 Found 138 hits with Last Name = 'fenaux' and Initial = 'm'
Found 138 hits with Last Name = 'fenaux' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 7
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CCR7 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50069756
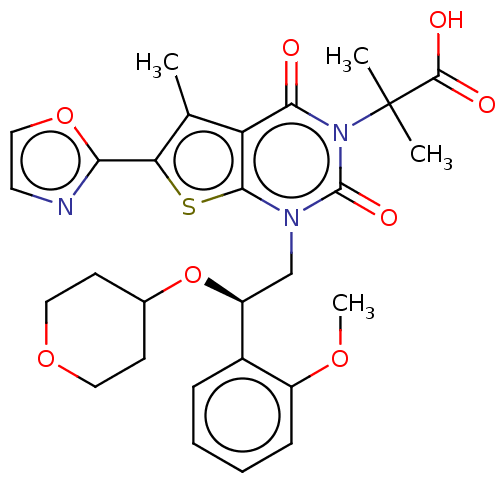
(CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...)Show SMILES COc1ccccc1[C@H](Cn1c2sc(c(C)c2c(=O)n(c1=O)C(C)(C)C(O)=O)-c1ncco1)OC1CCOCC1 |r| Show InChI InChI=1S/C28H31N3O8S/c1-16-21-24(32)31(28(2,3)26(33)34)27(35)30(25(21)40-22(16)23-29-11-14-38-23)15-20(39-17-9-12-37-13-10-17)18-7-5-6-8-19(18)36-4/h5-8,11,14,17,20H,9-10,12-13,15H2,1-4H3,(H,33,34)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50069756

(CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...)Show SMILES COc1ccccc1[C@H](Cn1c2sc(c(C)c2c(=O)n(c1=O)C(C)(C)C(O)=O)-c1ncco1)OC1CCOCC1 |r| Show InChI InChI=1S/C28H31N3O8S/c1-16-21-24(32)31(28(2,3)26(33)34)27(35)30(25(21)40-22(16)23-29-11-14-38-23)15-20(39-17-9-12-37-13-10-17)18-7-5-6-8-19(18)36-4/h5-8,11,14,17,20H,9-10,12-13,15H2,1-4H3,(H,33,34)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50511117
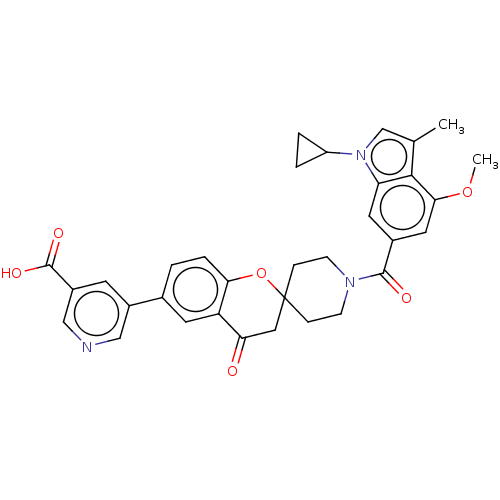
(CHEMBL4557289)Show SMILES COc1cc(cc2n(cc(C)c12)C1CC1)C(=O)N1CCC2(CC1)CC(=O)c1cc(ccc1O2)-c1cncc(c1)C(O)=O Show InChI InChI=1S/C33H31N3O6/c1-19-18-36(24-4-5-24)26-13-21(14-29(41-2)30(19)26)31(38)35-9-7-33(8-10-35)15-27(37)25-12-20(3-6-28(25)42-33)22-11-23(32(39)40)17-34-16-22/h3,6,11-14,16-18,24H,4-5,7-10,15H2,1-2H3,(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50511117

(CHEMBL4557289)Show SMILES COc1cc(cc2n(cc(C)c12)C1CC1)C(=O)N1CCC2(CC1)CC(=O)c1cc(ccc1O2)-c1cncc(c1)C(O)=O Show InChI InChI=1S/C33H31N3O6/c1-19-18-36(24-4-5-24)26-13-21(14-29(41-2)30(19)26)31(38)35-9-7-33(8-10-35)15-27(37)25-12-20(3-6-28(25)42-33)22-11-23(32(39)40)17-34-16-22/h3,6,11-14,16-18,24H,4-5,7-10,15H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 7
(Homo sapiens (Human)) | BDBM50306033
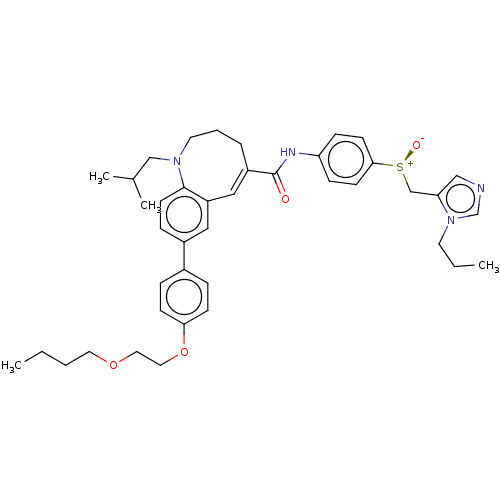
(Cenicriviroc | TAK-652 | TBR-652)Show SMILES CCCCOCCOc1ccc(cc1)-c1ccc2N(CC(C)C)CCC\C(=C/c2c1)C(=O)Nc1ccc(cc1)[S@@+]([O-])Cc1cncn1CCC |r,c:27| Show InChI InChI=1S/C41H52N4O4S/c1-5-7-22-48-23-24-49-38-15-10-32(11-16-38)33-12-19-40-35(25-33)26-34(9-8-21-44(40)28-31(3)4)41(46)43-36-13-17-39(18-14-36)50(47)29-37-27-42-30-45(37)20-6-2/h10-19,25-27,30-31H,5-9,20-24,28-29H2,1-4H3,(H,43,46)/b34-26+/t50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CCR7 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50306033

