 Found 1393 hits with Last Name = 'fauber' and Initial = 'bp'
Found 1393 hits with Last Name = 'fauber' and Initial = 'bp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to RORc (unknown origin) by radioligand binding assay |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50005410
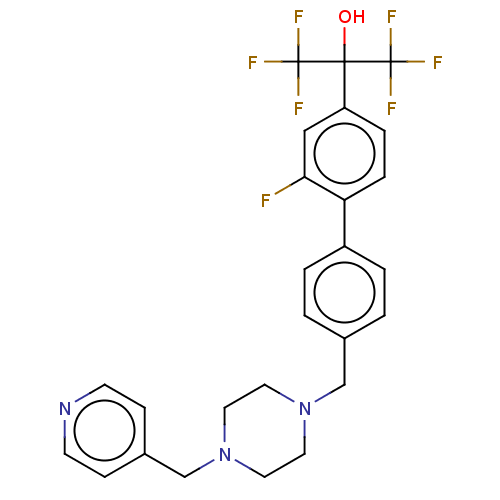
(CHEMBL139197 | CHEMBL2137199)Show SMILES OC(c1ccc(c(F)c1)-c1ccc(CN2CCN(Cc3ccncc3)CC2)cc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H24F7N3O/c27-23-15-21(24(37,25(28,29)30)26(31,32)33)5-6-22(23)20-3-1-18(2-4-20)16-35-11-13-36(14-12-35)17-19-7-9-34-10-8-19/h1-10,15,37H,11-14,16-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to RORc (unknown origin) by radioligand binding assay |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50444338
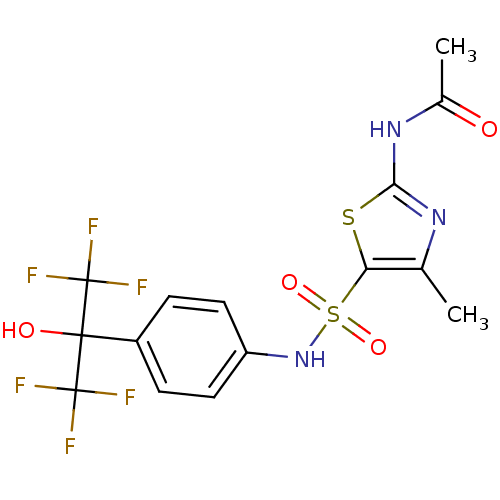
(CHEMBL3094388)Show SMILES CC(=O)Nc1nc(C)c(s1)S(=O)(=O)Nc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C15H13F6N3O4S2/c1-7-11(29-12(22-7)23-8(2)25)30(27,28)24-10-5-3-9(4-6-10)13(26,14(16,17)18)15(19,20)21/h3-6,24,26H,1-2H3,(H,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to RORc-LBD (unknown origin) by radioligand binding assay |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to RORa (unknown origin) by radioligand binding assay |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM50444338

(CHEMBL3094388)Show SMILES CC(=O)Nc1nc(C)c(s1)S(=O)(=O)Nc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C15H13F6N3O4S2/c1-7-11(29-12(22-7)23-8(2)25)30(27,28)24-10-5-3-9(4-6-10)13(26,14(16,17)18)15(19,20)21/h3-6,24,26H,1-2H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to RORa-LBD (unknown origin) by radioligand binding assay |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597908

(CHEMBL5181496)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(NC2CCCCC2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597908

(CHEMBL5181496)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(NC2CCCCC2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50044151

(CHEMBL3314028)Show SMILES Cc1noc(C)c1CN(CC(O)=O)c1ccc(CC(=O)NC(c2ccccc2)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C30H31N3O4/c1-20-9-13-25(14-10-20)30(24-7-5-4-6-8-24)31-28(34)17-23-11-15-26(16-12-23)33(19-29(35)36)18-27-21(2)32-37-22(27)3/h4-16,30H,17-19H2,1-3H3,(H,31,34)(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human RoRc-LBD expressed in cells assessed as SRC1 to 2 coactivator peptide recruitment |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50044152

