

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210579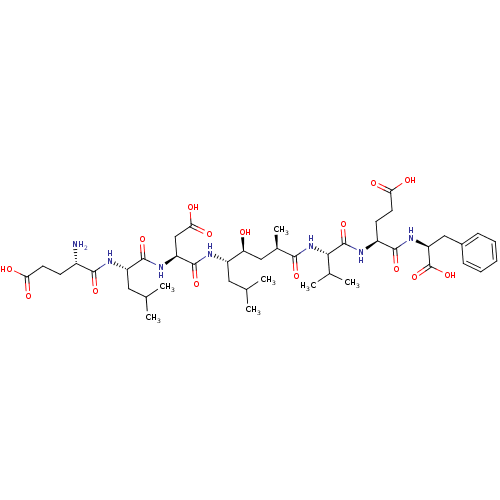 ((2S,5S,8S,11R,13S,14S,17S,20S,23S)-23-amino-2-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of active BACE1 (unknown origin) | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210579 ((2S,5S,8S,11R,13S,14S,17S,20S,23S)-23-amino-2-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of active BACE1 (unknown origin) | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169541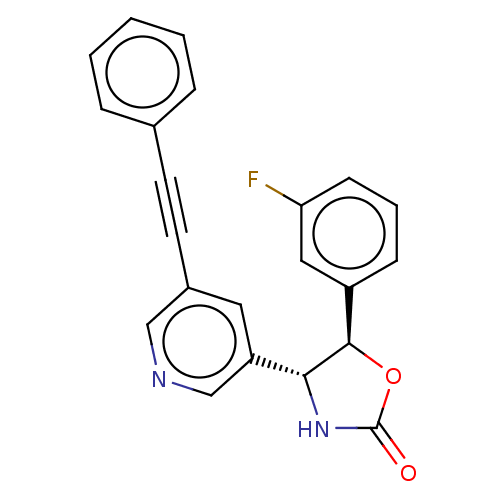 (CHEMBL3805542) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEPgamma in mGlu5 receptor (unknown origin) incubated for 60 mins by liquid scintillation counting method | ACS Med Chem Lett 7: 289-93 (2016) Article DOI: 10.1021/acsmedchemlett.5b00450 BindingDB Entry DOI: 10.7270/Q28G8NMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169542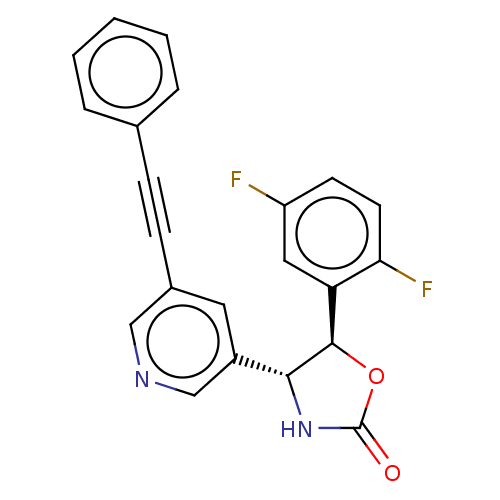 (CHEMBL3804846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEPgamma in mGlu5 receptor (unknown origin) incubated for 60 mins by