(Cenicriviroc | TAK-652 | TBR-652)Show SMILES CCCCOCCOc1ccc(cc1)-c1ccc2N(CC(C)C)CCC\C(=C/c2c1)C(=O)Nc1ccc(cc1)[S@@+]([O-])Cc1cncn1CCC |r,c:27| Show InChI InChI=1S/C41H52N4O4S/c1-5-7-22-48-23-24-49-38-15-10-32(11-16-38)33-12-19-40-35(25-33)26-34(9-8-21-44(40)28-31(3)4)41(46)43-36-13-17-39(18-14-36)50(47)29-37-27-42-30-45(37)20-6-2/h10-19,25-27,30-31H,5-9,20-24,28-29H2,1-4H3,(H,43,46)/b34-26+/t50-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CCR2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319585

(US10174007, Example 4 | US10787438, Example 4 | US...)Show SMILES C[C@H]1CCN1c1nc(cc(n1)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C16H19F3N4O2/c1-8-2-3-23(8)15-20-12(16(17,18)19)5-13(21-15)22-6-10-9(4-14(24)25)11(10)7-22/h5,8-11H,2-4,6-7H2,1H3,(H,24,25)/t8-,9-,10-,11+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50511118
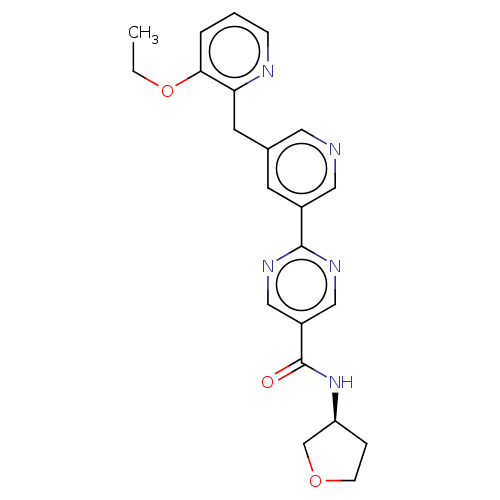
(CHEMBL4456029)Show SMILES CCOc1cccnc1Cc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C22H23N5O3/c1-2-30-20-4-3-6-24-19(20)9-15-8-16(11-23-10-15)21-25-12-17(13-26-21)22(28)27-18-5-7-29-14-18/h3-4,6,8,10-13,18H,2,5,7,9,14H2,1H3,(H,27,28)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged human DGAT2 expressed in SF9 cells after 1 hr by TopCount assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CCR2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50399710
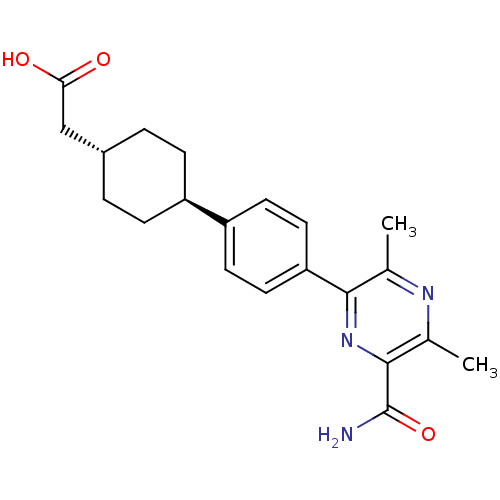
(CHEMBL2178953)Show SMILES Cc1nc(C)c(nc1C(N)=O)-c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:17.18,wD:20.22,(61.46,-10.56,;62.79,-9.79,;64.13,-10.56,;65.46,-9.79,;66.8,-10.56,;65.46,-8.26,;64.13,-7.48,;62.79,-8.25,;61.46,-7.48,;60.12,-8.25,;61.46,-5.94,;66.79,-7.49,;68.12,-8.26,;69.46,-7.49,;69.46,-5.94,;68.12,-5.17,;66.79,-5.94,;70.79,-5.16,;70.78,-3.63,;72.12,-2.86,;73.45,-3.62,;74.79,-2.85,;76.12,-3.62,;77.46,-2.85,;76.12,-5.16,;73.45,-5.17,;72.13,-5.93,)| Show InChI InChI=1S/C21H25N3O3/c1-12-19(24-20(21(22)27)13(2)23-12)17-9-7-16(8-10-17)15-5-3-14(4-6-15)11-18(25)26/h7-10,14-15H,3-6,11H2,1-2H3,(H2,22,27)(H,25,26)/t14-,15- | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged human DGAT2 expressed in SF9 cells after 1 hr by TopCount assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50030474
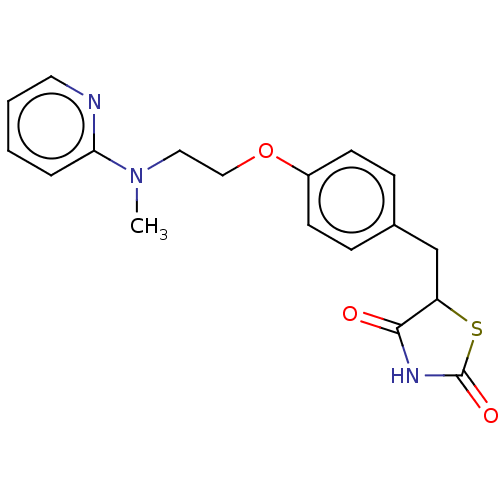
(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of mouse liver PPARalpha |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Rattus norvegicus) | BDBM50241178
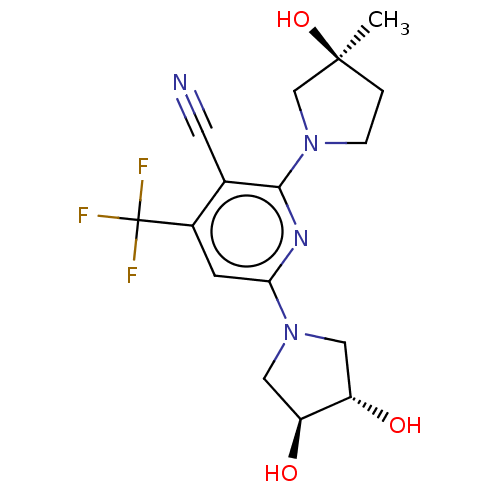
(CHEMBL4070442)Show SMILES C[C@]1(O)CCN(C1)c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H](O)[C@@H](O)C1 |r| Show InChI InChI=1S/C16H19F3N4O3/c1-15(26)2-3-22(8-15)14-9(5-20)10(16(17,18)19)4-13(21-14)23-6-11(24)12(25)7-23/h4,11-12,24-26H,2-3,6-8H2,1H3/t11-,12-,15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat KHK |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein
(Dengue virus) | BDBM50274119