(CHEMBL3314027)Show SMILES Cc1ccc([C@@H](NC(=O)Cc2ccc3oc(cc3c2)C(N2CC(N)C2)c2ccncc2)c2ccccc2)c(C)c1 |r| Show InChI InChI=1S/C34H34N4O2/c1-22-8-10-29(23(2)16-22)33(25-6-4-3-5-7-25)37-32(39)18-24-9-11-30-27(17-24)19-31(40-30)34(38-20-28(35)21-38)26-12-14-36-15-13-26/h3-17,19,28,33-34H,18,20-21,35H2,1-2H3,(H,37,39)/t33-,34?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human RoRc-LBD expressed in cells assessed as SRC1 to 2 coactivator peptide recruitment |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50044153
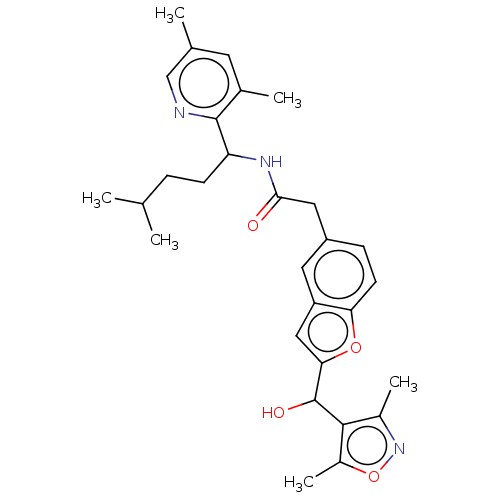
(CHEMBL3314026)Show SMILES CC(C)CCC(NC(=O)Cc1ccc2oc(cc2c1)C(O)c1c(C)noc1C)c1ncc(C)cc1C Show InChI InChI=1S/C29H35N3O4/c1-16(2)7-9-23(28-18(4)11-17(3)15-30-28)31-26(33)13-21-8-10-24-22(12-21)14-25(35-24)29(34)27-19(5)32-36-20(27)6/h8,10-12,14-16,23,29,34H,7,9,13H2,1-6H3,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human RoRc-LBD expressed in cells assessed as SRC1 to 2 coactivator peptide recruitment |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50044156

(CHEMBL3314023)Show SMILES Cc1ccc(C(NC(=O)Cc2ccc(OCC(O)c3cccnc3C)cc2)c2ccccc2)c(C)c1 Show InChI InChI=1S/C31H32N2O3/c1-21-11-16-27(22(2)18-21)31(25-8-5-4-6-9-25)33-30(35)19-24-12-14-26(15-13-24)36-20-29(34)28-10-7-17-32-23(28)3/h4-18,29,31,34H,19-20H2,1-3H3,(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human RoRc-LBD expressed in cells assessed as SRC1 to 2 coactivator peptide recruitment |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50044154
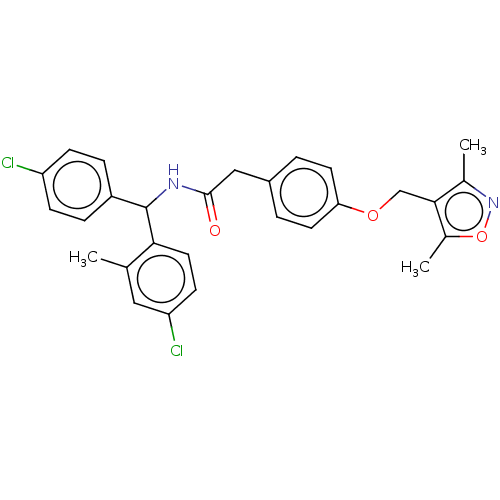
(CHEMBL3314025)Show SMILES Cc1noc(C)c1COc1ccc(CC(=O)NC(c2ccc(Cl)cc2)c2ccc(Cl)cc2C)cc1 Show InChI InChI=1S/C28H26Cl2N2O3/c1-17-14-23(30)10-13-25(17)28(21-6-8-22(29)9-7-21)31-27(33)15-20-4-11-24(12-5-20)34-16-26-18(2)32-35-19(26)3/h4-14,28H,15-16H2,1-3H3,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human RoRc-LBD expressed in cells assessed as SRC1 to 2 coactivator peptide recruitment |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597906
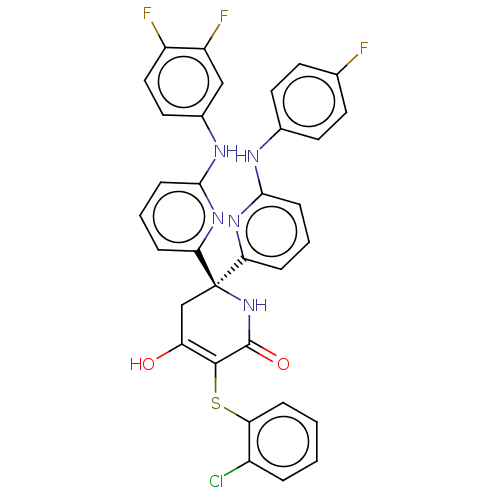
(CHEMBL5183539)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)N[C@](C1)(c1cccc(Nc2ccc(F)cc2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |r,c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597915

