 Found 105 hits with Last Name = 'fauchère' and Initial = 'jl'
Found 105 hits with Last Name = 'fauchère' and Initial = 'jl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50452012
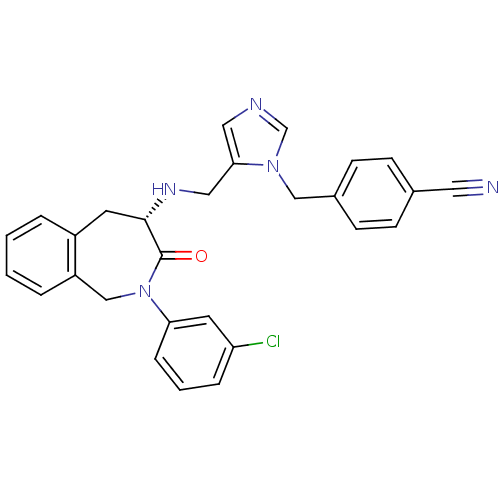
(CHEMBL2110187)Show SMILES Clc1cccc(c1)N1Cc2ccccc2C[C@H](NCc2cncn2Cc2ccc(cc2)C#N)C1=O |r| Show InChI InChI=1S/C28H24ClN5O/c29-24-6-3-7-25(13-24)34-18-23-5-2-1-4-22(23)12-27(28(34)35)32-16-26-15-31-19-33(26)17-21-10-8-20(14-30)9-11-21/h1-11,13,15,19,27,32H,12,16-18H2/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50092365
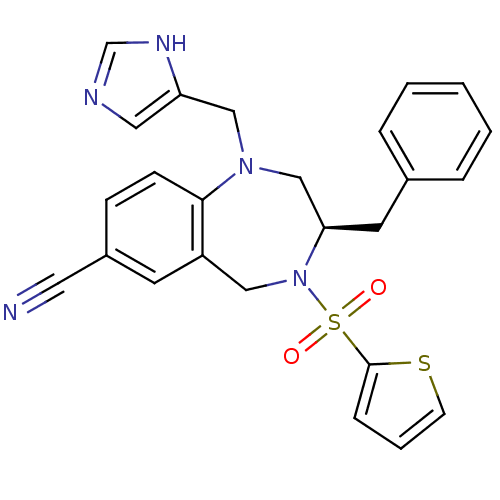
((R)-1-((1H-imidazol-5-yl)methyl)-3-benzyl-4-(thiop...)Show SMILES O=S(=O)(N1Cc2cc(ccc2N(Cc2cnc[nH]2)C[C@H]1Cc1ccccc1)C#N)c1cccs1 Show InChI InChI=1S/C25H23N5O2S2/c26-13-20-8-9-24-21(11-20)15-30(34(31,32)25-7-4-10-33-25)23(12-19-5-2-1-3-6-19)17-29(24)16-22-14-27-18-28-22/h1-11,14,18,23H,12,15-17H2,(H,27,28)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139576
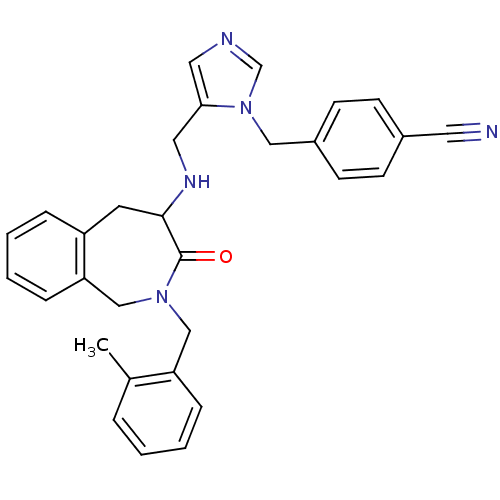
(4-(5-{[2-(2-Methyl-benzyl)-3-oxo-2,3,4,5-tetrahydr...)Show SMILES Cc1ccccc1CN1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C30H29N5O/c1-22-6-2-3-8-26(22)19-34-20-27-9-5-4-7-25(27)14-29(30(34)36)33-17-28-16-32-21-35(28)18-24-12-10-23(15-31)11-13-24/h2-13,16,21,29,33H,14,17-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50404819
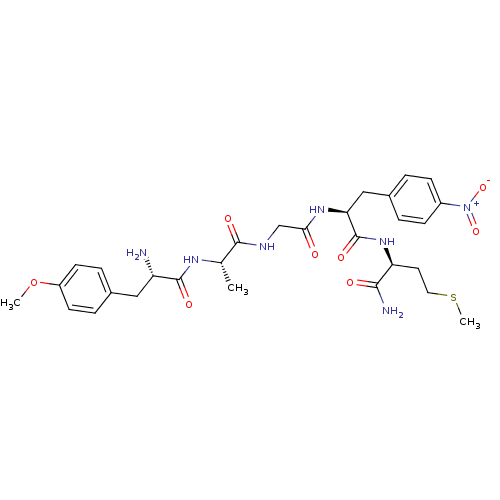
(CHEMBL33000)Show SMILES COc1ccc(C[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](Cc2ccc(cc2)[N+]([O-])=O)C(=O)N[C@@H](CCSC)C(N)=O)cc1 Show InChI InChI=1S/C29H39N7O8S/c1-17(33-28(40)22(30)14-18-6-10-21(44-2)11-7-18)27(39)32-16-25(37)34-24(15-19-4-8-20(9-5-19)36(42)43)29(41)35-23(26(31)38)12-13-45-3/h4-11,17,22-24H,12-16,30H2,1-3H3,(H2,31,38)(H,32,39)(H,33,40)(H,34,37)(H,35,41)/t17-,22-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity contractions of electrically stimulated mouse vas deferens was determined |
J Med Chem 25: 1428-31 (1983)
BindingDB Entry DOI: 10.7270/Q2N87C0G |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50404816
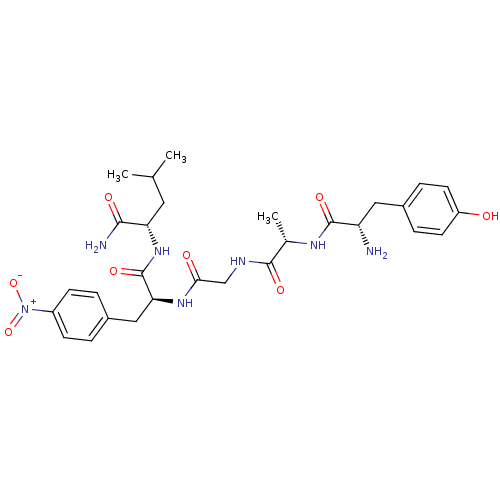
(CHEMBL434006)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C29H39N7O8/c1-16(2)12-23(26(31)39)35-29(42)24(14-19-4-8-20(9-5-19)36(43)44)34-25(38)15-32-27(40)17(3)33-28(41)22(30)13-18-6-10-21(37)11-7-18/h4-11,16-17,22-24,37H,12-15,30H2,1-3H3,(H2,31,39)(H,32,40)(H,33,41)(H,34,38)(H,35,42)/t17-,22-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity contractions of electrically stimulated mouse vas deferens was determined |
J Med Chem 25: 1428-31 (1983)
BindingDB Entry DOI: 10.7270/Q2N87C0G |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139567
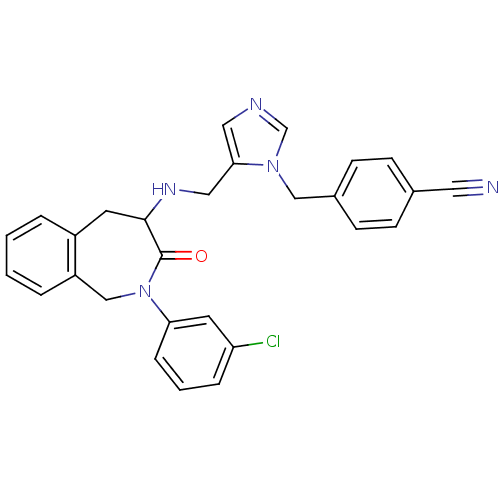
(4-(5-{[2-(3-Chloro-phenyl)-3-oxo-2,3,4,5-tetrahydr...)Show SMILES Clc1cccc(c1)N1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H24ClN5O/c29-24-6-3-7-25(13-24)34-18-23-5-2-1-4-22(23)12-27(28(34)35)32-16-26-15-31-19-33(26)17-21-10-8-20(14-30)9-11-21/h1-11,13,15,19,27,32H,12,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045033
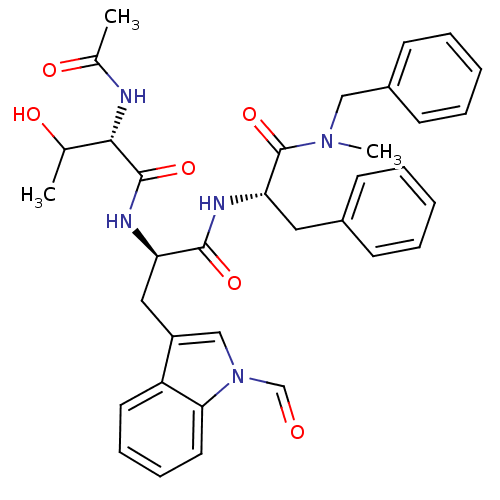
(1N-[1-[1-benzyl(methyl)carbamoyl-2-phenyl-(1S)-eth...)Show SMILES CC(O)[C@H](NC(C)=O)C(=O)N[C@H](Cc1cn(C=O)c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(C)Cc1ccccc1 Show InChI InChI=1S/C35H39N5O6/c1-23(42)32(36-24(2)43)34(45)37-29(19-27-21-40(22-41)31-17-11-10-16-28(27)31)33(44)38-30(18-25-12-6-4-7-13-25)35(46)39(3)20-26-14-8-5-9-15-26/h4-17,21-23,29-30,32,42H,18-20H2,1-3H3,(H,36,43)(H,37,45)(H,38,44)/t23?,29-,30+,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-1 receptor transfected on CHO cells using [125I]-Tyr] SP as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139588