liquid scintillation counting method | ACS Med Chem Lett 7: 289-93 (2016) Article DOI: 10.1021/acsmedchemlett.5b00450 BindingDB Entry DOI: 10.7270/Q28G8NMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50094979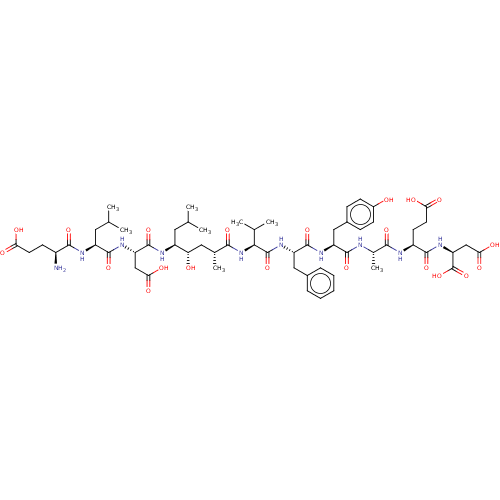 (CHEMBL3589002) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50094977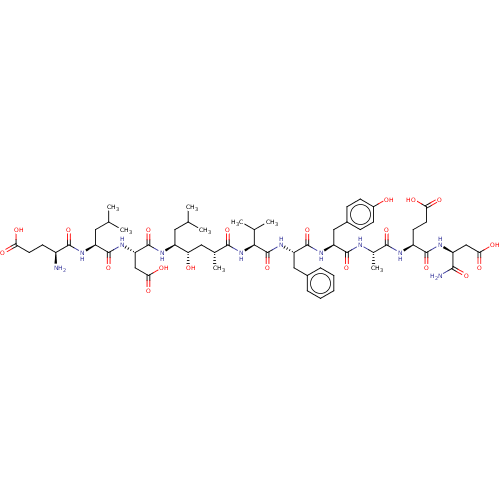 (CHEMBL3589000) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50094978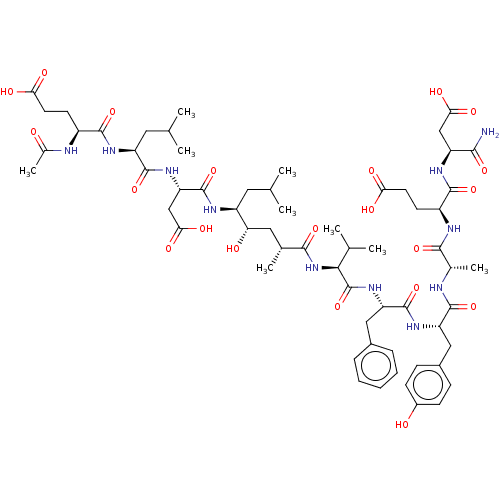 (CHEMBL3589001) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50094976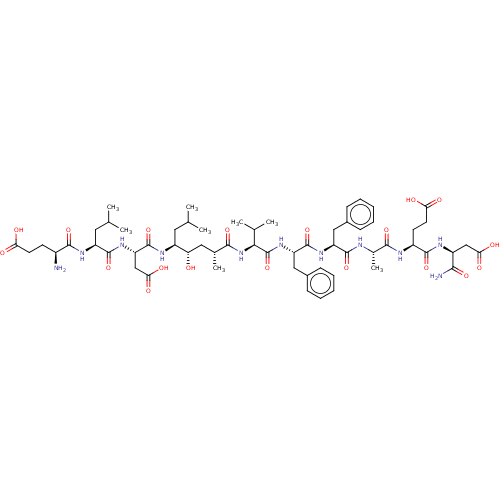 (CHEMBL3588999) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 2 hrs by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50210579 ((2S,5S,8S,11R,13S,14S,17S,20S,23S)-23-amino-2-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552436 (CHEMBL4749654) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552434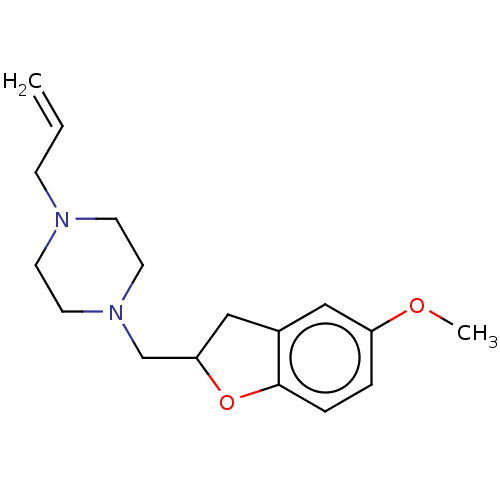 (CHEMBL4747180) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169480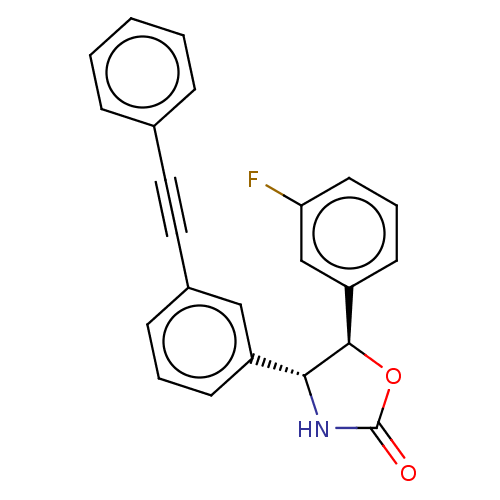 (CHEMBL3805340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEP in mGlu5 receptor (unknown origin) incubated for 60 mins by liquid scintillation counting method | ACS Med Chem Lett 7: 289-93 (2016) Article DOI: 10.1021/acsmedchemlett.5b00450 BindingDB Entry DOI: 10.7270/Q28G8NMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50094976 (CHEMBL3588999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of pro-BACE1 (unknown origin) | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169544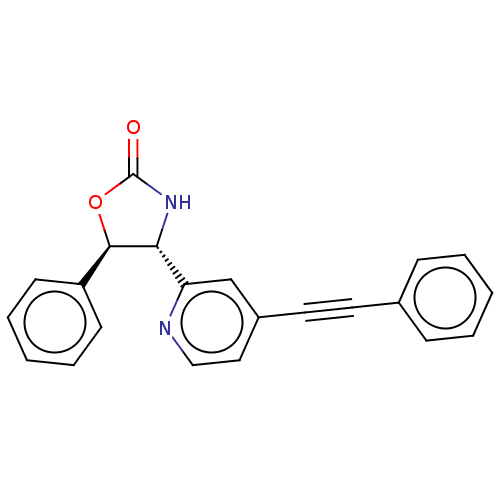 (CHEMBL3805655) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEPgamma in mGlu5 receptor (unknown origin) incubated for 60 mins by liquid scintillation counting method | ACS Med Chem Lett 7: 289-93 (2016) Article DOI: 10.1021/acsmedchemlett.5b00450 BindingDB Entry DOI: 10.7270/Q28G8NMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169457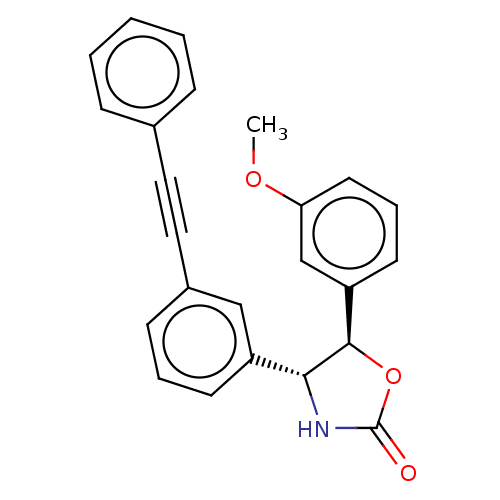 (CHEMBL3805652) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEPgamma in mGlu5 receptor (unknown origin) incubated for 60 mins by liquid scintillation counting method | ACS Med Chem Lett 7: 289-93 (2016) Article DOI: 10.1021/acsmedchemlett.5b00450 BindingDB Entry DOI: 10.7270/Q28G8NMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50094979 (CHEMBL3589002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50094977 (CHEMBL3589000) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552435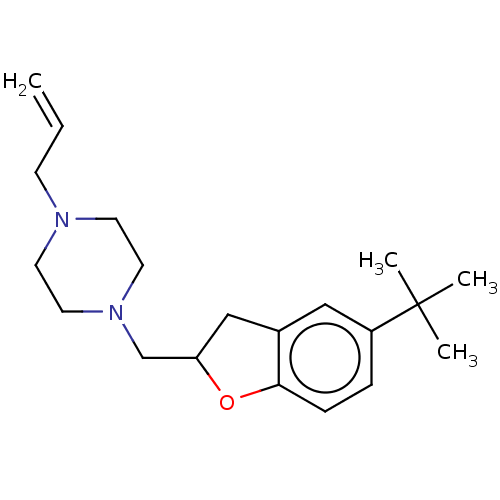 (CHEMBL4756432) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50094978 (CHEMBL3589001) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Apparent inhibition of human recombinant BACE2 using Mca-SEVNLDAEFK-DNP substrate assessed as substrate hydrolysis after 1 hr by HPLC-FLU analysis | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169543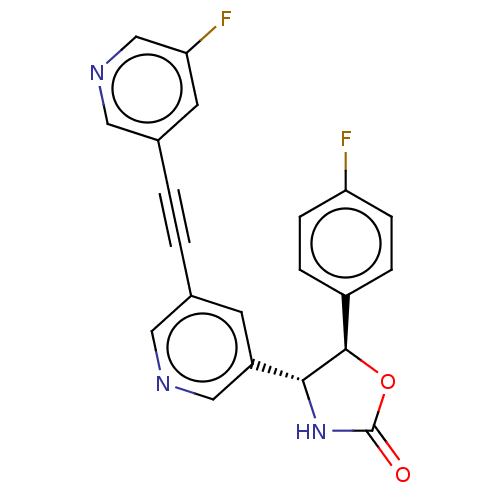 (CHEMBL3806078) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEPgamma in mGlu5 receptor (unknown origin) incubated for 60 mins by liquid scintillation counting method | ACS Med Chem Lett 7: 289-93 (2016) Article