 Found 382 hits with Last Name = 'fells' and Initial = 'ji'
Found 382 hits with Last Name = 'fells' and Initial = 'ji' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysophosphatidic acid receptor 2
(Homo sapiens (Human)) | BDBM50271765
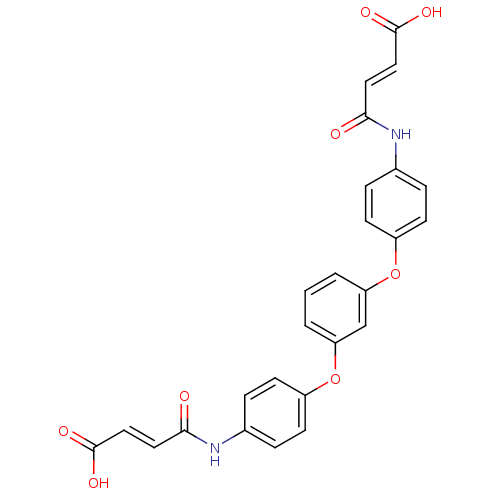
(3-(4-{3-[4-(3-Carboxy-acryloylamino)-phenoxy]-phen...)Show SMILES OC(=O)\C=C\C(=O)Nc1ccc(Oc2cccc(Oc3ccc(NC(=O)\C=C\C(O)=O)cc3)c2)cc1 Show InChI InChI=1S/C26H20N2O8/c29-23(12-14-25(31)32)27-17-4-8-19(9-5-17)35-21-2-1-3-22(16-21)36-20-10-6-18(7-11-20)28-24(30)13-15-26(33)34/h1-16H,(H,27,29)(H,28,30)(H,31,32)(H,33,34)/b14-12+,15-13+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA2 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... |
Bioorg Med Chem 16: 6207-17 (2008)
Article DOI: 10.1016/j.bmc.2008.04.035
BindingDB Entry DOI: 10.7270/Q270817C |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 2
(Homo sapiens (Human)) | BDBM50271763
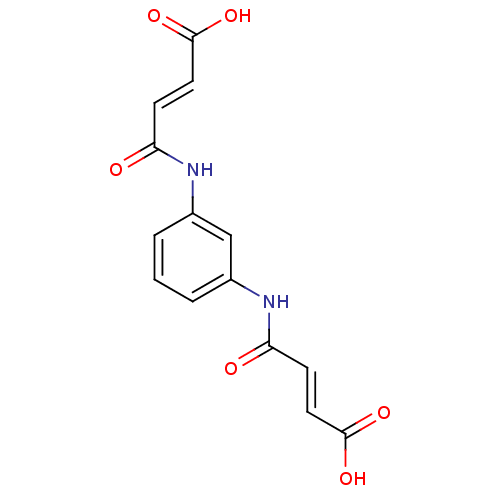
(3-[3-(3-Carboxy-acryloylamino)-phenylcarbamoyl]-ac...)Show SMILES OC(=O)\C=C\C(=O)Nc1cccc(NC(=O)\C=C\C(O)=O)c1 Show InChI InChI=1S/C14H12N2O6/c17-11(4-6-13(19)20)15-9-2-1-3-10(8-9)16-12(18)5-7-14(21)22/h1-8H,(H,15,17)(H,16,18)(H,19,20)(H,21,22)/b6-4+,7-5+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA2 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... |
Bioorg Med Chem 16: 6207-17 (2008)
Article DOI: 10.1016/j.bmc.2008.04.035
BindingDB Entry DOI: 10.7270/Q270817C |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 2
(Homo sapiens (Human)) | BDBM50271764
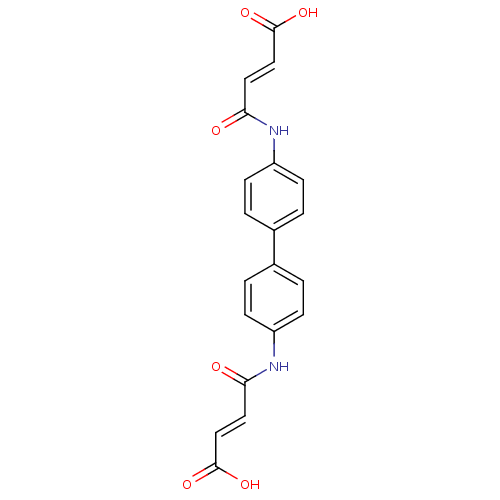
(3-[4'-(3-Carboxy-acryloylamino)-biphenyl-4-ylcarba...)Show SMILES OC(=O)\C=C\C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)\C=C\C(O)=O)cc1 Show InChI InChI=1S/C20H16N2O6/c23-17(9-11-19(25)26)21-15-5-1-13(2-6-15)14-3-7-16(8-4-14)22-18(24)10-12-20(27)28/h1-12H,(H,21,23)(H,22,24)(H,25,26)(H,27,28)/b11-9+,12-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA2 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... |
Bioorg Med Chem 16: 6207-17 (2008)
Article DOI: 10.1016/j.bmc.2008.04.035
BindingDB Entry DOI: 10.7270/Q270817C |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50304580
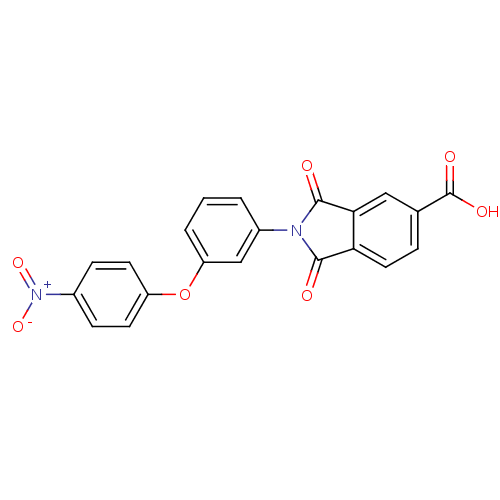
(2-(3-(4-nitrophenoxy)phenyl)-1,3-dioxoisoindoline-...)Show SMILES OC(=O)c1ccc2C(=O)N(C(=O)c2c1)c1cccc(Oc2ccc(cc2)[N+]([O-])=O)c1 Show InChI InChI=1S/C21H12N2O7/c24-19-17-9-4-12(21(26)27)10-18(17)20(25)22(19)14-2-1-3-16(11-14)30-15-7-5-13(6-8-15)23(28)29/h1-11H,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Binding affinity to LPA1 |
Bioorg Med Chem 17: 7457-64 (2009)
Article DOI: 10.1016/j.bmc.2009.09.022
BindingDB Entry DOI: 10.7270/Q2GB244M |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50271763