(4-(5-{[2-(3-Methoxy-phenyl)-3-oxo-2,3,4,5-tetrahyd...)Show SMILES COc1cccc(c1)N1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C29H27N5O2/c1-36-27-8-4-7-25(14-27)34-19-24-6-3-2-5-23(24)13-28(29(34)35)32-17-26-16-31-20-33(26)18-22-11-9-21(15-30)10-12-22/h2-12,14,16,20,28,32H,13,17-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139580
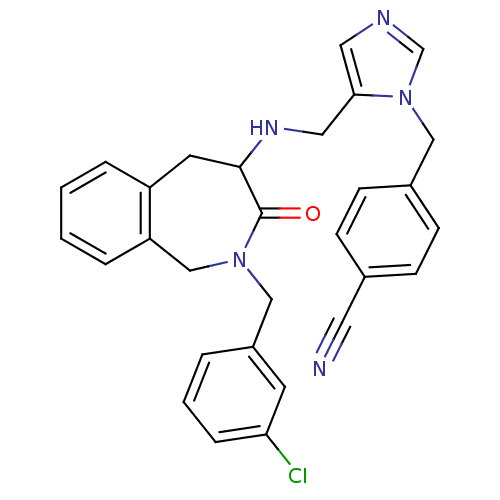
(4-(5-{[2-(3-Chloro-benzyl)-3-oxo-2,3,4,5-tetrahydr...)Show SMILES Clc1cccc(CN2Cc3ccccc3CC(NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 Show InChI InChI=1S/C29H26ClN5O/c30-26-7-3-4-23(12-26)18-34-19-25-6-2-1-5-24(25)13-28(29(34)36)33-16-27-15-32-20-35(27)17-22-10-8-21(14-31)9-11-22/h1-12,15,20,28,33H,13,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139568
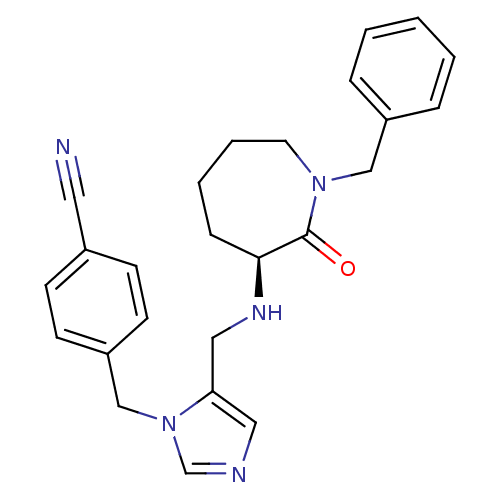
(4-{5-[((S)-1-Benzyl-2-oxo-azepan-3-ylamino)-methyl...)Show SMILES O=C1[C@H](CCCCN1Cc1ccccc1)NCc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C25H27N5O/c26-14-20-9-11-22(12-10-20)18-30-19-27-15-23(30)16-28-24-8-4-5-13-29(25(24)31)17-21-6-2-1-3-7-21/h1-3,6-7,9-12,15,19,24,28H,4-5,8,13,16-18H2/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139570

(4-(5-{[2-(3,5-Difluoro-benzyl)-3-oxo-2,3,4,5-tetra...)Show SMILES Fc1cc(F)cc(CN2Cc3ccccc3CC(NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 Show InChI InChI=1S/C29H25F2N5O/c30-25-9-22(10-26(31)12-25)17-35-18-24-4-2-1-3-23(24)11-28(29(35)37)34-15-27-14-33-19-36(27)16-21-7-5-20(13-32)6-8-21/h1-10,12,14,19,28,34H,11,15-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139591

(4-(5-{[2-(3-Fluoro-phenyl)-3-oxo-2,3,4,5-tetrahydr...)Show SMILES Fc1cccc(c1)N1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H24FN5O/c29-24-6-3-7-25(13-24)34-18-23-5-2-1-4-22(23)12-27(28(34)35)32-16-26-15-31-19-33(26)17-21-10-8-20(14-30)9-11-21/h1-11,13,15,19,27,32H,12,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50452137
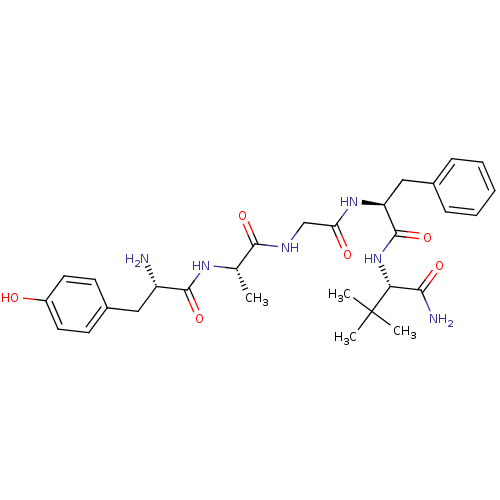
(CHEMBL2372195)Show SMILES C[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C(N)=O)C(C)(C)C Show InChI InChI=1S/C29H40N6O6/c1-17(33-27(40)21(30)14-19-10-12-20(36)13-11-19)26(39)32-16-23(37)34-22(15-18-8-6-5-7-9-18)28(41)35-24(25(31)38)29(2,3)4/h5-13,17,21-22,24,36H,14-16,30H2,1-4H3,(H2,31,38)(H,32,39)(H,33,40)(H,34,37)(H,35,41)/t17-,21-,22-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity contractions of electrically stimulated mouse vas deferens was determined |
J Med Chem 25: 1428-31 (1983)
BindingDB Entry DOI: 10.7270/Q2N87C0G |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139585