(CHEMBL5185237)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Nc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597915

(CHEMBL5185237)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Nc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597916

(CHEMBL5201115)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Oc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597916

(CHEMBL5201115)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Oc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597916

(CHEMBL5201115)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Oc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597896
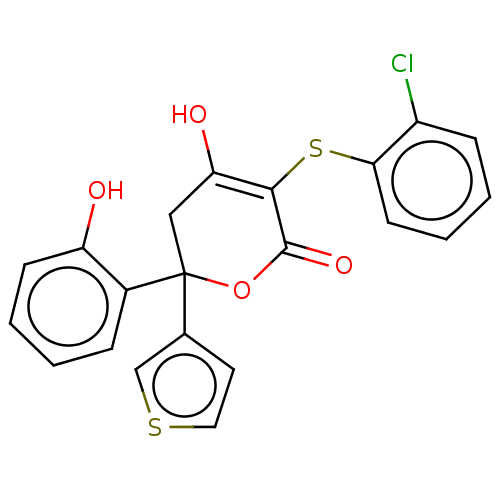
(CHEMBL5181996)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)OC(C1)(c1ccsc1)c1ccccc1O |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597896

(CHEMBL5181996)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)OC(C1)(c1ccsc1)c1ccccc1O |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597916

(CHEMBL5201115)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Oc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50388706
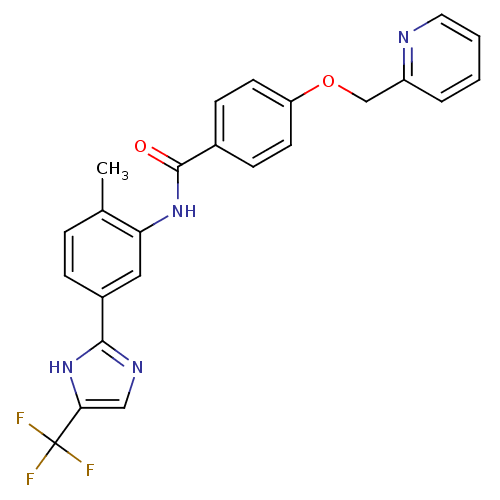
(CHEMBL2059863)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1ncc([nH]1)C(F)(F)F Show InChI InChI=1S/C24H19F3N4O2/c1-15-5-6-17(22-29-13-21(31-22)24(25,26)27)12-20(15)30-23(32)16-7-9-19(10-8-16)33-14-18-4-2-3-11-28-18/h2-13H,14H2,1H3,(H,29,31)(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Smo expressed in mouse NIH/3T3 cells after 20 hrs by Gli reporter gene assay |
Bioorg Med Chem Lett 22: 4907-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.104
BindingDB Entry DOI: 10.7270/Q2W37XCG |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597915