(3-[3-(3-Carboxy-acryloylamino)-phenylcarbamoyl]-ac...)Show SMILES OC(=O)\C=C\C(=O)Nc1cccc(NC(=O)\C=C\C(O)=O)c1 Show InChI InChI=1S/C14H12N2O6/c17-11(4-6-13(19)20)15-9-2-1-3-10(8-9)16-12(18)5-7-14(21)22/h1-8H,(H,15,17)(H,16,18)(H,19,20)(H,21,22)/b6-4+,7-5+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA1 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... |
Bioorg Med Chem 16: 6207-17 (2008)
Article DOI: 10.1016/j.bmc.2008.04.035
BindingDB Entry DOI: 10.7270/Q270817C |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 3
(Homo sapiens (Human)) | BDBM50304580

(2-(3-(4-nitrophenoxy)phenyl)-1,3-dioxoisoindoline-...)Show SMILES OC(=O)c1ccc2C(=O)N(C(=O)c2c1)c1cccc(Oc2ccc(cc2)[N+]([O-])=O)c1 Show InChI InChI=1S/C21H12N2O7/c24-19-17-9-4-12(21(26)27)10-18(17)20(25)22(19)14-2-1-3-16(11-14)30-15-7-5-13(6-8-15)23(28)29/h1-11H,(H,26,27) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Binding affinity to LPA3 |
Bioorg Med Chem 17: 7457-64 (2009)
Article DOI: 10.1016/j.bmc.2009.09.022
BindingDB Entry DOI: 10.7270/Q2GB244M |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 5
(Homo sapiens (Human)) | BDBM50304580