(4-(5-{[2-(3,5-Difluoro-phenyl)-3-oxo-2,3,4,5-tetra...)Show SMILES Fc1cc(F)cc(c1)N1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H23F2N5O/c29-23-10-24(30)12-25(11-23)35-17-22-4-2-1-3-21(22)9-27(28(35)36)33-15-26-14-32-18-34(26)16-20-7-5-19(13-31)6-8-20/h1-8,10-12,14,18,27,33H,9,15-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139592

(4-(5-{[2-(2-Chloro-benzyl)-3-oxo-2,3,4,5-tetrahydr...)Show SMILES Clc1ccccc1CN1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C29H26ClN5O/c30-27-8-4-3-7-25(27)19-34-18-24-6-2-1-5-23(24)13-28(29(34)36)33-16-26-15-32-20-35(26)17-22-11-9-21(14-31)10-12-22/h1-12,15,20,28,33H,13,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139579
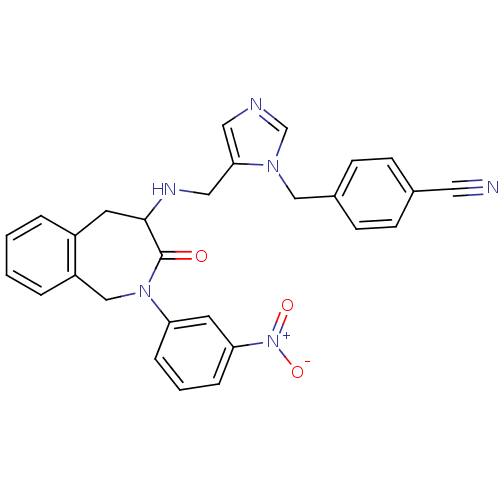
(4-(5-{[2-(3-Nitro-phenyl)-3-oxo-2,3,4,5-tetrahydro...)Show SMILES [O-][N+](=O)c1cccc(c1)N1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H24N6O3/c29-14-20-8-10-21(11-9-20)17-32-19-30-15-26(32)16-31-27-12-22-4-1-2-5-23(22)18-33(28(27)35)24-6-3-7-25(13-24)34(36)37/h1-11,13,15,19,27,31H,12,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139582

(4-(5-{[2-(3,5-Dichloro-phenyl)-3-oxo-2,3,4,5-tetra...)Show SMILES Clc1cc(Cl)cc(c1)N1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H23Cl2N5O/c29-23-10-24(30)12-25(11-23)35-17-22-4-2-1-3-21(22)9-27(28(35)36)33-15-26-14-32-18-34(26)16-20-7-5-19(13-31)6-8-20/h1-8,10-12,14,18,27,33H,9,15-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139583

(4-(5-{[2-(3-Bromo-phenyl)-3-oxo-2,3,4,5-tetrahydro...)Show SMILES Brc1cccc(c1)N1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H24BrN5O/c29-24-6-3-7-25(13-24)34-18-23-5-2-1-4-22(23)12-27(28(34)35)32-16-26-15-31-19-33(26)17-21-10-8-20(14-30)9-11-21/h1-11,13,15,19,27,32H,12,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139575
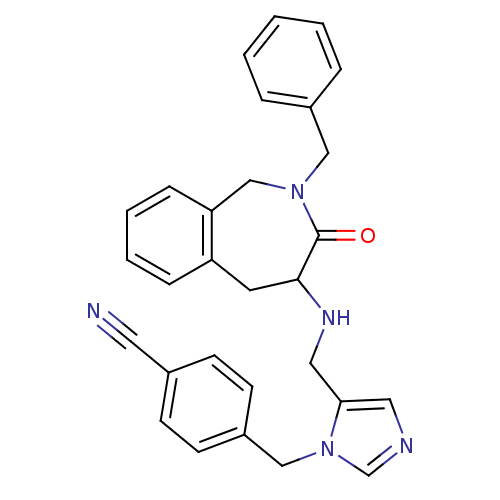
(4-{5-[(2-Benzyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[...)Show SMILES O=C1C(Cc2ccccc2CN1Cc1ccccc1)NCc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C29H27N5O/c30-15-22-10-12-24(13-11-22)19-34-21-31-16-27(34)17-32-28-14-25-8-4-5-9-26(25)20-33(29(28)35)18-23-6-2-1-3-7-23/h1-13,16,21,28,32H,14,17-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045040
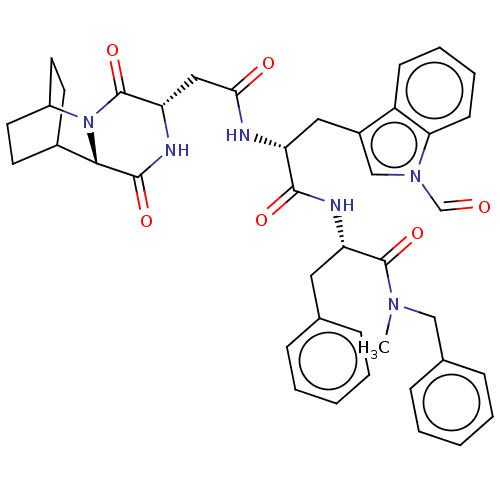
(CHEMBL3144343 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C=O)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:43.46,11.11,39.42,22.24,THB:41:43:46.45:48.49,(10.33,-13,;9.17,-14.02,;9.47,-15.53,;10.93,-16.02,;11.23,-17.53,;12.69,-18.03,;13.85,-17.01,;13.55,-15.5,;12.09,-15.01,;7.71,-13.52,;6.56,-14.54,;7.41,-12.01,;8.57,-11,;8.26,-9.49,;9.42,-8.47,;9.12,-6.96,;7.66,-6.47,;6.5,-7.48,;6.81,-8.99,;5.95,-11.52,;5.65,-10.01,;5.99,-8.51,;4.19,-9.52,;3.03,-10.53,;3.34,-12.04,;4.73,-12.69,;4.56,-14.22,;5.69,-15.26,;5.35,-16.76,;3.05,-14.52,;2.26,-15.84,;.72,-15.82,;-.03,-14.48,;.75,-13.16,;2.29,-13.18,;3.89,-8.01,;2.43,-7.51,;1.27,-8.53,;2.13,-6,;.67,-5.51,;.37,-4,;-1.09,-3.51,;-1.39,-2,;-2.25,-4.52,;-3.61,-3.99,;-5.11,-4.65,;-4.91,-6.06,;-3.34,-5.39,;-3.08,-3.45,;-3.54,-2.32,;-1.95,-6.03,;-.49,-6.53,;-.19,-8.04,)| Show InChI InChI=1S/C41H44N6O6/c1-45(23-27-12-6-3-7-13-27)40(52)33(20-26-10-4-2-5-11-26)43-38(50)32(21-29-24-46(25-48)35-15-9-8-14-31(29)35)42-36(49)22-34-41(53)47-30-18-16-28(17-19-30)37(47)39(51)44-34/h2-15,24-25,28,30,32-34,37H,16-23H2,1H3,(H,42,49)(H,43,50)(H,44,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor transfected on CHO cells using [125I]-His] NKA as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139590
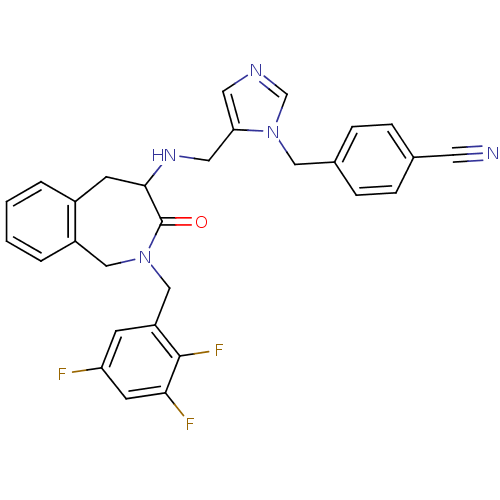
(4-(5-{[3-Oxo-2-(2,3,5-trifluoro-benzyl)-2,3,4,5-te...)Show SMILES Fc1cc(F)c(F)c(CN2Cc3ccccc3CC(NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 Show InChI InChI=1S/C29H24F3N5O/c30-24-9-23(28(32)26(31)11-24)17-36-16-22-4-2-1-3-21(22)10-27(29(36)38)35-14-25-13-34-18-37(25)15-20-7-5-19(12-33)6-8-20/h1-9,11,13,18,27,35H,10,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139573
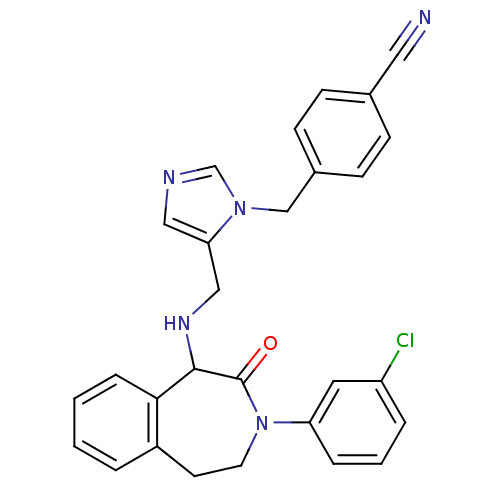
(4-(5-{[3-(3-Chloro-phenyl)-2-oxo-2,3,4,5-tetrahydr...)Show SMILES Clc1cccc(c1)N1CCc2ccccc2C(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H24ClN5O/c29-23-5-3-6-24(14-23)34-13-12-22-4-1-2-7-26(22)27(28(34)35)32-17-25-16-31-19-33(25)18-21-10-8-20(15-30)9-11-21/h1-11,14,16,19,27,32H,12-13,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50452011