DOI: 10.1021/acsmedchemlett.5b00450 BindingDB Entry DOI: 10.7270/Q28G8NMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557298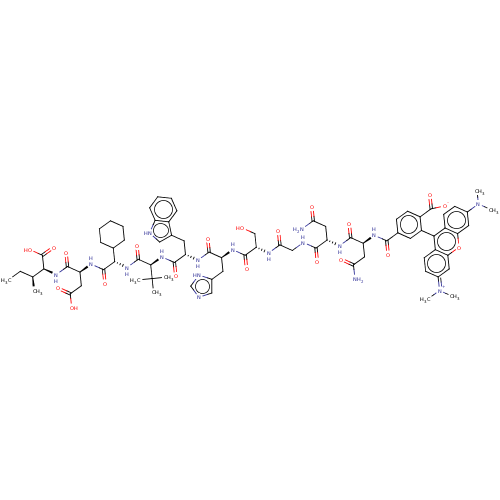 (CHEMBL4750927) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557293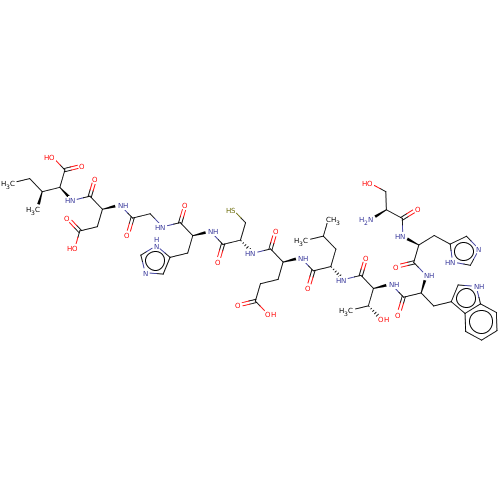 (CHEMBL4781637) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552430 (CHEMBL4798369) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50552439 (CHEMBL4568949) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50402966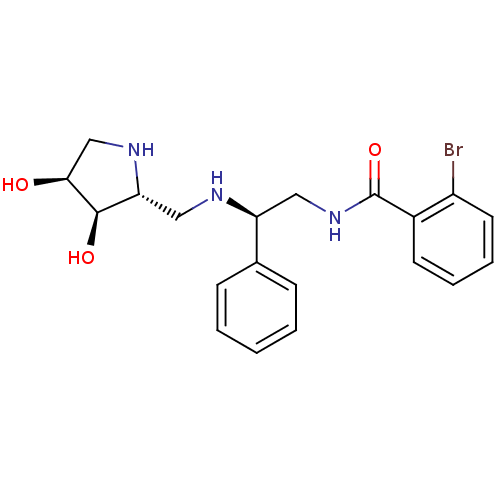 (CHEMBL2206824) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557299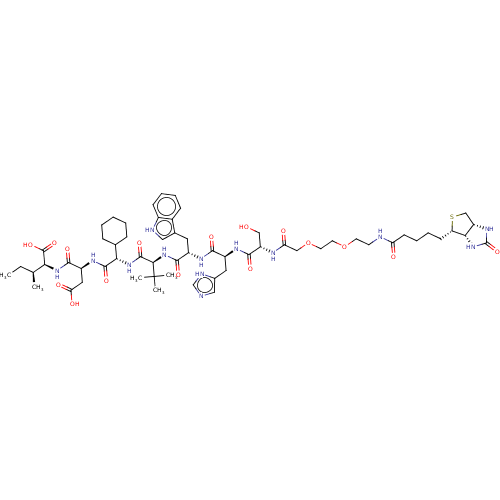 (CHEMBL4799812) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) by SPR analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557299 (CHEMBL4799812) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50402972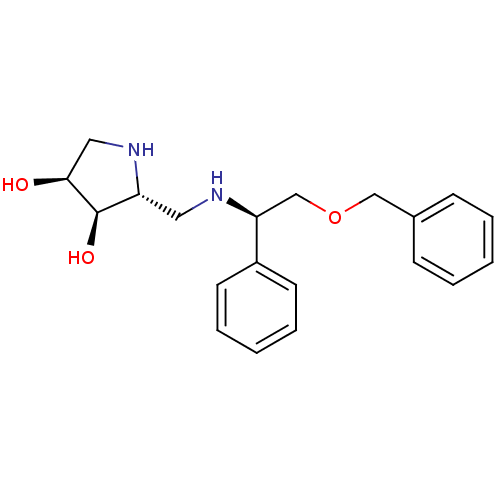 (CHEMBL2206818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557295 (CHEMBL4758599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557297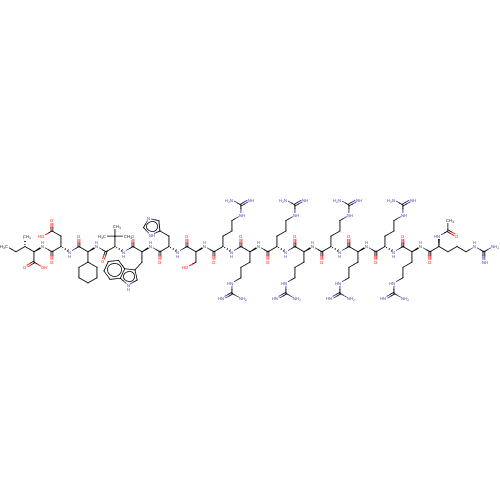 (CHEMBL4747962) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557294 (CHEMBL4749490) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Syntenin-1 (Homo sapiens) | BDBM50557296 (CHEMBL4792861) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00382 BindingDB Entry DOI: 10.7270/Q2V98CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50402967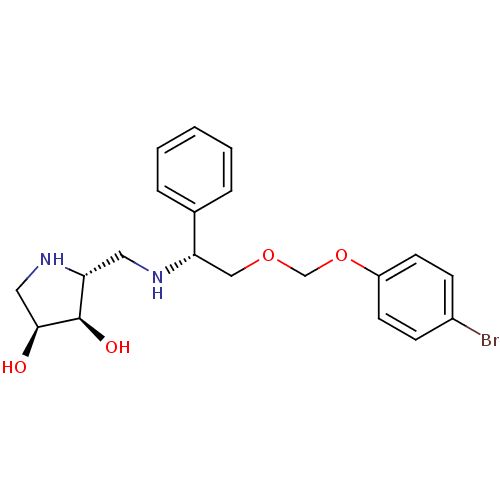 (CHEMBL2206823) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50402968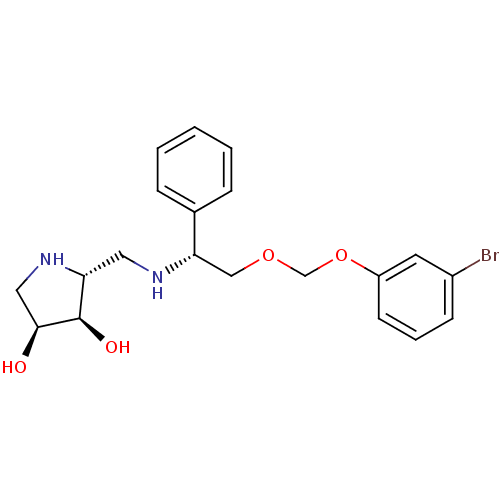 (CHEMBL2206822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552441 (CHEMBL4747747) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50402969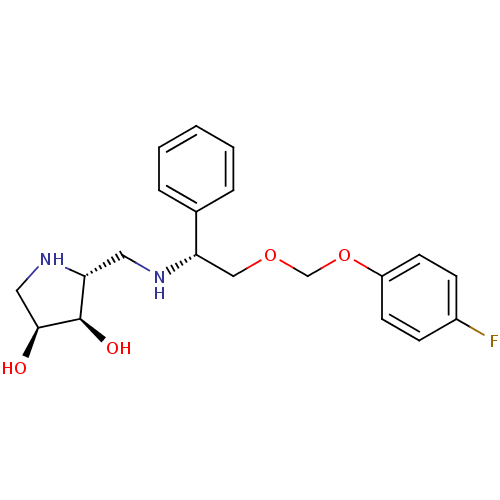 (CHEMBL2206821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552429 (CHEMBL4752345) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50402971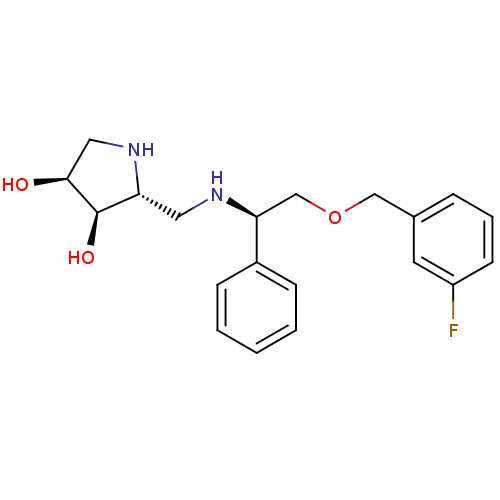 (CHEMBL2206819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50169002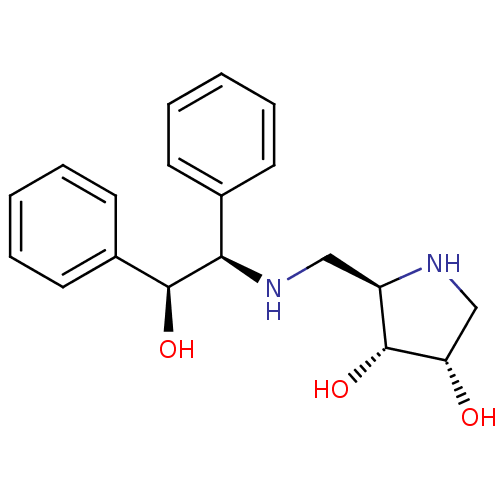 ((2R,3R,4S)-2-[((S)-Phenyl-1-(R)-2-hydroxy-2-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552444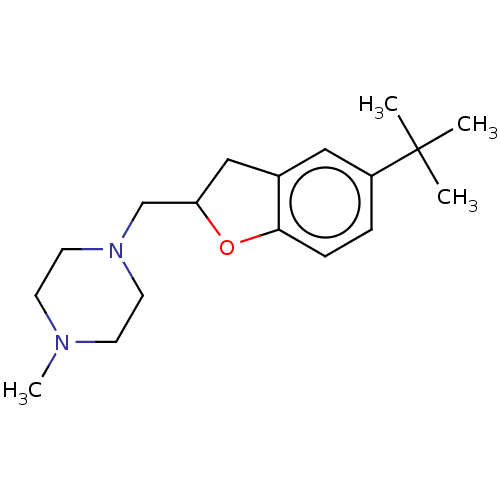 (CHEMBL4764535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50552442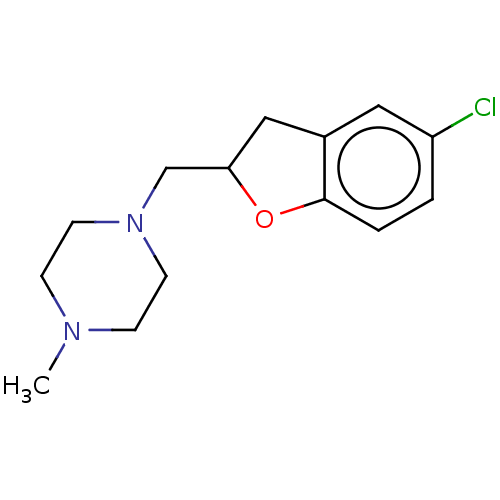 (CHEMBL4792753) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552443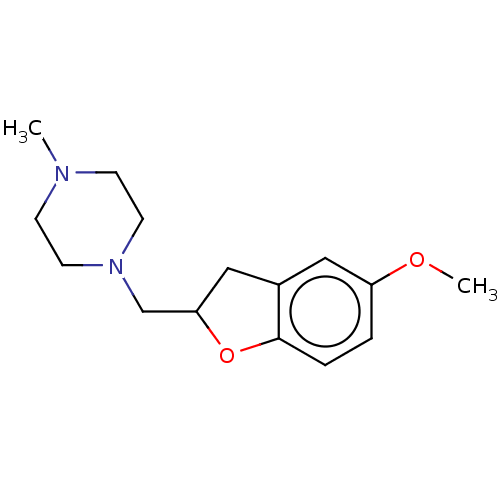 (CHEMBL4758983) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50552442 (CHEMBL4792753) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115924 BindingDB Entry DOI: 10.7270/Q2833WNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50402970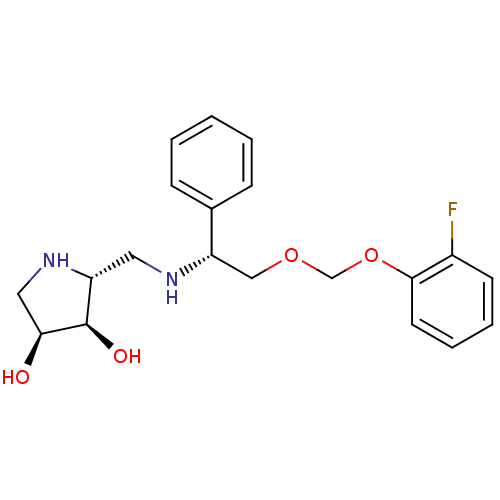 (CHEMBL2206820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50234567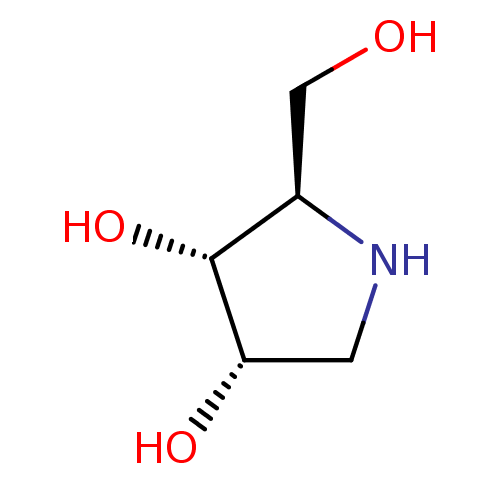 (1,4-dideoxy-1,4-imino-D-ribitol | CHEMBL261634 | I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50402989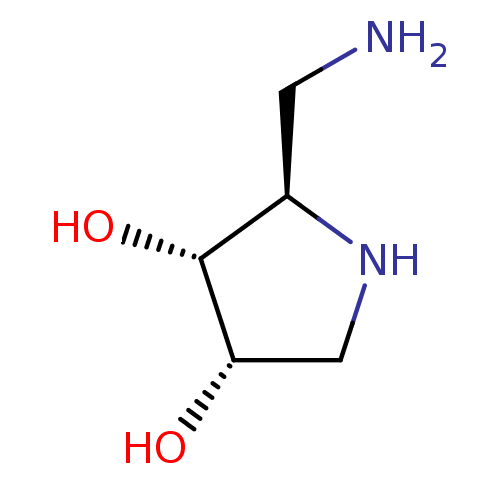 (CHEMBL2206825) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 248 total ) | Next | Last >> |