(2-(3-(4-nitrophenoxy)phenyl)-1,3-dioxoisoindoline-...)Show SMILES OC(=O)c1ccc2C(=O)N(C(=O)c2c1)c1cccc(Oc2ccc(cc2)[N+]([O-])=O)c1 Show InChI InChI=1S/C21H12N2O7/c24-19-17-9-4-12(21(26)27)10-18(17)20(25)22(19)14-2-1-3-16(11-14)30-15-7-5-13(6-8-15)23(28)29/h1-11H,(H,26,27) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 292 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Binding affinity to LPA5 |
Bioorg Med Chem 17: 7457-64 (2009)
Article DOI: 10.1016/j.bmc.2009.09.022
BindingDB Entry DOI: 10.7270/Q2GB244M |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 3
(Homo sapiens (Human)) | BDBM50271765

(3-(4-{3-[4-(3-Carboxy-acryloylamino)-phenoxy]-phen...)Show SMILES OC(=O)\C=C\C(=O)Nc1ccc(Oc2cccc(Oc3ccc(NC(=O)\C=C\C(O)=O)cc3)c2)cc1 Show InChI InChI=1S/C26H20N2O8/c29-23(12-14-25(31)32)27-17-4-8-19(9-5-17)35-21-2-1-3-22(16-21)36-20-10-6-18(7-11-20)28-24(30)13-15-26(33)34/h1-16H,(H,27,29)(H,28,30)(H,31,32)(H,33,34)/b14-12+,15-13+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA3 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... |
Bioorg Med Chem 16: 6207-17 (2008)
Article DOI: 10.1016/j.bmc.2008.04.035
BindingDB Entry DOI: 10.7270/Q270817C |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50304581
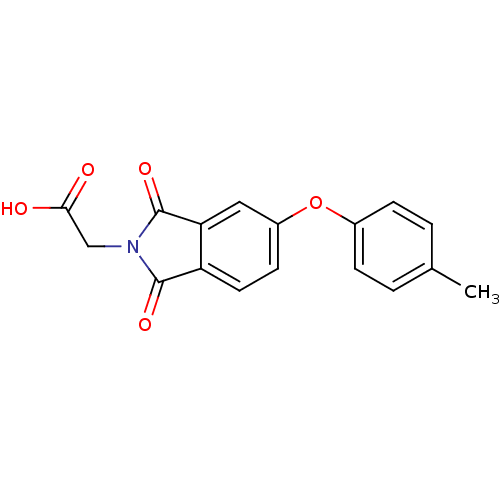
(2-(1,3-dioxo-5-(p-tolyloxy)isoindolin-2-yl)acetic ...)Show InChI InChI=1S/C17H13NO5/c1-10-2-4-11(5-3-10)23-12-6-7-13-14(8-12)17(22)18(16(13)21)9-15(19)20/h2-8H,9H2,1H3,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Binding affinity to LPA1 |
Bioorg Med Chem 17: 7457-64 (2009)
Article DOI: 10.1016/j.bmc.2009.09.022
BindingDB Entry DOI: 10.7270/Q2GB244M |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 3
(Homo sapiens (Human)) | BDBM50241460
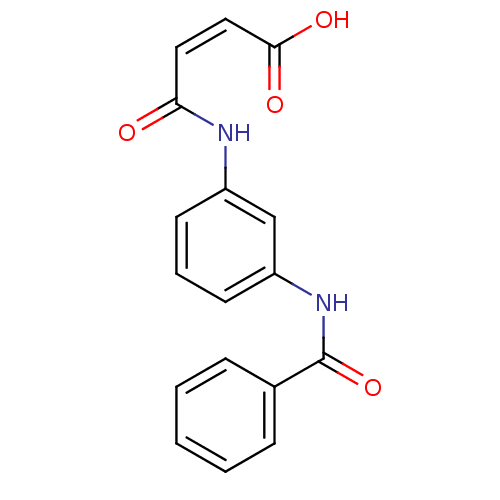
(4-(3-benzamidophenylamino)-4-oxobut-2-enoic acid |...)Show InChI InChI=1S/C17H14N2O4/c20-15(9-10-16(21)22)18-13-7-4-8-14(11-13)19-17(23)12-5-2-1-3-6-12/h1-11H,(H,18,20)(H,19,23)(H,21,22)/b10-9- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 317 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA3 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... |
Bioorg Med Chem 16: 6207-17 (2008)
Article DOI: 10.1016/j.bmc.2008.04.035
BindingDB Entry DOI: 10.7270/Q270817C |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 3
(Homo sapiens (Human)) | BDBM50304581