(CHEMBL2110188)Show SMILES O=C1[C@H](Cc2ccccc2CN1c1ccccc1)NCc1cncn1Cc1ccc(cc1)C#N |r| Show InChI InChI=1S/C28H25N5O/c29-15-21-10-12-22(13-11-21)18-32-20-30-16-26(32)17-31-27-14-23-6-4-5-7-24(23)19-33(28(27)34)25-8-2-1-3-9-25/h1-13,16,20,27,31H,14,17-19H2/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50404817
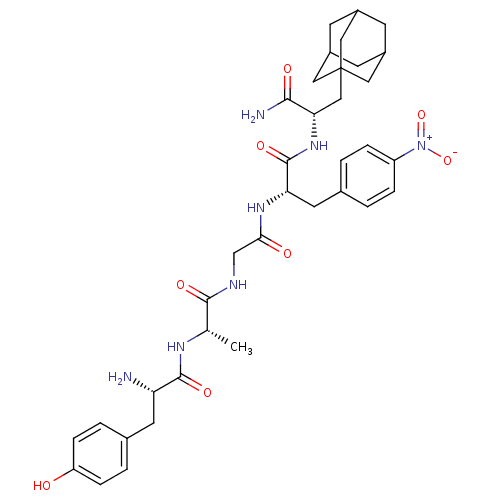
(CHEMBL431895)Show SMILES C[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](CC12CC3CC(CC(C3)C1)C2)C(N)=O |TLB:45:44:47:40.39.41,45:40:47:44.46.43,THB:43:42:39:44.46.45,43:44:39:42.47.41| Show InChI InChI=1S/C36H47N7O8/c1-20(40-34(48)28(37)13-21-4-8-27(44)9-5-21)33(47)39-19-31(45)41-29(14-22-2-6-26(7-3-22)43(50)51)35(49)42-30(32(38)46)18-36-15-23-10-24(16-36)12-25(11-23)17-36/h2-9,20,23-25,28-30,44H,10-19,37H2,1H3,(H2,38,46)(H,39,47)(H,40,48)(H,41,45)(H,42,49)/t20-,23?,24?,25?,28-,29-,30-,36?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity contractions of electrically stimulated mouse vas deferens was determined |
J Med Chem 25: 1428-31 (1983)
BindingDB Entry DOI: 10.7270/Q2N87C0G |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045036
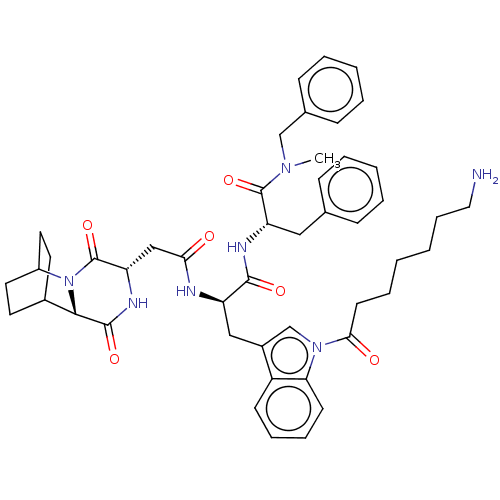
(7-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)CCCCCCN)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:50.53,46.49,22.24,wD:11.11,THB:48:50:53.52:55.56,(11.12,-10.02,;9.98,-8.97,;11.14,-7.96,;12.6,-8.45,;13.76,-7.43,;15.21,-7.93,;15.52,-9.44,;14.36,-10.45,;12.9,-9.96,;8.52,-8.48,;8.22,-6.97,;7.37,-9.5,;7.67,-11.01,;9.13,-11.5,;10.29,-10.48,;11.74,-10.98,;12.05,-12.49,;10.89,-13.5,;9.43,-13.01,;5.91,-9,;4.75,-10.02,;5.05,-11.53,;3.29,-9.52,;2.14,-10.54,;2.44,-12.05,;3.84,-12.69,;3.66,-14.22,;4.79,-15.27,;6.26,-14.81,;4.45,-16.77,;5.58,-17.81,;5.25,-19.32,;6.38,-20.36,;6.04,-21.86,;7.17,-22.91,;6.83,-24.41,;2.15,-14.53,;1.36,-15.85,;-.18,-15.83,;-.93,-14.49,;-.15,-13.16,;1.39,-13.18,;2.99,-8.01,;4.15,-7,;5.61,-7.49,;3.85,-5.49,;2.39,-4.99,;2.09,-3.48,;.63,-2.99,;.32,-1.48,;-.53,-4.01,;-1.89,-3.47,;-3.39,-4.13,;-3.19,-5.54,;-1.63,-4.87,;-1.36,-2.93,;-1.82,-1.8,;-.23,-5.52,;1.23,-6.01,;1.53,-7.52,)| Show InChI InChI=1S/C47H57N7O6/c1-52(29-32-16-8-5-9-17-32)46(59)38(26-31-14-6-4-7-15-31)50-44(57)37(27-34-30-53(40-19-12-11-18-36(34)40)42(56)20-10-2-3-13-25-48)49-41(55)28-39-47(60)54-35-23-21-33(22-24-35)43(54)45(58)51-39/h4-9,11-12,14-19,30,33,35,37-39,43H,2-3,10,13,20-29,48H2,1H3,(H,49,55)(H,50,57)(H,51,58)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-1 receptor |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139569