(CHEMBL4126343)Show SMILES Nc1ncnc2n(ccc12)C1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@]1(O)C#N |r| Show InChI InChI=1S/C12H16N5O13P3/c13-4-12(19)8(18)7(3-27-32(23,24)30-33(25,26)29-31(20,21)22)28-11(12)17-2-1-6-9(14)15-5-16-10(6)17/h1-2,5,7-8,11,18-19H,3H2,(H,23,24)(H,25,26)(H2,14,15,16)(H2,20,21,22)/t7-,8-,11?,12-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus 4 NS5 full length RdRp activity using RNA as template assessed as inhibition of NTPs incorporation after 90 mins by FAPA a... |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50274120

(CHEMBL3417270)Show SMILES Nc1ccn([C@@H]2O[C@@](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)(N=[N+]=[N-])[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H15N6O14P3/c10-4-1-2-15(8(18)12-4)7-5(16)6(17)9(27-7,13-14-11)3-26-31(22,23)29-32(24,25)28-30(19,20)21/h1-2,5-7,16-17H,3H2,(H,22,23)(H,24,25)(H2,10,12,18)(H2,19,20,21)/t5-,6+,7-,9-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus 2 NS5 full length RdRp activity using RNA as template/primer assessed as inhibition of [alpha3P]GTP incorporation by SDS-P... |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM50241178

(CHEMBL4070442)Show SMILES C[C@]1(O)CCN(C1)c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H](O)[C@@H](O)C1 |r| Show InChI InChI=1S/C16H19F3N4O3/c1-15(26)2-3-22(8-15)14-9(5-20)10(16(17,18)19)4-13(21-14)23-6-11(24)12(25)7-23/h4,11-12,24-26H,2-3,6-8H2,1H3/t11-,12-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50511110