(2-(1,3-dioxo-5-(p-tolyloxy)isoindolin-2-yl)acetic ...)Show InChI InChI=1S/C17H13NO5/c1-10-2-4-11(5-3-10)23-12-6-7-13-14(8-12)17(22)18(16(13)21)9-15(19)20/h2-8H,9H2,1H3,(H,19,20) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Binding affinity to LPA3 |
Bioorg Med Chem 17: 7457-64 (2009)
Article DOI: 10.1016/j.bmc.2009.09.022
BindingDB Entry DOI: 10.7270/Q2GB244M |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 3
(Homo sapiens (Human)) | BDBM50271764

(3-[4'-(3-Carboxy-acryloylamino)-biphenyl-4-ylcarba...)Show SMILES OC(=O)\C=C\C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)\C=C\C(O)=O)cc1 Show InChI InChI=1S/C20H16N2O6/c23-17(9-11-19(25)26)21-15-5-1-13(2-6-15)14-3-7-16(8-4-14)22-18(24)10-12-20(27)28/h1-12H,(H,21,23)(H,22,24)(H,25,26)(H,27,28)/b11-9+,12-10+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA3 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... |
Bioorg Med Chem 16: 6207-17 (2008)
Article DOI: 10.1016/j.bmc.2008.04.035
BindingDB Entry DOI: 10.7270/Q270817C |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50271765

(3-(4-{3-[4-(3-Carboxy-acryloylamino)-phenoxy]-phen...)Show SMILES OC(=O)\C=C\C(=O)Nc1ccc(Oc2cccc(Oc3ccc(NC(=O)\C=C\C(O)=O)cc3)c2)cc1 Show InChI InChI=1S/C26H20N2O8/c29-23(12-14-25(31)32)27-17-4-8-19(9-5-17)35-21-2-1-3-22(16-21)36-20-10-6-18(7-11-20)28-24(30)13-15-26(33)34/h1-16H,(H,27,29)(H,28,30)(H,31,32)(H,33,34)/b14-12+,15-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA1 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... |
Bioorg Med Chem 16: 6207-17 (2008)
Article DOI: 10.1016/j.bmc.2008.04.035
BindingDB Entry DOI: 10.7270/Q270817C |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606543
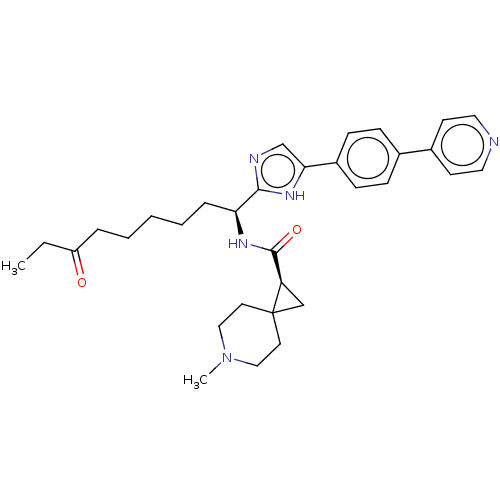
(CHEMBL5218926)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc(cc1)-c1ccncc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606556
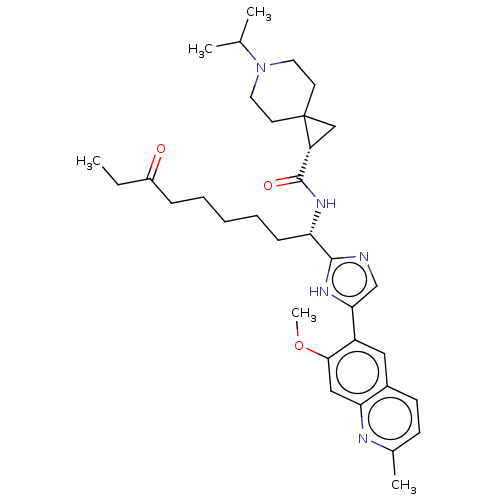
(CHEMBL5218854)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC1)C(C)C)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606557
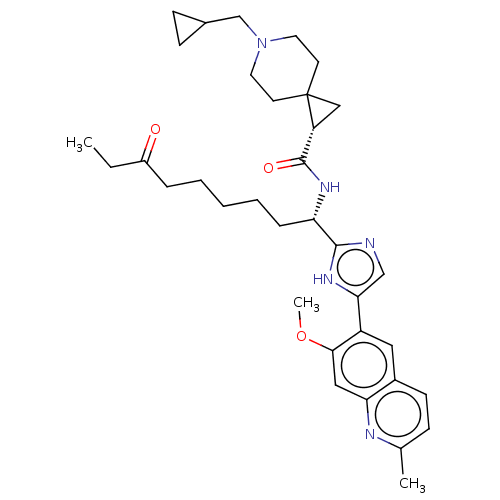
(CHEMBL5219294)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606558
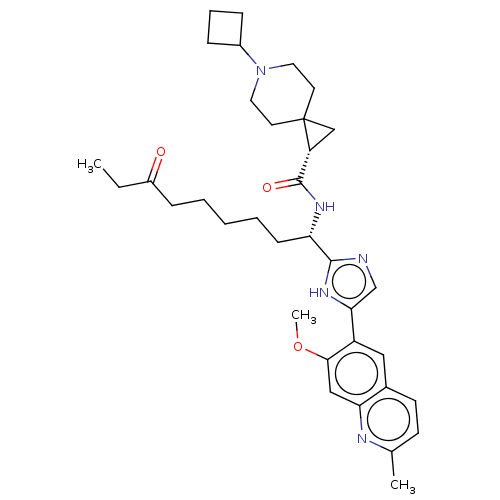
(CHEMBL5219825)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC1)C1CCC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606559