(4-(5-{[3-(3-Bromo-benzyl)-2-oxo-2,3,4,5-tetrahydro...)Show SMILES Brc1cccc(CN2CCc3ccccc3C(NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 Show InChI InChI=1S/C29H26BrN5O/c30-25-6-3-4-23(14-25)19-34-13-12-24-5-1-2-7-27(24)28(29(34)36)33-17-26-16-32-20-35(26)18-22-10-8-21(15-31)9-11-22/h1-11,14,16,20,28,33H,12-13,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
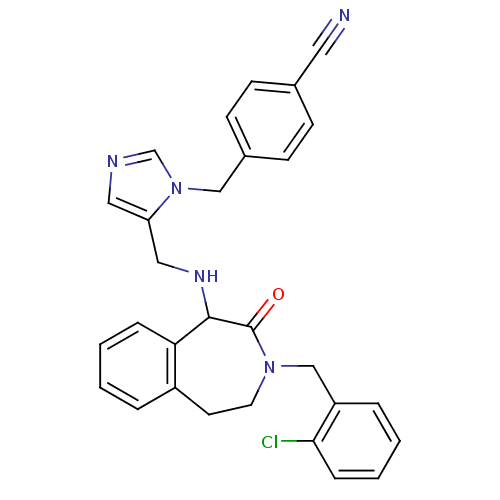
(Homo sapiens (Human)) | BDBM50139578
(4-(5-{[3-(2-Chloro-benzyl)-2-oxo-2,3,4,5-tetrahydr...)Show SMILES Clc1ccccc1CN1CCc2ccccc2C(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C29H26ClN5O/c30-27-8-4-2-6-24(27)19-34-14-13-23-5-1-3-7-26(23)28(29(34)36)33-17-25-16-32-20-35(25)18-22-11-9-21(15-31)10-12-22/h1-12,16,20,28,33H,13-14,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139577

(4-(5-{[3-(3-Chloro-benzyl)-2-oxo-2,3,4,5-tetrahydr...)Show SMILES Clc1cccc(CN2CCc3ccccc3C(NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 Show InChI InChI=1S/C29H26ClN5O/c30-25-6-3-4-23(14-25)19-34-13-12-24-5-1-2-7-27(24)28(29(34)36)33-17-26-16-32-20-35(26)18-22-10-8-21(15-31)9-11-22/h1-11,14,16,20,28,33H,12-13,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
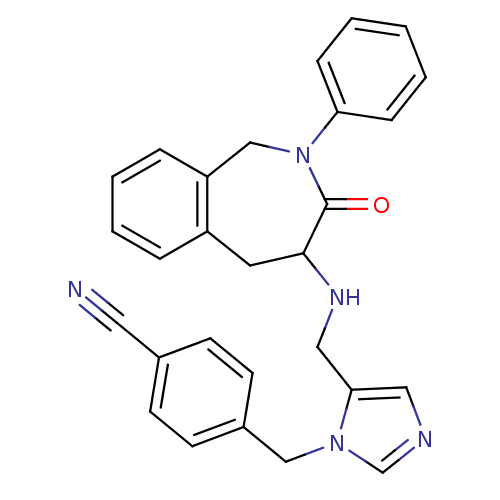
(Homo sapiens (Human)) | BDBM50139574
(4-{5-[(3-Oxo-2-phenyl-2,3,4,5-tetrahydro-1H-benzo[...)Show SMILES O=C1C(Cc2ccccc2CN1c1ccccc1)NCc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C28H25N5O/c29-15-21-10-12-22(13-11-21)18-32-20-30-16-26(32)17-31-27-14-23-6-4-5-7-24(23)19-33(28(27)34)25-8-2-1-3-9-25/h1-13,16,20,27,31H,14,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139586

(4-(5-{[3-(3,5-Difluoro-benzyl)-2-oxo-2,3,4,5-tetra...)Show SMILES Fc1cc(F)cc(CN2CCc3ccccc3C(NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 Show InChI InChI=1S/C29H25F2N5O/c30-24-11-22(12-25(31)13-24)18-35-10-9-23-3-1-2-4-27(23)28(29(35)37)34-16-26-15-33-19-36(26)17-21-7-5-20(14-32)6-8-21/h1-8,11-13,15,19,28,34H,9-10,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025903
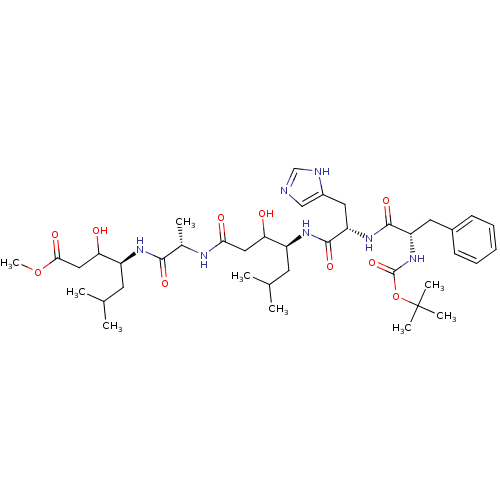
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C40H63N7O10/c1-23(2)15-28(32(48)19-34(50)43-25(5)36(52)44-29(16-24(3)4)33(49)20-35(51)56-9)45-38(54)31(18-27-21-41-22-42-27)46-37(53)30(17-26-13-11-10-12-14-26)47-39(55)57-40(6,7)8/h10-14,21-25,28-33,48-49H,15-20H2,1-9H3,(H,41,42)(H,43,50)(H,44,52)(H,45,54)(H,46,53)(H,47,55)/t25-,28-,29-,30-,31-,32?,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024167
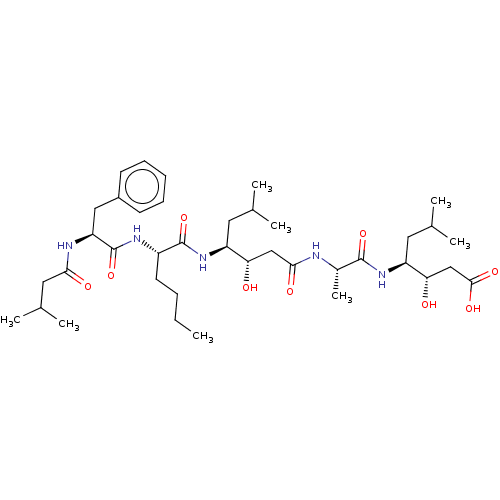
(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024187
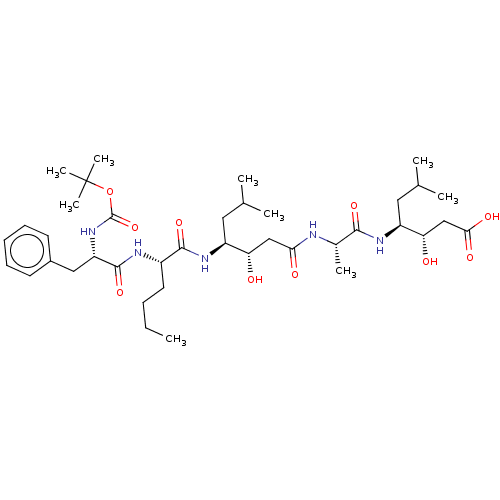
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O10/c1-10-11-17-27(41-37(52)30(20-26-15-13-12-14-16-26)44-38(53)54-39(7,8)9)36(51)43-28(18-23(2)3)31(45)21-33(47)40-25(6)35(50)42-29(19-24(4)5)32(46)22-34(48)49/h12-16,23-25,27-32,45-46H,10-11,17-22H2,1-9H3,(H,40,47)(H,41,52)(H,42,50)(H,43,51)(H,44,53)(H,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139571
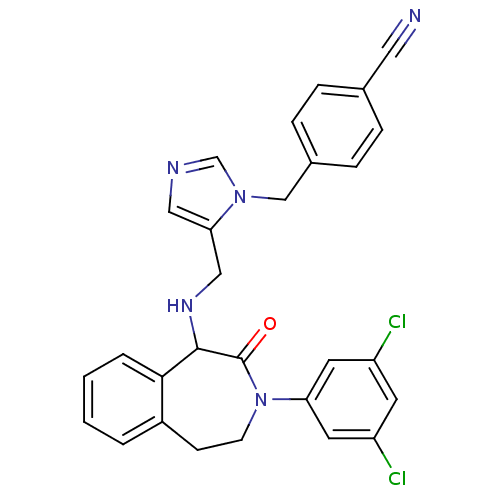
(4-(5-{[3-(3,5-Dichloro-phenyl)-2-oxo-2,3,4,5-tetra...)Show SMILES Clc1cc(Cl)cc(c1)N1CCc2ccccc2C(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H23Cl2N5O/c29-22-11-23(30)13-24(12-22)35-10-9-21-3-1-2-4-26(21)27(28(35)36)33-16-25-15-32-18-34(25)17-20-7-5-19(14-31)6-8-20/h1-8,11-13,15,18,27,33H,9-10,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50404815
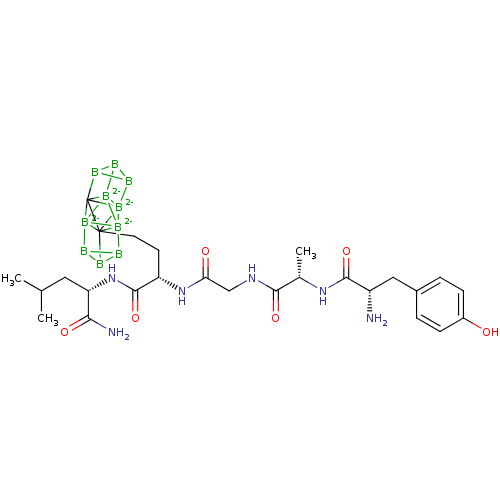
(CHEMBL218872)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC[C]1234B5B6B5[B--]115[B--]789B%10B%11B%10[C]217[B--]38%11[B--]4659)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C26H37B10N6O6/c1-13(2)10-19(21(38)45)42-24(48)18(41-20(44)12-39-22(46)14(3)40-23(47)17(37)11-15-4-6-16(43)7-5-15)8-9-26-25-27-29-30(27)35(25)33(25,26,29)36(26,35)32-28(26)31(32)34(25,26,35)36/h4-7,13-14,17-19,43H,8-12,37H2,1-3H3,(H2,38,45)(H,39,46)(H,40,47)(H,41,44)(H,42,48)/q-8/t14-,17-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC sid
UniChem
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity contractions of electrically stimulated mouse vas deferens was determined |
J Med Chem 25: 1428-31 (1983)
BindingDB Entry DOI: 10.7270/Q2N87C0G |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045039
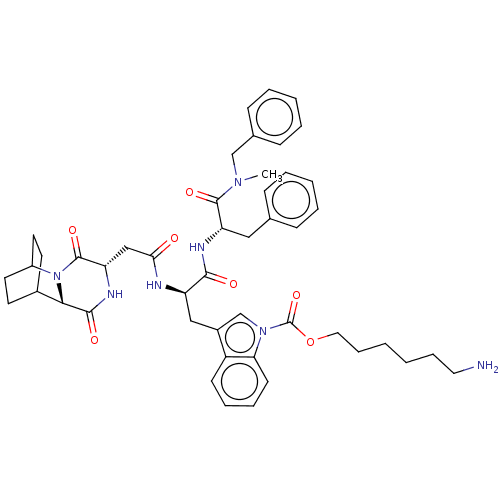
(6-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)OCCCCCCN)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:51.54,47.50,22.24,wD:11.12,THB:49:51:54.53:56.57,(11.28,-9.71,;10.14,-8.66,;11.3,-7.64,;12.76,-8.14,;13.92,-7.12,;15.38,-7.61,;15.68,-9.13,;14.52,-10.14,;13.06,-9.65,;8.69,-8.17,;8.38,-6.66,;7.53,-9.18,;7.83,-10.69,;9.29,-11.19,;10.45,-10.17,;11.91,-10.66,;12.21,-12.17,;11.05,-13.19,;9.59,-12.7,;6.07,-8.69,;4.91,-9.71,;5.22,-11.22,;3.46,-9.21,;2.3,-10.23,;2.6,-11.74,;4,-12.38,;3.82,-13.91,;4.95,-14.96,;6.42,-14.5,;4.61,-16.46,;5.75,-17.5,;5.41,-19.01,;6.54,-20.05,;6.2,-21.55,;7.33,-22.6,;6.99,-24.1,;8.13,-25.14,;2.31,-14.22,;1.52,-15.54,;-.02,-15.52,;-.77,-14.18,;.02,-12.85,;1.56,-12.87,;3.15,-7.7,;4.31,-6.69,;5.77,-7.18,;4.01,-5.18,;2.55,-4.68,;2.25,-3.17,;.79,-2.68,;.49,-1.17,;-.37,-3.7,;-1.73,-3.16,;-3.23,-3.82,;-3.03,-5.23,;-1.46,-4.56,;-1.2,-2.62,;-1.66,-1.49,;-.07,-5.21,;1.39,-5.7,;1.69,-7.21,)| Show InChI InChI=1S/C47H57N7O7/c1-52(29-32-16-8-5-9-17-32)45(58)38(26-31-14-6-4-7-15-31)50-43(56)37(49-41(55)28-39-46(59)54-35-22-20-33(21-23-35)42(54)44(57)51-39)27-34-30-53(40-19-11-10-18-36(34)40)47(60)61-25-13-3-2-12-24-48/h4-11,14-19,30,33,35,37-39,42H,2-3,12-13,20-29,48H2,1H3,(H,49,55)(H,50,56)(H,51,57)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421779

