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Met |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50188928
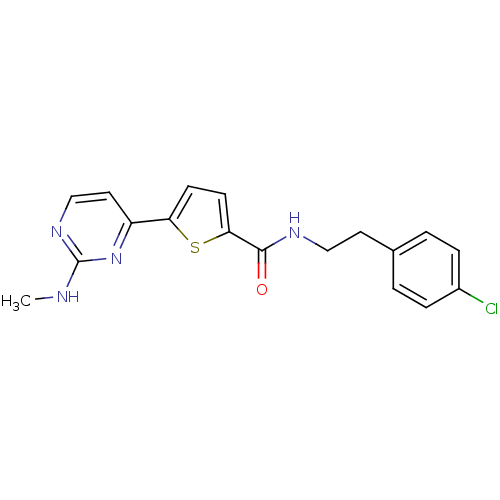
(CHEMBL376004 | N-(4-chlorophenethyl)-5-(2-(methyla...)Show InChI InChI=1S/C18H17ClN4OS/c1-20-18-22-11-9-14(23-18)15-6-7-16(25-15)17(24)21-10-8-12-2-4-13(19)5-3-12/h2-7,9,11H,8,10H2,1H3,(H,21,24)(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 in presence of 0.2 uM ATP |
Bioorg Med Chem Lett 16: 4163-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.092
BindingDB Entry DOI: 10.7270/Q24M945C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603976

(CHEMBL5200247) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data