(CHEMBL5219875)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccc(CC)nc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606560
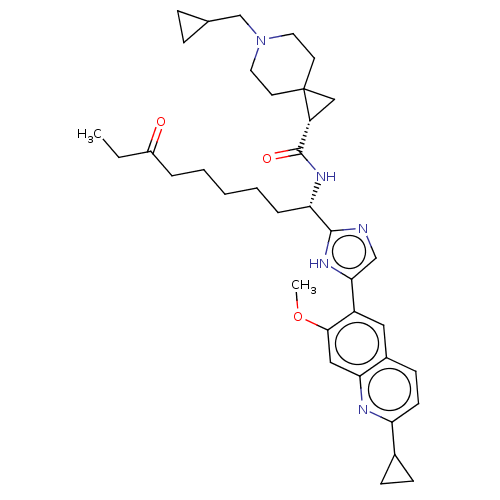
(CHEMBL5220360)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccc(nc2cc1OC)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606561
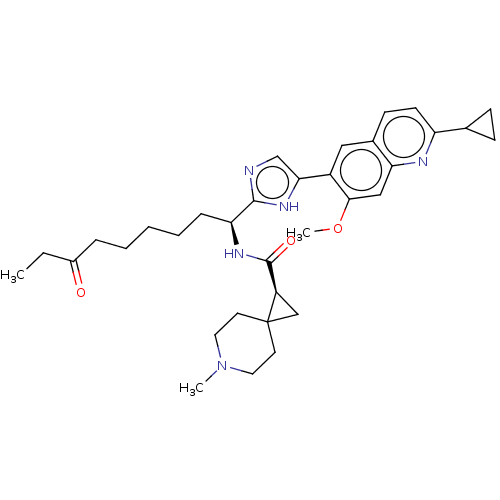
(CHEMBL5218537)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(nc2cc1OC)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606562
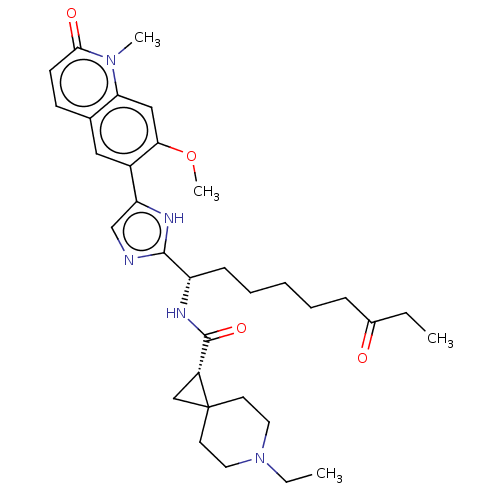
(CHEMBL5220969)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccc(=O)n(C)c3cc2OC)CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50568207