(CHEMBL5185237)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Nc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597913
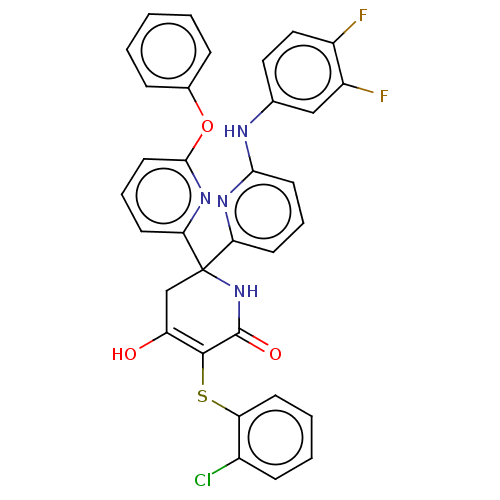
(CHEMBL5194549)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(Nc2ccc(F)c(F)c2)n1)c1cccc(Oc2ccccc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597913

(CHEMBL5194549)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(Nc2ccc(F)c(F)c2)n1)c1cccc(Oc2ccccc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597915

(CHEMBL5185237)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccc(cc1)N1CCOCC1)c1cccc(Nc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50388707

(CHEMBL2059864)Show SMILES Cc1c[nH]c(n1)-c1ccc(C)c(NC(=O)c2ccc(OCc3ccccn3)cc2)c1 Show InChI InChI=1S/C24H22N4O2/c1-16-6-7-19(23-26-14-17(2)27-23)13-22(16)28-24(29)18-8-10-21(11-9-18)30-15-20-5-3-4-12-25-20/h3-14H,15H2,1-2H3,(H,26,27)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Smo expressed in mouse NIH/3T3 cells after 20 hrs by Gli reporter gene assay |
Bioorg Med Chem Lett 22: 4907-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.104
BindingDB Entry DOI: 10.7270/Q2W37XCG |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597909

(CHEMBL5196929)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(NC2CCOCC2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597909

(CHEMBL5196929)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(NC2CCOCC2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597908

(CHEMBL5181496)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(NC2CCCCC2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50597908

(CHEMBL5181496)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(NC2CCCCC2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597904
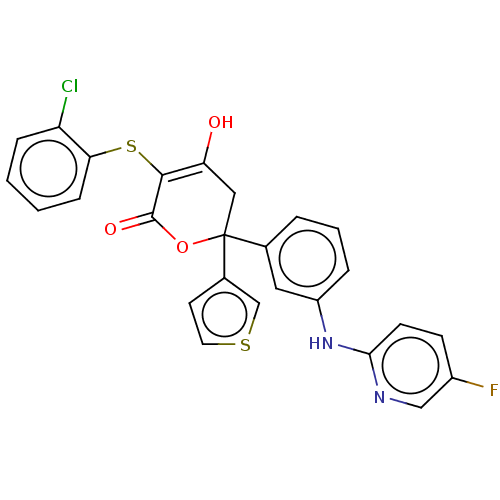
(CHEMBL5176448)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)OC(C1)(c1ccsc1)c1cccc(Nc2ccc(F)cn2)c1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597904

(CHEMBL5176448)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)OC(C1)(c1ccsc1)c1cccc(Nc2ccc(F)cn2)c1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597905
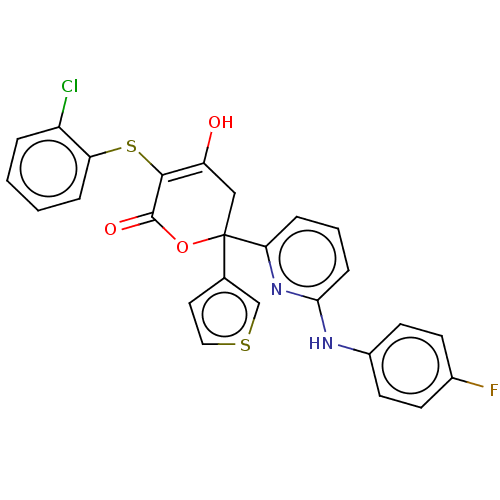
(CHEMBL5182266)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)OC(C1)(c1ccsc1)c1cccc(Nc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597905

(CHEMBL5182266)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)OC(C1)(c1ccsc1)c1cccc(Nc2ccc(F)cc2)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM197160