(CHEMBL4530491)Show SMILES CN1CCN(CC1)c1nc(CNc2ncc(Cl)cn2)cc(Oc2ccc(CS(N)(=O)=O)cc2)n1 Show InChI InChI=1S/C21H25ClN8O3S/c1-29-6-8-30(9-7-29)21-27-17(13-26-20-24-11-16(22)12-25-20)10-19(28-21)33-18-4-2-15(3-5-18)14-34(23,31)32/h2-5,10-12H,6-9,13-14H2,1H3,(H2,23,31,32)(H,24,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 579 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged human DGAT2 expressed in SF9 cells after 1 hr by TopCount assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50274124
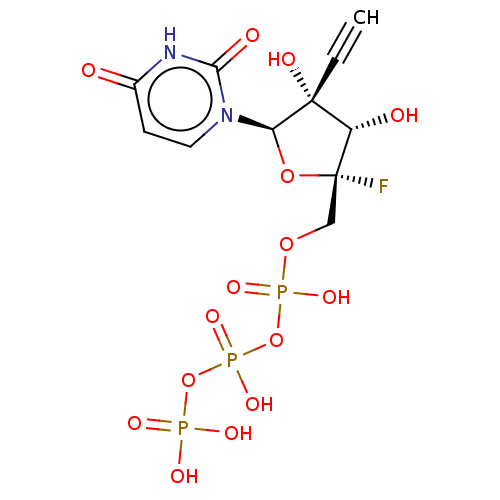
(CHEMBL4125966 | US10793591, Compound 12)Show SMILES O[C@@H]1[C@@](F)(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@@H](n2ccc(=O)[nH]c2=O)[C@@]1(O)C#C |r| Show InChI InChI=1S/C11H14FN2O15P3/c1-2-10(18)7(16)11(12,27-8(10)14-4-3-6(15)13-9(14)17)5-26-31(22,23)29-32(24,25)28-30(19,20)21/h1,3-4,7-8,16,18H,5H2,(H,22,23)(H,24,25)(H,13,15,17)(H2,19,20,21)/t7-,8+,10+,11+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus RdRp activity using 5'-(TCAG)20(TCCAAG)14(TCAG)20-3' as template measured after 120 mins |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50103521
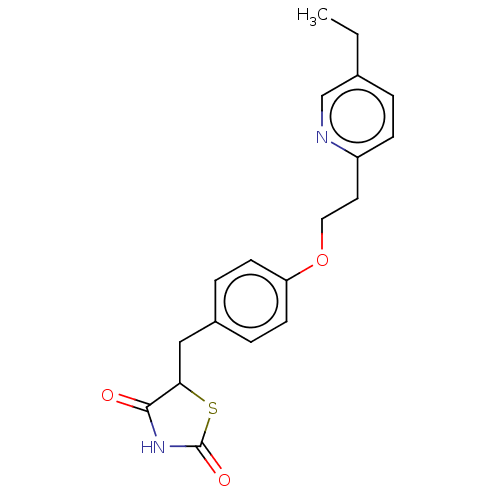
(Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of mouse liver PPARalpha |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50164648
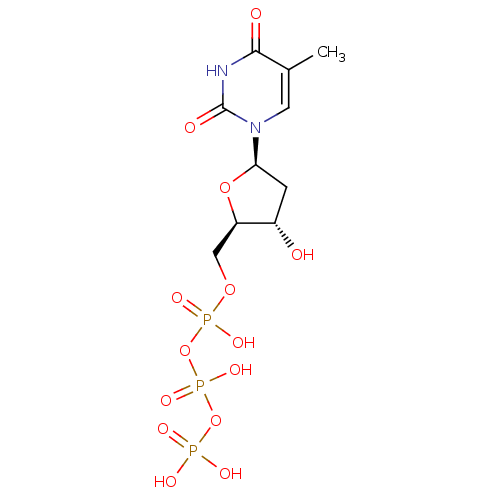
(2'-deoxythymidine triphosphate | 5'-TTP | CHEMBL36...)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H17N2O14P3/c1-5-3-12(10(15)11-9(5)14)8-2-6(13)7(24-8)4-23-28(19,20)26-29(21,22)25-27(16,17)18/h3,6-8,13H,2,4H2,1H3,(H,19,20)(H,21,22)(H,11,14,15)(H2,16,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase-beta |
Bioorg Med Chem Lett 27: 1840-1847 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.037
BindingDB Entry DOI: 10.7270/Q2M047Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein
(Dengue virus) | BDBM50274121