(CHEMBL308300 | CHEMBL3142338)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C1CCCC1 |r| Show InChI InChI=1S/C41H67N5O10/c1-24(2)19-29(32(47)22-34(49)42-26(5)37(51)43-30(20-25(3)4)33(48)23-35(50)55-9)44-39(53)36(28-17-13-14-18-28)46-38(52)31(21-27-15-11-10-12-16-27)45-40(54)56-41(6,7)8/h10-12,15-16,24-26,28-33,36,47-48H,13-14,17-23H2,1-9H3,(H,42,49)(H,43,51)(H,44,53)(H,45,54)(H,46,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421775

(CHEMBL3142326 | CHEMBL73556)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C39H65N5O9/c1-10-14-28(42-39(52)31(41-34(47)19-25(6)7)20-27-15-12-11-13-16-27)38(51)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(50)43-30(18-24(4)5)33(46)22-36(49)53-9/h11-13,15-16,23-26,28-33,45-46H,10,14,17-22H2,1-9H3,(H,40,48)(H,41,47)(H,42,52)(H,43,50)(H,44,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045036

(7-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)CCCCCCN)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:50.53,46.49,22.24,wD:11.11,THB:48:50:53.52:55.56,(11.12,-10.02,;9.98,-8.97,;11.14,-7.96,;12.6,-8.45,;13.76,-7.43,;15.21,-7.93,;15.52,-9.44,;14.36,-10.45,;12.9,-9.96,;8.52,-8.48,;8.22,-6.97,;7.37,-9.5,;7.67,-11.01,;9.13,-11.5,;10.29,-10.48,;11.74,-10.98,;12.05,-12.49,;10.89,-13.5,;9.43,-13.01,;5.91,-9,;4.75,-10.02,;5.05,-11.53,;3.29,-9.52,;2.14,-10.54,;2.44,-12.05,;3.84,-12.69,;3.66,-14.22,;4.79,-15.27,;6.26,-14.81,;4.45,-16.77,;5.58,-17.81,;5.25,-19.32,;6.38,-20.36,;6.04,-21.86,;7.17,-22.91,;6.83,-24.41,;2.15,-14.53,;1.36,-15.85,;-.18,-15.83,;-.93,-14.49,;-.15,-13.16,;1.39,-13.18,;2.99,-8.01,;4.15,-7,;5.61,-7.49,;3.85,-5.49,;2.39,-4.99,;2.09,-3.48,;.63,-2.99,;.32,-1.48,;-.53,-4.01,;-1.89,-3.47,;-3.39,-4.13,;-3.19,-5.54,;-1.63,-4.87,;-1.36,-2.93,;-1.82,-1.8,;-.23,-5.52,;1.23,-6.01,;1.53,-7.52,)| Show InChI InChI=1S/C47H57N7O6/c1-52(29-32-16-8-5-9-17-32)46(59)38(26-31-14-6-4-7-15-31)50-44(57)37(27-34-30-53(40-19-12-11-18-36(34)40)42(56)20-10-2-3-13-25-48)49-41(55)28-39-47(60)54-35-23-21-33(22-24-35)43(54)45(58)51-39/h4-9,11-12,14-19,30,33,35,37-39,43H,2-3,10,13,20-29,48H2,1H3,(H,49,55)(H,50,57)(H,51,58)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045039