(GNE-140 (6))Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)N[C@@](C1)(c1ccsc1)c1ccc(cc1)N1CCOCC1 |r,c:1| Show InChI InChI=1S/C25H23ClN2O3S2/c26-20-3-1-2-4-22(20)33-23-21(29)15-25(27-24(23)30,18-9-14-32-16-18)17-5-7-19(8-6-17)28-10-12-31-13-11-28/h1-9,14,16,29H,10-13,15H2,(H,27,30)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
ACS Med Chem Lett 7: 896-901 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00190
BindingDB Entry DOI: 10.7270/Q2V40ZP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50534318
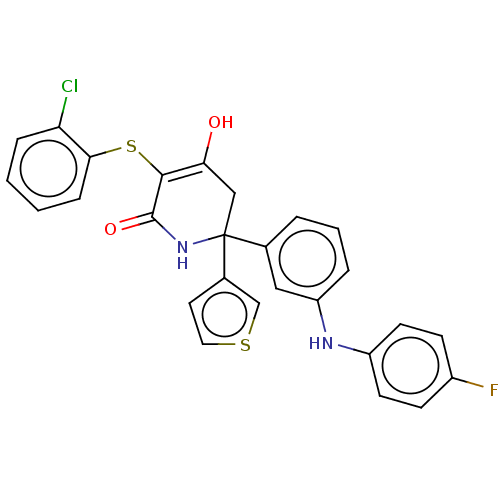
(CHEMBL4576717)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccsc1)c1cccc(Nc2ccc(F)cc2)c1 |c:1| Show InChI InChI=1S/C27H20ClFN2O2S2/c28-22-6-1-2-7-24(22)35-25-23(32)15-27(31-26(25)33,18-12-13-34-16-18)17-4-3-5-21(14-17)30-20-10-8-19(29)9-11-20/h1-14,16,30,32H,15H2,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
ACS Med Chem Lett 7: 896-901 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00190
BindingDB Entry DOI: 10.7270/Q2V40ZP9 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597907
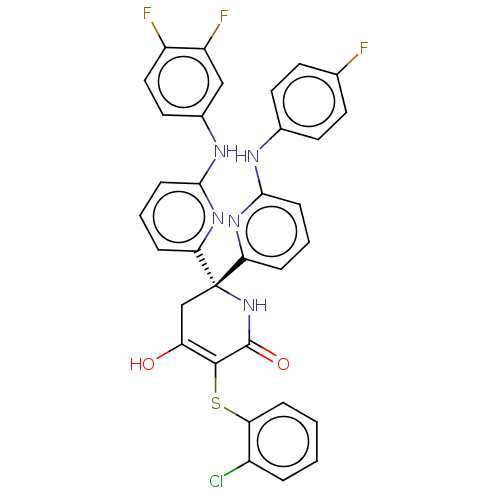
(CHEMBL5189879)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)N[C@@](C1)(c1cccc(Nc2ccc(F)cc2)n1)c1cccc(Nc2ccc(F)c(F)c2)n1 |r,c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597914
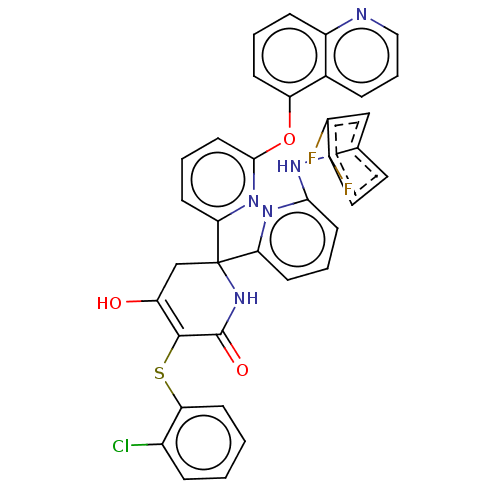
(CHEMBL5188480)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(Nc2ccc(F)c(F)c2)n1)c1cccc(Oc2cccc3ncccc23)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597914