(CHEMBL4127092)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@@H](n2ccc(=O)[nH]c2=O)[C@@]1(O)C#C |r| Show InChI InChI=1S/C11H15N2O15P3/c1-2-11(17)8(15)6(26-9(11)13-4-3-7(14)12-10(13)16)5-25-30(21,22)28-31(23,24)27-29(18,19)20/h1,3-4,6,8-9,15,17H,5H2,(H,21,22)(H,23,24)(H,12,14,16)(H2,18,19,20)/t6-,8-,9-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus RdRp activity using 5'-(TCAG)20(TCCAAG)14(TCAG)20-3' as template measured after 120 mins |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50274122
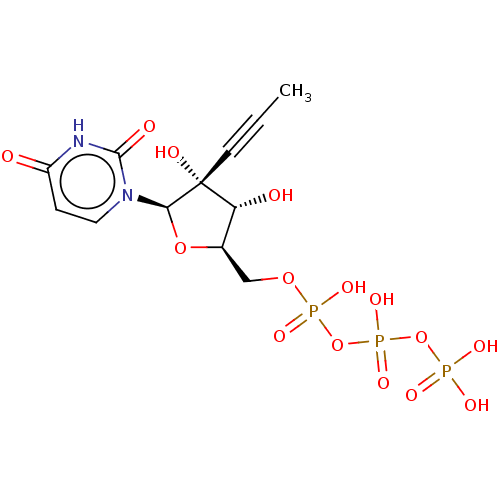
(CHEMBL4127819)Show SMILES CC#C[C@@]1(O)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C12H17N2O15P3/c1-2-4-12(18)9(16)7(27-10(12)14-5-3-8(15)13-11(14)17)6-26-31(22,23)29-32(24,25)28-30(19,20)21/h3,5,7,9-10,16,18H,6H2,1H3,(H,22,23)(H,24,25)(H,13,15,17)(H2,19,20,21)/t7-,9-,10-,12-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus RdRp activity using 5'-(TCAG)20(TCCAAG)14(TCAG)20-3' as template measured after 120 mins |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50274125
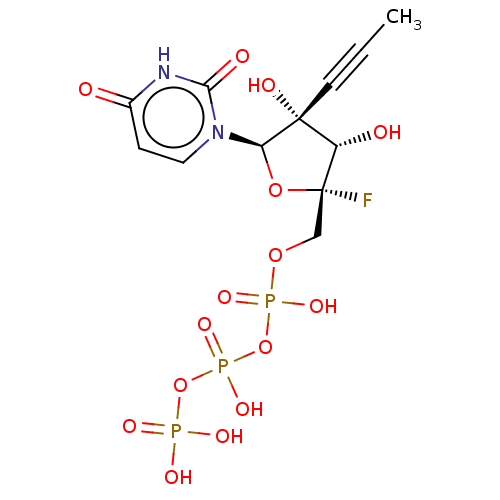
(CHEMBL4127565)Show SMILES CC#C[C@@]1(O)[C@H](O)[C@@](F)(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C12H16FN2O15P3/c1-2-4-11(19)8(17)12(13,28-9(11)15-5-3-7(16)14-10(15)18)6-27-32(23,24)30-33(25,26)29-31(20,21)22/h3,5,8-9,17,19H,6H2,1H3,(H,23,24)(H,25,26)(H,14,16,18)(H2,20,21,22)/t8-,9+,11+,12+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus RdRp activity using 5'-(TCAG)20(TCCAAG)14(TCAG)20-3' as template measured after 120 mins |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50511111

(CHEMBL4476176)Show SMILES CCOC(=O)c1sc2n(CCc3ccccc3)c(=O)n(CCO)c(=O)c2c1C Show InChI InChI=1S/C20H22N2O5S/c1-3-27-19(25)16-13(2)15-17(24)21(11-12-23)20(26)22(18(15)28-16)10-9-14-7-5-4-6-8-14/h4-8,23H,3,9-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50271224
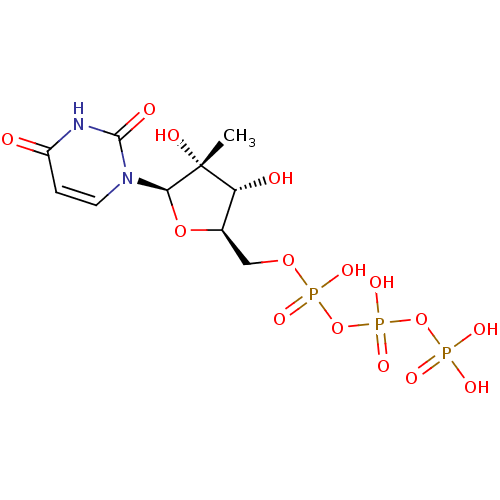
(2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H17N2O15P3/c1-10(16)7(14)5(25-8(10)12-3-2-6(13)11-9(12)15)4-24-29(20,21)27-30(22,23)26-28(17,18)19/h2-3,5,7-8,14,16H,4H2,1H3,(H,20,21)(H,22,23)(H,11,13,15)(H2,17,18,19)/t5-,7-,8-,10-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus RdRp activity using 5'-(TCAG)20(TCCAAG)14(TCAG)20-3' as template measured after 120 mins |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50274118
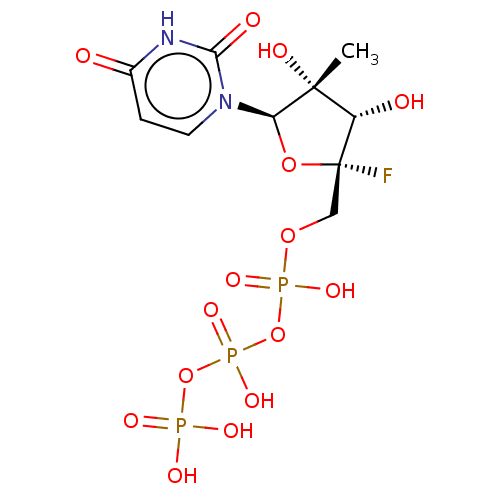
(CHEMBL4127030 | US10793591, Compound 7a)Show SMILES C[C@@]1(O)[C@H](O)[C@@](F)(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H16FN2O15P3/c1-9(17)6(15)10(11,26-7(9)13-3-2-5(14)12-8(13)16)4-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-3,6-7,15,17H,4H2,1H3,(H,21,22)(H,23,24)(H,12,14,16)(H2,18,19,20)/t6-,7+,9+,10+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus RdRp activity using 5'-(TCAG)20(TCCAAG)14(TCAG)20-3' as template measured after 120 mins |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50511111