(6-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)OCCCCCCN)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:51.54,47.50,22.24,wD:11.12,THB:49:51:54.53:56.57,(11.28,-9.71,;10.14,-8.66,;11.3,-7.64,;12.76,-8.14,;13.92,-7.12,;15.38,-7.61,;15.68,-9.13,;14.52,-10.14,;13.06,-9.65,;8.69,-8.17,;8.38,-6.66,;7.53,-9.18,;7.83,-10.69,;9.29,-11.19,;10.45,-10.17,;11.91,-10.66,;12.21,-12.17,;11.05,-13.19,;9.59,-12.7,;6.07,-8.69,;4.91,-9.71,;5.22,-11.22,;3.46,-9.21,;2.3,-10.23,;2.6,-11.74,;4,-12.38,;3.82,-13.91,;4.95,-14.96,;6.42,-14.5,;4.61,-16.46,;5.75,-17.5,;5.41,-19.01,;6.54,-20.05,;6.2,-21.55,;7.33,-22.6,;6.99,-24.1,;8.13,-25.14,;2.31,-14.22,;1.52,-15.54,;-.02,-15.52,;-.77,-14.18,;.02,-12.85,;1.56,-12.87,;3.15,-7.7,;4.31,-6.69,;5.77,-7.18,;4.01,-5.18,;2.55,-4.68,;2.25,-3.17,;.79,-2.68,;.49,-1.17,;-.37,-3.7,;-1.73,-3.16,;-3.23,-3.82,;-3.03,-5.23,;-1.46,-4.56,;-1.2,-2.62,;-1.66,-1.49,;-.07,-5.21,;1.39,-5.7,;1.69,-7.21,)| Show InChI InChI=1S/C47H57N7O7/c1-52(29-32-16-8-5-9-17-32)45(58)38(26-31-14-6-4-7-15-31)50-43(56)37(49-41(55)28-39-46(59)54-35-22-20-33(21-23-35)42(54)44(57)51-39)27-34-30-53(40-19-11-10-18-36(34)40)47(60)61-25-13-3-2-12-24-48/h4-11,14-19,30,33,35,37-39,42H,2-3,12-13,20-29,48H2,1H3,(H,49,55)(H,50,56)(H,51,57)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139572

(4-{5-[(2-Cyclohexylmethyl-3-oxo-2,3,4,5-tetrahydro...)Show SMILES O=C1C(Cc2ccccc2CN1CC1CCCCC1)NCc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C29H33N5O/c30-15-22-10-12-24(13-11-22)19-34-21-31-16-27(34)17-32-28-14-25-8-4-5-9-26(25)20-33(29(28)35)18-23-6-2-1-3-7-23/h4-5,8-13,16,21,23,28,32H,1-3,6-7,14,17-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50404820

(CHEMBL431501)Show SMILES C[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC12CC3CC(CC(C3)C1)C2)C(N)=O |TLB:42:41:44:37.36.38,42:37:44:41.43.40,THB:40:39:36:41.43.42,40:41:36:39.44.38| Show InChI InChI=1S/C36H48N6O6/c1-21(40-34(47)28(37)14-23-7-9-27(43)10-8-23)33(46)39-20-31(44)41-29(15-22-5-3-2-4-6-22)35(48)42-30(32(38)45)19-36-16-24-11-25(17-36)13-26(12-24)18-36/h2-10,21,24-26,28-30,43H,11-20,37H2,1H3,(H2,38,45)(H,39,46)(H,40,47)(H,41,44)(H,42,48)/t21-,24?,25?,26?,28-,29-,30-,36?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity contractions of electrically stimulated mouse vas deferens was determined |
J Med Chem 25: 1428-31 (1983)
BindingDB Entry DOI: 10.7270/Q2N87C0G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50226299

(CHEMBL3142798)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C39H65N5O10/c1-11-15-27(41-37(51)30(20-26-16-13-12-14-17-26)44-38(52)54-39(7,8)9)36(50)43-28(18-23(2)3)31(45)21-33(47)40-25(6)35(49)42-29(19-24(4)5)32(46)22-34(48)53-10/h12-14,16-17,23-25,27-32,45-46H,11,15,18-22H2,1-10H3,(H,40,47)(H,41,51)(H,42,49)(H,43,50)(H,44,52)/t25-,27-,28-,29-,30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139587
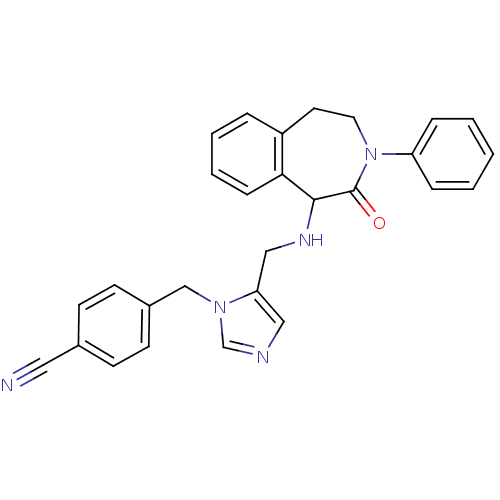
(4-{5-[(2-Oxo-3-phenyl-2,3,4,5-tetrahydro-1H-benzo[...)Show SMILES O=C1C(NCc2cncn2Cc2ccc(cc2)C#N)c2ccccc2CCN1c1ccccc1 Show InChI InChI=1S/C28H25N5O/c29-16-21-10-12-22(13-11-21)19-32-20-30-17-25(32)18-31-27-26-9-5-4-6-23(26)14-15-33(28(27)34)24-7-2-1-3-8-24/h1-13,17,20,27,31H,14-15,18-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045040