(CHEMBL5188480)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1cccc(Nc2ccc(F)c(F)c2)n1)c1cccc(Oc2cccc3ncccc23)n1 |c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50597902
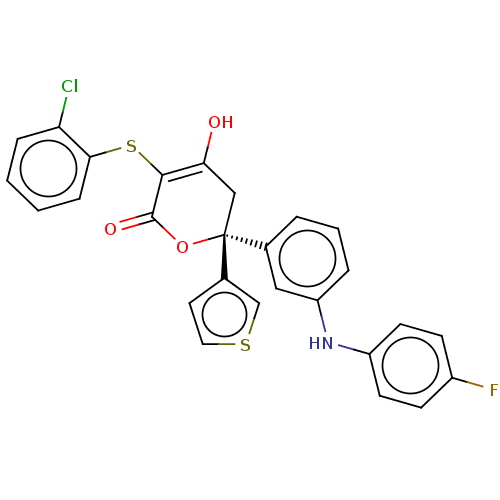
(CHEMBL5177632)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)O[C@@](C1)(c1ccsc1)c1cccc(Nc2ccc(F)cc2)c1 |r,c:1| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128576
BindingDB Entry DOI: 10.7270/Q2251P69 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50534305
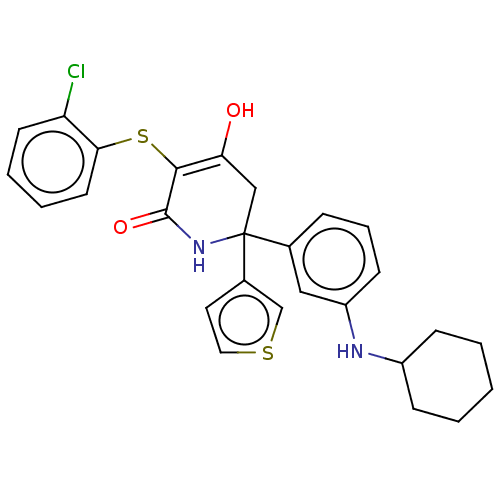
(CHEMBL4435570)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)NC(C1)(c1ccsc1)c1cccc(NC2CCCCC2)c1 |c:1| Show InChI InChI=1S/C27H27ClN2O2S2/c28-22-11-4-5-12-24(22)34-25-23(31)16-27(30-26(25)32,19-13-14-33-17-19)18-7-6-10-21(15-18)29-20-8-2-1-3-9-20/h4-7,10-15,17,20,29,31H,1-3,8-9,16H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
ACS Med Chem Lett 7: 896-901 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00190
BindingDB Entry DOI: 10.7270/Q2V40ZP9 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50388705
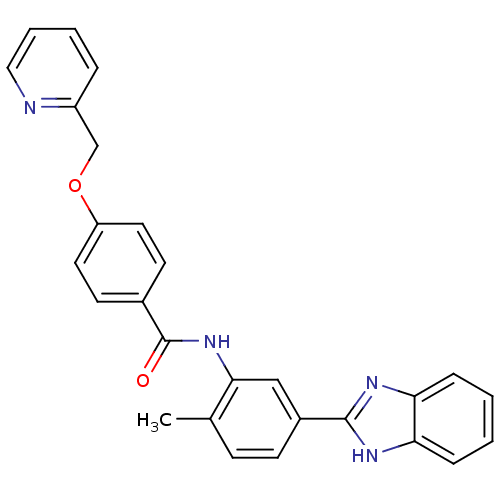
(CHEMBL2059859)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C27H22N4O2/c1-18-9-10-20(26-29-23-7-2-3-8-24(23)30-26)16-25(18)31-27(32)19-11-13-22(14-12-19)33-17-21-6-4-5-15-28-21/h2-16H,17H2,1H3,(H,29,30)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Smo expressed in mouse NIH/3T3 cells after 20 hrs by Gli reporter gene assay |
Bioorg Med Chem Lett 22: 4907-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.104
BindingDB Entry DOI: 10.7270/Q2W37XCG |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50534309
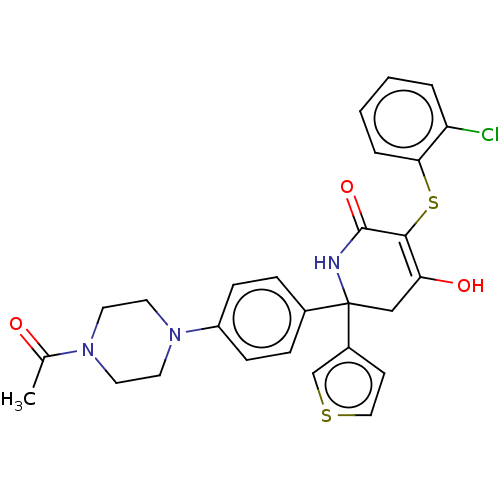
(CHEMBL4467769)Show SMILES CC(=O)N1CCN(CC1)c1ccc(cc1)C1(CC(O)=C(Sc2ccccc2Cl)C(=O)N1)c1ccsc1 |t:20| Show InChI InChI=1S/C27H26ClN3O3S2/c1-18(32)30-11-13-31(14-12-30)21-8-6-19(7-9-21)27(20-10-15-35-17-20)16-23(33)25(26(34)29-27)36-24-5-3-2-4-22(24)28/h2-10,15,17,33H,11-14,16H2,1H3,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
ACS Med Chem Lett 7: 896-901 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00190
BindingDB Entry DOI: 10.7270/Q2V40ZP9 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50388708
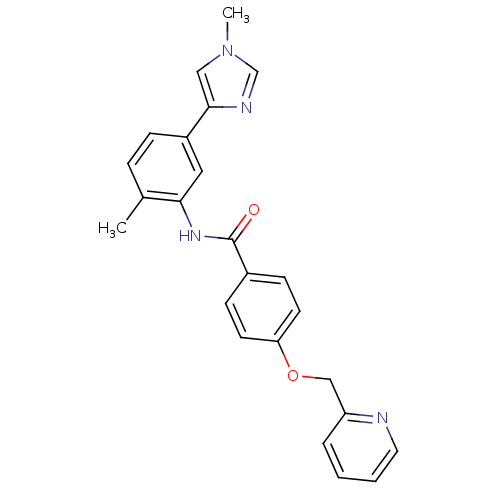
(CHEMBL2059866)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1cn(C)cn1 Show InChI InChI=1S/C24H22N4O2/c1-17-6-7-19(23-14-28(2)16-26-23)13-22(17)27-24(29)18-8-10-21(11-9-18)30-15-20-5-3-4-12-25-20/h3-14,16H,15H2,1-2H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Smo expressed in mouse NIH/3T3 cells after 20 hrs by Gli reporter gene assay |
Bioorg Med Chem Lett 22: 4907-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.104
BindingDB Entry DOI: 10.7270/Q2W37XCG |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM197160