(CHEMBL4856631)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2c(ccn(C)c2=O)c1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606548
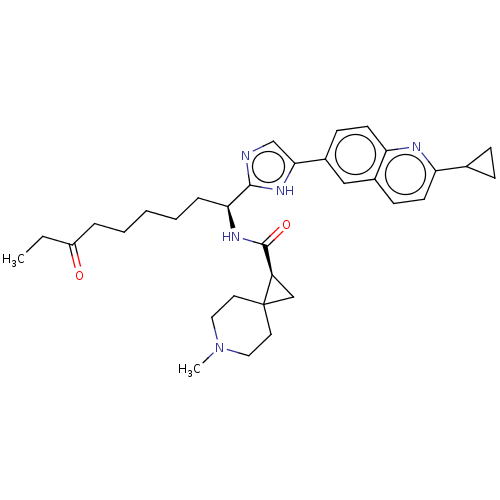
(CHEMBL5218918)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2nc(ccc2c1)C1CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606555
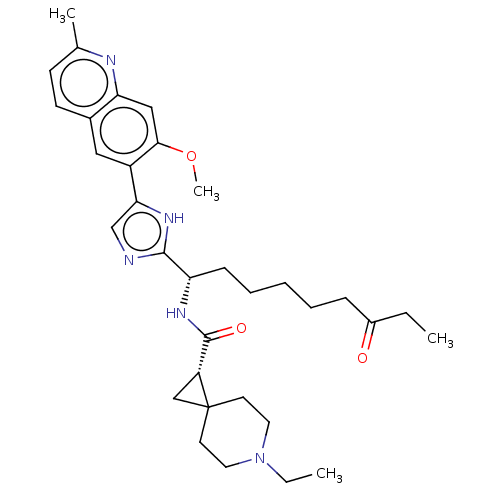
(CHEMBL5220926)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccc(C)nc3cc2OC)CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606556

(CHEMBL5218854)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC1)C(C)C)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606557

(CHEMBL5219294)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606558

(CHEMBL5219825)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC1)C1CCC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606559

(CHEMBL5219875)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccc(CC)nc2cc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606560

(CHEMBL5220360)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccc(nc2cc1OC)C1CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606561

(CHEMBL5218537)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(nc2cc1OC)C1CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606562