(CHEMBL3144343 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C=O)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:43.46,11.11,39.42,22.24,THB:41:43:46.45:48.49,(10.33,-13,;9.17,-14.02,;9.47,-15.53,;10.93,-16.02,;11.23,-17.53,;12.69,-18.03,;13.85,-17.01,;13.55,-15.5,;12.09,-15.01,;7.71,-13.52,;6.56,-14.54,;7.41,-12.01,;8.57,-11,;8.26,-9.49,;9.42,-8.47,;9.12,-6.96,;7.66,-6.47,;6.5,-7.48,;6.81,-8.99,;5.95,-11.52,;5.65,-10.01,;5.99,-8.51,;4.19,-9.52,;3.03,-10.53,;3.34,-12.04,;4.73,-12.69,;4.56,-14.22,;5.69,-15.26,;5.35,-16.76,;3.05,-14.52,;2.26,-15.84,;.72,-15.82,;-.03,-14.48,;.75,-13.16,;2.29,-13.18,;3.89,-8.01,;2.43,-7.51,;1.27,-8.53,;2.13,-6,;.67,-5.51,;.37,-4,;-1.09,-3.51,;-1.39,-2,;-2.25,-4.52,;-3.61,-3.99,;-5.11,-4.65,;-4.91,-6.06,;-3.34,-5.39,;-3.08,-3.45,;-3.54,-2.32,;-1.95,-6.03,;-.49,-6.53,;-.19,-8.04,)| Show InChI InChI=1S/C41H44N6O6/c1-45(23-27-12-6-3-7-13-27)40(52)33(20-26-10-4-2-5-11-26)43-38(50)32(21-29-24-46(25-48)35-15-9-8-14-31(29)35)42-36(49)22-34-41(53)47-30-18-16-28(17-19-30)37(47)39(51)44-34/h2-15,24-25,28,30,32-34,37H,16-23H2,1H3,(H,42,49)(H,43,50)(H,44,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139566
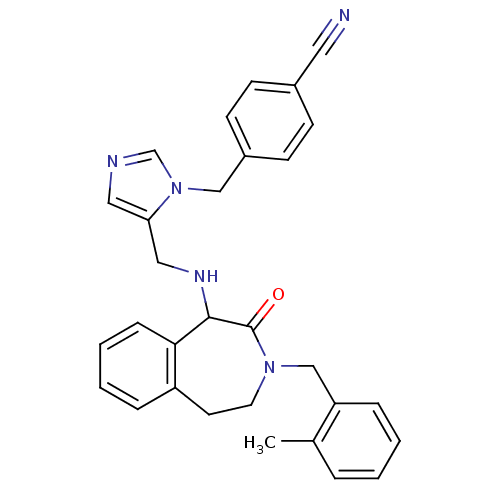
(4-(5-{[3-(2-Methyl-benzyl)-2-oxo-2,3,4,5-tetrahydr...)Show SMILES Cc1ccccc1CN1CCc2ccccc2C(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C30H29N5O/c1-22-6-2-3-8-26(22)20-34-15-14-25-7-4-5-9-28(25)29(30(34)36)33-18-27-17-32-21-35(27)19-24-12-10-23(16-31)11-13-24/h2-13,17,21,29,33H,14-15,18-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139584
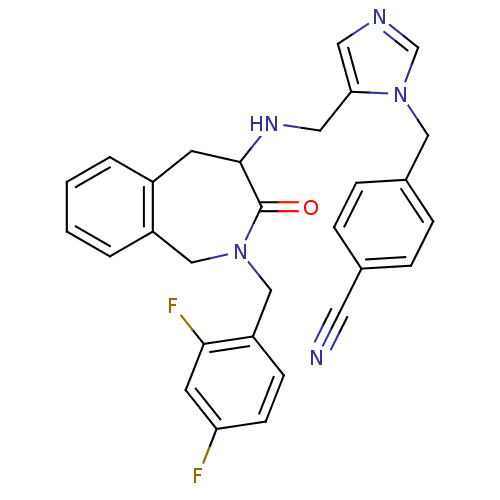
(4-(5-{[2-(2,4-Difluoro-benzyl)-3-oxo-2,3,4,5-tetra...)Show SMILES Fc1ccc(CN2Cc3ccccc3CC(NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c(F)c1 Show InChI InChI=1S/C29H25F2N5O/c30-25-10-9-24(27(31)12-25)18-35-17-23-4-2-1-3-22(23)11-28(29(35)37)34-15-26-14-33-19-36(26)16-21-7-5-20(13-32)6-8-21/h1-10,12,14,19,28,34H,11,15-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139589

(4-(5-{[3-(3,5-Difluoro-phenyl)-2-oxo-2,3,4,5-tetra...)Show SMILES Fc1cc(F)cc(c1)N1CCc2ccccc2C(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H23F2N5O/c29-22-11-23(30)13-24(12-22)35-10-9-21-3-1-2-4-26(21)27(28(35)36)33-16-25-15-32-18-34(25)17-20-7-5-19(14-31)6-8-20/h1-8,11-13,15,18,27,33H,9-10,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045037
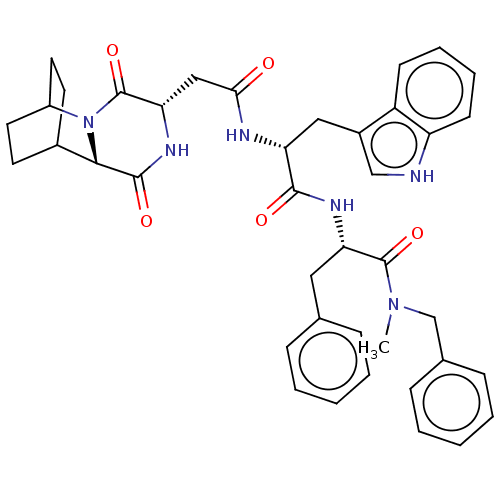
(CHEMBL3144352 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:41.44,37.40,wD:11.11,22.24,THB:39:41:44.43:46.47,(10.7,-11.55,;10.4,-10.04,;11.55,-9.02,;13.01,-9.52,;14.17,-8.5,;15.63,-8.99,;15.93,-10.5,;14.77,-11.52,;13.31,-11.03,;8.94,-9.55,;8.63,-8.04,;7.78,-10.56,;8.08,-12.07,;6.93,-13.09,;7.23,-14.6,;6.07,-15.62,;4.61,-15.12,;4.31,-13.61,;5.47,-12.6,;6.32,-10.07,;5.16,-11.09,;4.52,-12.48,;3.71,-10.59,;3.4,-9.08,;4.56,-8.07,;6.06,-8.4,;6.85,-7.08,;5.83,-5.92,;6.01,-4.39,;4.78,-3.47,;3.36,-4.08,;3.18,-5.61,;4.42,-6.53,;2.55,-11.61,;1.09,-11.12,;.79,-9.61,;-.07,-12.13,;-1.53,-11.64,;-1.83,-10.13,;-3.29,-9.63,;-3.59,-8.12,;-4.44,-10.65,;-5.8,-10.12,;-7.3,-10.77,;-7.1,-12.18,;-5.54,-11.51,;-5.28,-9.58,;-5.73,-8.44,;-4.14,-12.16,;-2.68,-12.66,;-2.38,-14.17,)| Show InChI InChI=1S/C40H44N6O5/c1-45(24-26-12-6-3-7-13-26)39(50)33(20-25-10-4-2-5-11-25)43-37(48)32(21-28-23-41-31-15-9-8-14-30(28)31)42-35(47)22-34-40(51)46-29-18-16-27(17-19-29)36(46)38(49)44-34/h2-15,23,27,29,32-34,36,41H,16-22,24H2,1H3,(H,42,47)(H,43,48)(H,44,49) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor transfected on CHO cells using [125I]-His] NKA as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50045037

(CHEMBL3144352 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:41.44,37.40,wD:11.11,22.24,THB:39:41:44.43:46.47,(10.7,-11.55,;10.4,-10.04,;11.55,-9.02,;13.01,-9.52,;14.17,-8.5,;15.63,-8.99,;15.93,-10.5,;14.77,-11.52,;13.31,-11.03,;8.94,-9.55,;8.63,-8.04,;7.78,-10.56,;8.08,-12.07,;6.93,-13.09,;7.23,-14.6,;6.07,-15.62,;4.61,-15.12,;4.31,-13.61,;5.47,-12.6,;6.32,-10.07,;5.16,-11.09,;4.52,-12.48,;3.71,-10.59,;3.4,-9.08,;4.56,-8.07,;6.06,-8.4,;6.85,-7.08,;5.83,-5.92,;6.01,-4.39,;4.78,-3.47,;3.36,-4.08,;3.18,-5.61,;4.42,-6.53,;2.55,-11.61,;1.09,-11.12,;.79,-9.61,;-.07,-12.13,;-1.53,-11.64,;-1.83,-10.13,;-3.29,-9.63,;-3.59,-8.12,;-4.44,-10.65,;-5.8,-10.12,;-7.3,-10.77,;-7.1,-12.18,;-5.54,-11.51,;-5.28,-9.58,;-5.73,-8.44,;-4.14,-12.16,;-2.68,-12.66,;-2.38,-14.17,)| Show InChI InChI=1S/C40H44N6O5/c1-45(24-26-12-6-3-7-13-26)39(50)33(20-25-10-4-2-5-11-25)43-37(48)32(21-28-23-41-31-15-9-8-14-30(28)31)42-35(47)22-34-40(51)46-29-18-16-27(17-19-29)36(46)38(49)44-34/h2-15,23,27,29,32-34,36,41H,16-22,24H2,1H3,(H,42,47)(H,43,48)(H,44,49) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against rat NK-2 receptor transfected on CHO cells using [125I]-His] NKA as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data