(GNE-140 (6))Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)N[C@@](C1)(c1ccsc1)c1ccc(cc1)N1CCOCC1 |r,c:1| Show InChI InChI=1S/C25H23ClN2O3S2/c26-20-3-1-2-4-22(20)33-23-21(29)15-25(27-24(23)30,18-9-14-32-16-18)17-5-7-19(8-6-17)28-10-12-31-13-11-28/h1-9,14,16,29H,10-13,15H2,(H,27,30)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHB by UV endpoint assay |
ACS Med Chem Lett 7: 896-901 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00190
BindingDB Entry DOI: 10.7270/Q2V40ZP9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50035909
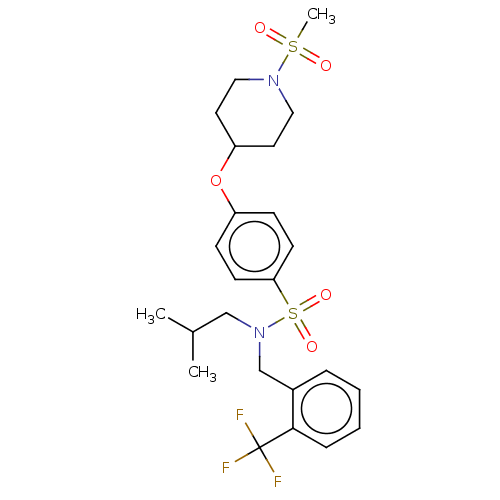
(CHEMBL3361052)Show SMILES CC(C)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(OC2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C24H31F3N2O5S2/c1-18(2)16-29(17-19-6-4-5-7-23(19)24(25,26)27)36(32,33)22-10-8-20(9-11-22)34-21-12-14-28(15-13-21)35(3,30)31/h4-11,18,21H,12-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5769-76 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.037
BindingDB Entry DOI: 10.7270/Q2125V8F |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50090104
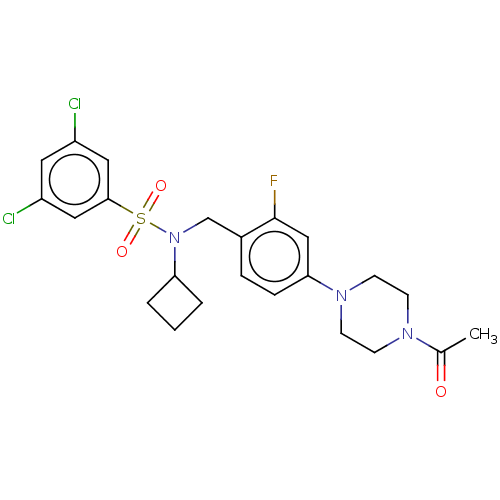
(CHEMBL3581543 | US9216988, 97)Show SMILES CC(=O)N1CCN(CC1)c1ccc(CN(C2CCC2)S(=O)(=O)c2cc(Cl)cc(Cl)c2)c(F)c1 Show InChI InChI=1S/C20H21ClFNO2/c21-17-5-3-16(4-6-17)20(25)10-13-23(14-11-20)12-9-19(24)15-1-7-18(22)8-2-15/h1-8,25H,9-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-25-hydroxycholesterol from N-terminal 6xHis-tagged human RORc ligand binding domain (241 to 486) expressed in bacterial express... |
ACS Med Chem Lett 6: 276-81 (2015)
Article DOI: 10.1021/ml500420y
BindingDB Entry DOI: 10.7270/Q2TT4SPB |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50044247