(CHEMBL4476176)Show SMILES CCOC(=O)c1sc2n(CCc3ccccc3)c(=O)n(CCO)c(=O)c2c1C Show InChI InChI=1S/C20H22N2O5S/c1-3-27-19(25)16-13(2)15-17(24)21(11-12-23)20(26)22(18(15)28-16)10-9-14-7-5-4-6-8-14/h4-8,23H,3,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50274117
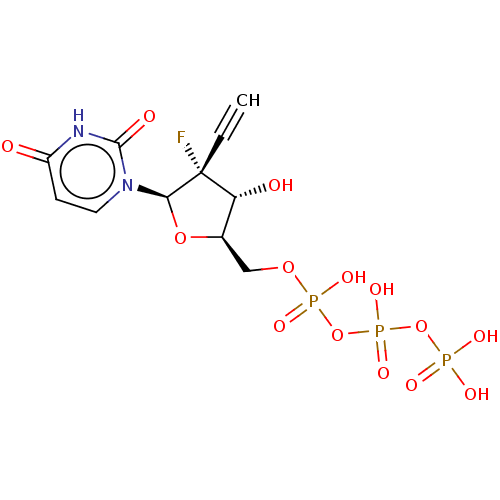
(CHEMBL4127921)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@@H](n2ccc(=O)[nH]c2=O)[C@@]1(F)C#C |r| Show InChI InChI=1S/C11H14FN2O14P3/c1-2-11(12)8(16)6(26-9(11)14-4-3-7(15)13-10(14)17)5-25-30(21,22)28-31(23,24)27-29(18,19)20/h1,3-4,6,8-9,16H,5H2,(H,21,22)(H,23,24)(H,13,15,17)(H2,18,19,20)/t6-,8-,9-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus RdRp activity using 5'-(TCAG)20(TCCAAG)14(TCAG)20-3' as template measured after 120 mins |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50333129
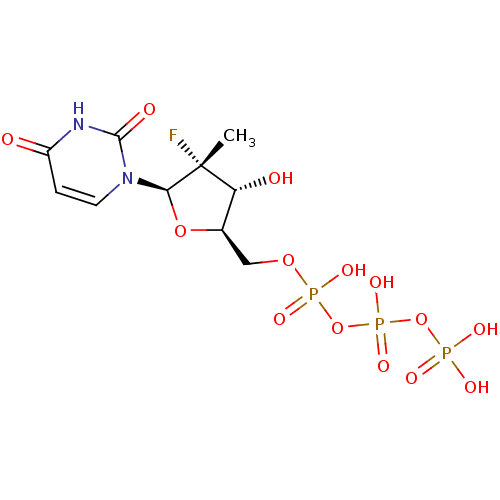
(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...)Show SMILES C[C@@]1(F)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H16FN2O14P3/c1-10(11)7(15)5(25-8(10)13-3-2-6(14)12-9(13)16)4-24-29(20,21)27-30(22,23)26-28(17,18)19/h2-3,5,7-8,15H,4H2,1H3,(H,20,21)(H,22,23)(H,12,14,16)(H2,17,18,19)/t5-,7-,8-,10-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus RdRp activity using 5'-(TCAG)20(TCCAAG)14(TCAG)20-3' as template measured after 120 mins |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50511113
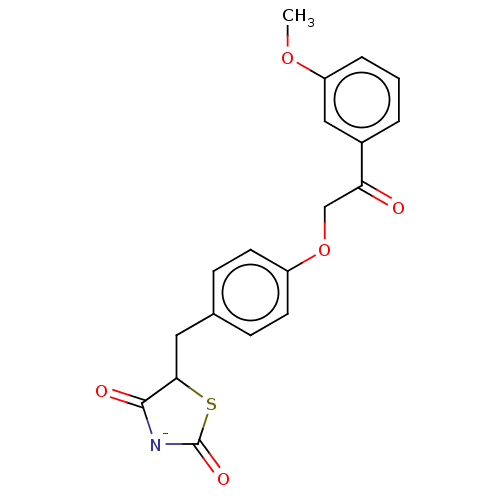
(CHEMBL4593380)Show SMILES [K;v0+].[#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#16]-[#6](=O)-[#7-]-[#6]-2=O)cc1 Show InChI InChI=1S/C19H17NO5S.K/c1-24-15-4-2-3-13(10-15)16(21)11-25-14-7-5-12(6-8-14)9-17-18(22)20-19(23)26-17;/h2-8,10,17H,9,11H2,1H3,(H,20,22,23);/q;+1/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of mouse liver PPARalpha |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus) | BDBM50274123