(CHEMBL5220969)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccc(=O)n(C)c3cc2OC)CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606566
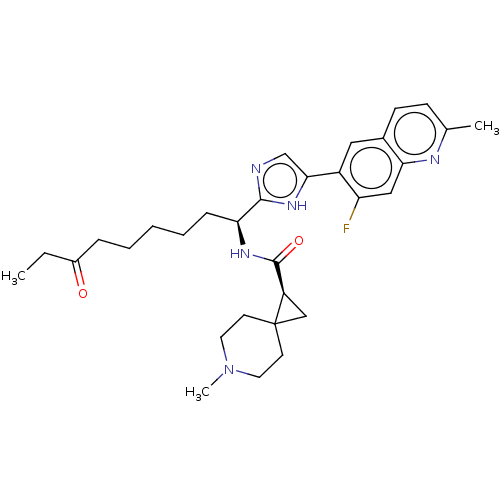
(CHEMBL5220233)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1F |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606568
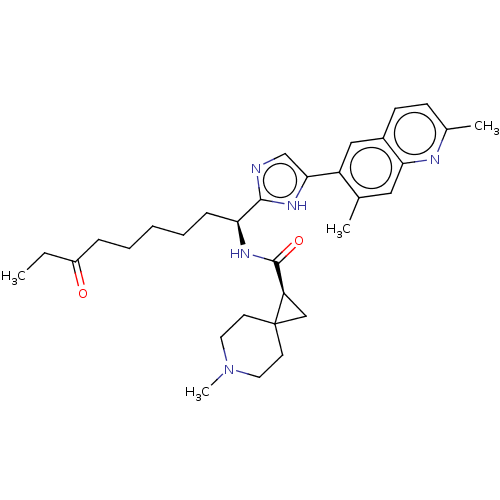
(CHEMBL5219115)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1C |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606571
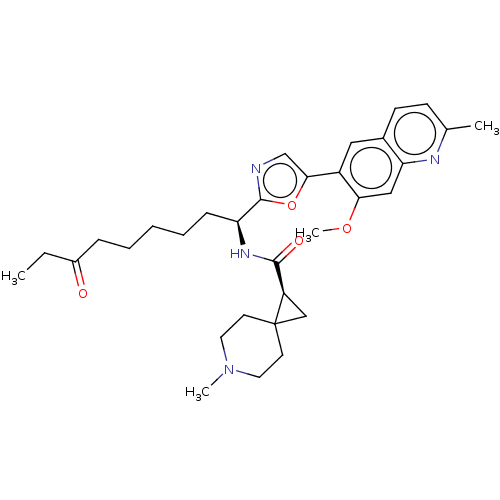
(CHEMBL5220278)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc(o1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50606572
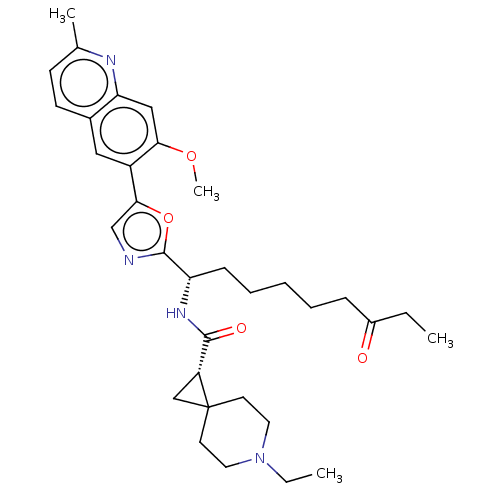
(CHEMBL5218693)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc(o2)-c2cc3ccc(C)nc3cc2OC)CC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606565
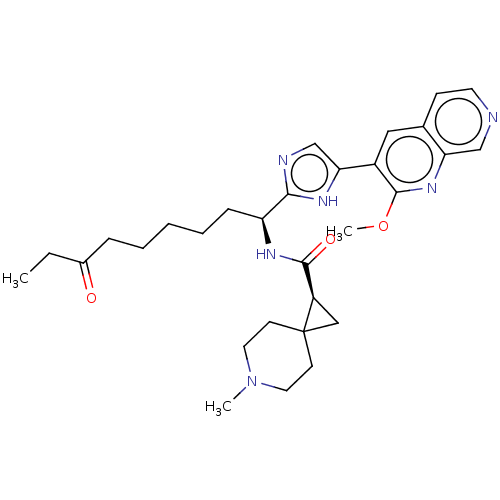
(CHEMBL5219039)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccncc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606566

(CHEMBL5220233)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606568

(CHEMBL5219115)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606569

(CHEMBL5220254)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1CC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606572

(CHEMBL5218693)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc(o2)-c2cc3ccc(C)nc3cc2OC)CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50606557

(CHEMBL5219294)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50606558

(CHEMBL5219825)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC1)C1CCC1)c1ncc([nH]1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50606559

(CHEMBL5219875)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccc(CC)nc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50606560

(CHEMBL5220360)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccc(nc2cc1OC)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50568207

(CHEMBL4856631)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2c(ccn(C)c2=O)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606546

(CHEMBL5218556)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2nc(C)ccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606548

(CHEMBL5218918)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2nc(ccc2c1)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606550

(CHEMBL5220616)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2nc(ccc2c1)N1CCCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606553

(CHEMBL5218586)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2cccnc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606555

(CHEMBL5220926)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccc(C)nc3cc2OC)CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data