(CHEMBL3314036 | US9216988, 159)Show SMILES CC(C)CN(Cc1ccc(OC2CCN(CC2)S(C)(=O)=O)cc1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C24H34N2O5S2/c1-20(2)17-26(33(29,30)19-22-7-5-4-6-8-22)18-21-9-11-23(12-10-21)31-24-13-15-25(16-14-24)32(3,27)28/h4-12,20,24H,13-19H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H2]25-hydroxycholesterol from RoRc-LBD (unknown origin) |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50089931
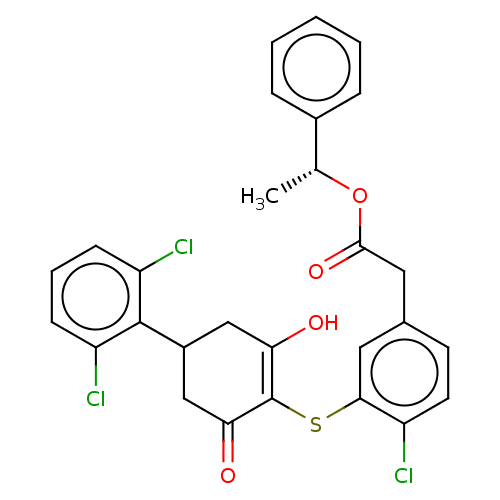
(CHEMBL3581197)Show SMILES C[C@@H](OC(=O)Cc1ccc(Cl)c(SC2=C(O)CC(CC2=O)c2c(Cl)cccc2Cl)c1)c1ccccc1 |r,c:13| Show InChI InChI=1S/C28H23Cl3O4S/c1-16(18-6-3-2-4-7-18)35-26(34)13-17-10-11-20(29)25(12-17)36-28-23(32)14-19(15-24(28)33)27-21(30)8-5-9-22(27)31/h2-12,16,19,32H,13-15H2,1H3/t16-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human LDH-A by biochemical assay |
Bioorg Med Chem Lett 25: 75-82 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.008
BindingDB Entry DOI: 10.7270/Q2BR8TXC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data