(CHEMBL4129313)Show SMILES CC#C[C@@]1(F)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C12H16FN2O14P3/c1-2-4-12(13)9(17)7(27-10(12)15-5-3-8(16)14-11(15)18)6-26-31(22,23)29-32(24,25)28-30(19,20)21/h3,5,7,9-10,17H,6H2,1H3,(H,22,23)(H,24,25)(H,14,16,18)(H2,19,20,21)/t7-,9-,10-,12-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of Dengue virus RdRp activity using 5'-(TCAG)20(TCCAAG)14(TCAG)20-3' as template measured after 120 mins |
Bioorg Med Chem Lett 28: 2324-2327 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.069
BindingDB Entry DOI: 10.7270/Q24T6MWH |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50237537
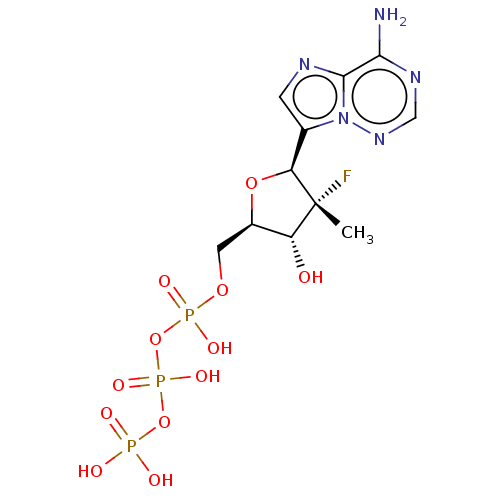
(CHEMBL4093601)Show SMILES C[C@@]1(F)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1c1cnc2c(N)ncnn12 |r| Show InChI InChI=1S/C11H17FN5O12P3/c1-11(12)7(18)6(3-26-31(22,23)29-32(24,25)28-30(19,20)21)27-8(11)5-2-14-10-9(13)15-4-16-17(5)10/h2,4,6-8,18H,3H2,1H3,(H,22,23)(H,24,25)(H2,13,15,16)(H2,19,20,21)/t6-,7-,8+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase-beta |
Bioorg Med Chem Lett 27: 1840-1847 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.037
BindingDB Entry DOI: 10.7270/Q2M047Q1 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50399710

(CHEMBL2178953)Show SMILES Cc1nc(C)c(nc1C(N)=O)-c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:17.18,wD:20.22,(61.46,-10.56,;62.79,-9.79,;64.13,-10.56,;65.46,-9.79,;66.8,-10.56,;65.46,-8.26,;64.13,-7.48,;62.79,-8.25,;61.46,-7.48,;60.12,-8.25,;61.46,-5.94,;66.79,-7.49,;68.12,-8.26,;69.46,-7.49,;69.46,-5.94,;68.12,-5.17,;66.79,-5.94,;70.79,-5.16,;70.78,-3.63,;72.12,-2.86,;73.45,-3.62,;74.79,-2.85,;76.12,-3.62,;77.46,-2.85,;76.12,-5.16,;73.45,-5.17,;72.13,-5.93,)| Show InChI InChI=1S/C21H25N3O3/c1-12-19(24-20(21(22)27)13(2)23-12)17-9-7-16(8-10-17)15-5-3-14(4-6-15)11-18(25)26/h7-10,14-15H,3-6,11H2,1-2H3,(H2,22,27)(H,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged human DGAT1 expressed in SF9 cells after 1 hr by TopCount assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM50241205

(CHEMBL1463512)Show InChI InChI=1S/C9H5F3N2O/c10-9(11,12)7-8(15)14-6-4-2-1-3-5(6)13-7/h1-4H,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM50241206
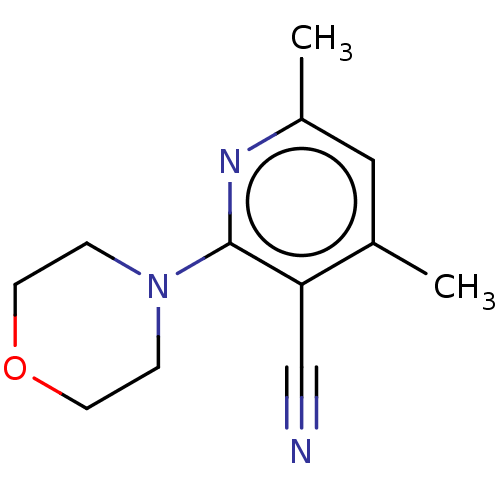
(CHEMBL1500542)Show InChI InChI=1S/C12H15N3O/c1-9-7-10(2)14-12(11(9)8-13)15-3-5-16-6-4-15/h7H,3-6H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor alpha
(Homo sapiens (Human)) | US20240059682, Example 9
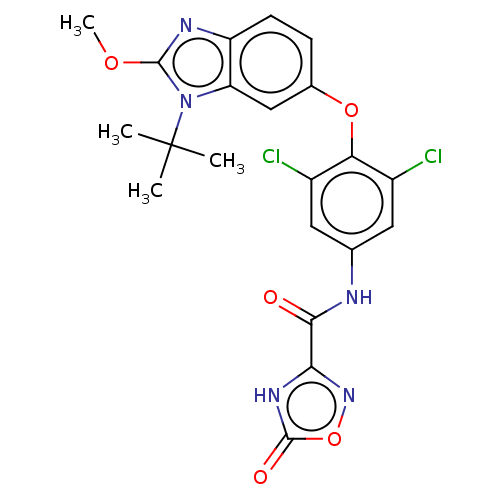
(N-(4-((1-(tert-butyl)-2-methoxy-1H-benzo[d]imidazo...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor beta
(Homo sapiens (Human)) | US20240059682, Example 10

(N-(4-((3-(tert-butyl)-2-oxo-2,3-dihydro-1H-benzo[d...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor alpha
(Homo sapiens (Human)) | US20240059682, Example 10

(N-(4-((3-(tert-butyl)-2-oxo-2,3-dihydro-1H-benzo[d...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor beta
(Homo sapiens (Human)) | US20240059682, Example 11

(N-(3,5-dichloro-4-((2-methoxy-1-(1-methylcycloprop...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor alpha
(Homo sapiens (Human)) | US20240059682, Example 11

(N-(3,5-dichloro-4-((2-methoxy-1-(1-methylcycloprop...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor beta
(Homo sapiens (Human)) | US20240059682, Example 12

(2-(3,5-dichloro-4-((2-methoxy-1-(1-methylcycloprop...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor alpha
(Homo sapiens (Human)) | US20240059682, Example 12

(2-(3,5-dichloro-4-((2-methoxy-1-(1-methylcycloprop...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor beta
(Homo sapiens (Human)) | US20240059682, Example 13
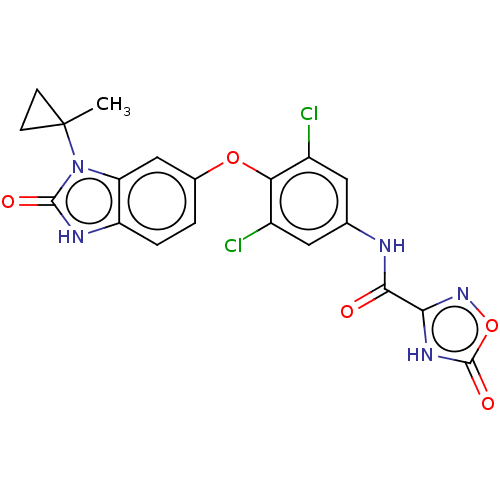
(N-(3,5-dichloro-4-((3-(1-methylcyclopropyl)-2-oxo-...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor alpha
(Homo sapiens (Human)) | US20240059682, Example 13

(N-(3,5-dichloro-4-((3-(1-methylcyclopropyl)-2-oxo-...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor beta
(Homo sapiens (Human)) | US20240059682, Example 14
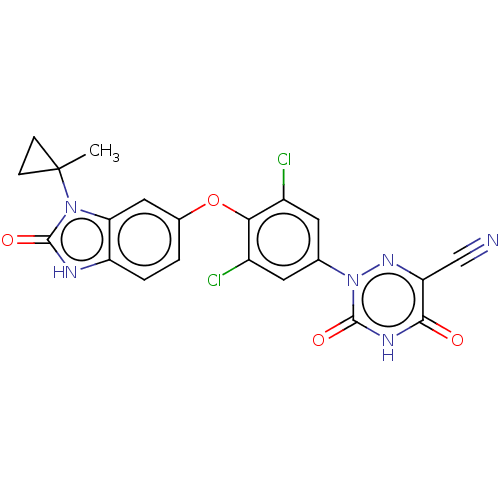
(2-(3,5-dichloro-4-((3-(1-methylcyclopropyl)-2-oxo-...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor alpha
(Homo sapiens (Human)) | US20240059682, Example 14

(2-(3,5-dichloro-4-((3-(1-methylcyclopropyl)-2-oxo-...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor beta
(Homo sapiens (Human)) | US20240059682, Example 15
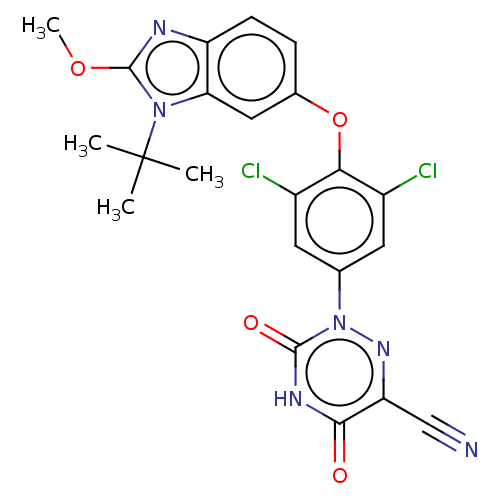
(2-(4-((1-(tert-butyl)-2-methoxy-1H-benzo[d] imidaz...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Thyroid hormone receptor alpha
(Homo sapiens (Human)) | US20240059682, Example 15

(2-(4-((1-(tert-butyl)-2-methoxy-1H-benzo[d] imidaz...) | GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data