

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50453020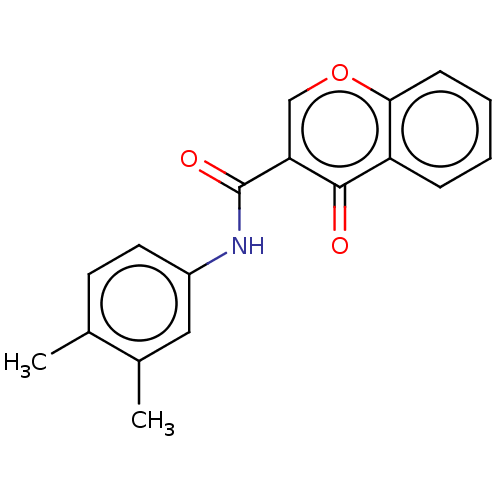 (CHEMBL4209203) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Non-competitive inhibition of human microsomal MAO-B expressed in recombinant baculovirus infected insect BTI-TN-5B1-4 cells assessed as reduction in... | J Med Chem 59: 5879-93 (2016) Article DOI: 10.1021/acs.jmedchem.6b00527 BindingDB Entry DOI: 10.7270/Q2X92FSJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50453019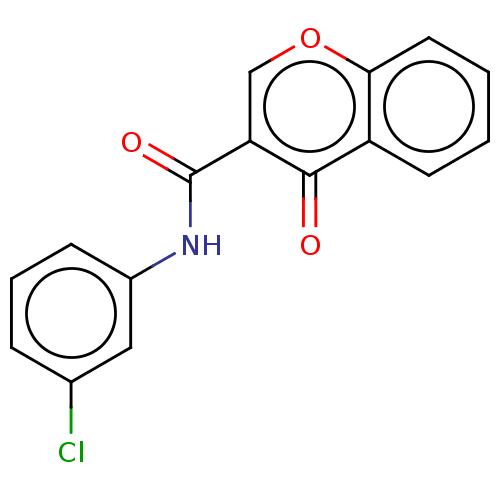 (CHEMBL4206812) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Competitive inhibition of human microsomal MAO-B expressed in recombinant baculovirus infected insect BTI-TN-5B1-4 cells assessed as reduction in H2O... | J Med Chem 59: 5879-93 (2016) Article DOI: 10.1021/acs.jmedchem.6b00527 BindingDB Entry DOI: 10.7270/Q2X92FSJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50504743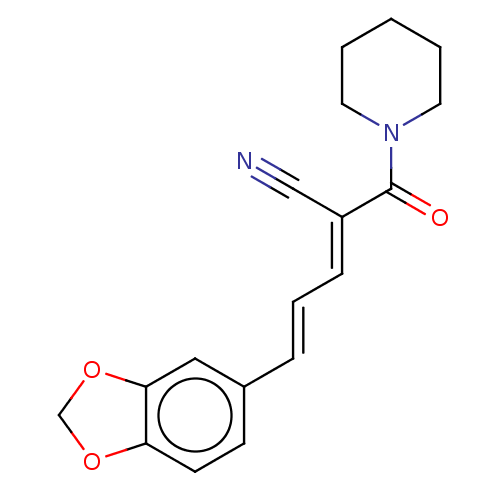 (CHEMBL4464272) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Competitive inhibition of human microsomal MAO-B expressed in baculovirus infected BTI-TN-5B1-4 cells assessed as inhibition constant using kynuramin... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111770 BindingDB Entry DOI: 10.7270/Q21N84DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577843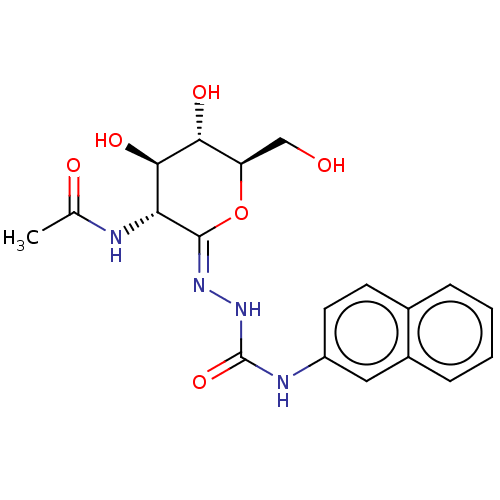 (CHEMBL4853460) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of N-terminal His-tagged human OGA using 4-Mu-GlcNAc as substrate assessed as inhibition constant by measuring liberation of n... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50504739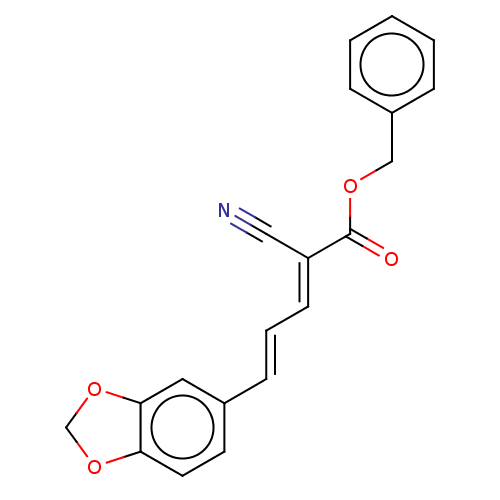 (CHEMBL4585553) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Competitive inhibition of human microsomal MAO-B expressed in baculovirus infected BTI-TN-5B1-4 cells assessed as inhibition constant using kynuramin... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111770 BindingDB Entry DOI: 10.7270/Q21N84DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase (Bos taurus) | BDBM50577843 (CHEMBL4853460) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50531967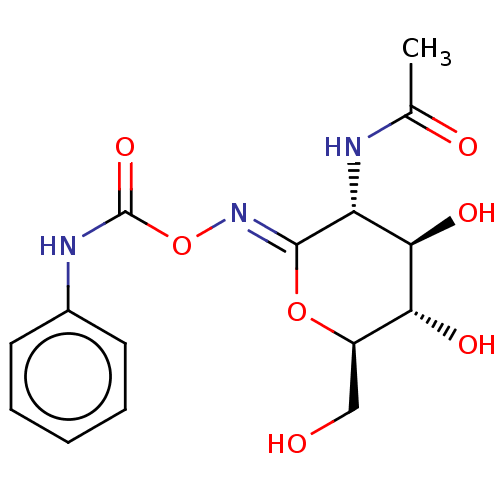 (Pugnac) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of OGA (unknown origin) using pNP-O-GlcNAc as substrate assessed as inhibition constant incubated for 30 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50504740 (CHEMBL4514572) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Competitive inhibition of human microsomal MAO-B expressed in baculovirus infected BTI-TN-5B1-4 cells assessed as inhibition constant using kynuramin... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111770 BindingDB Entry DOI: 10.7270/Q21N84DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577842 (CHEMBL4866781) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of N-terminal His-tagged human OGA using 4-Mu-GlcNAc as substrate assessed as inhibition constant by measuring liberation of n... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase (Bos taurus) | BDBM50577838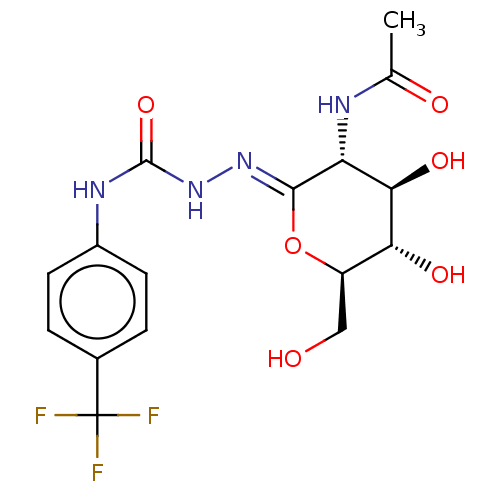 (CHEMBL4874886) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase (Bos taurus) | BDBM50577836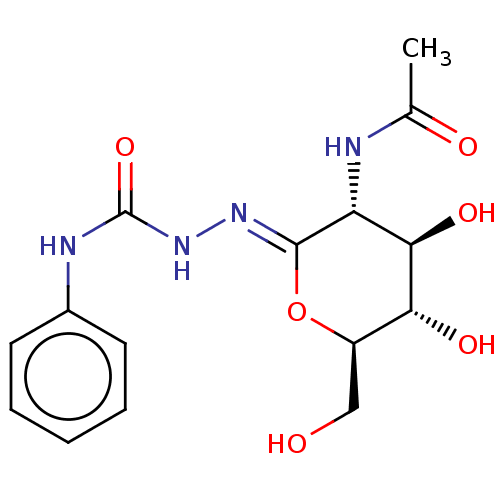 (CHEMBL4861823) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HexA/HexB (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase (Bos taurus) | BDBM50577840 (CHEMBL4864246) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577837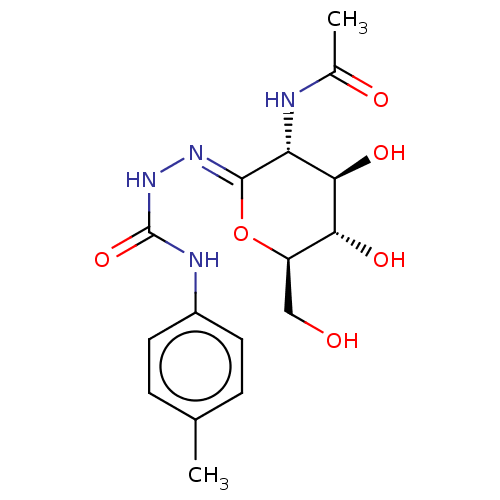 (CHEMBL4875285) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577838 (CHEMBL4874886) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase (Bos taurus) | BDBM50577842 (CHEMBL4866781) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577836 (CHEMBL4861823) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Bos taurus) | BDBM50577836 (CHEMBL4861823) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577839 (CHEMBL4851727) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase (Bos taurus) | BDBM50577837 (CHEMBL4875285) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase (Bos taurus) | BDBM50577839 (CHEMBL4851727) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 413 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577840 (CHEMBL4864246) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 506 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577840 (CHEMBL4864246) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 509 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of N-terminal His-tagged human OGA using 4-Mu-GlcNAc as substrate assessed as inhibition constant by measuring liberation of n... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577844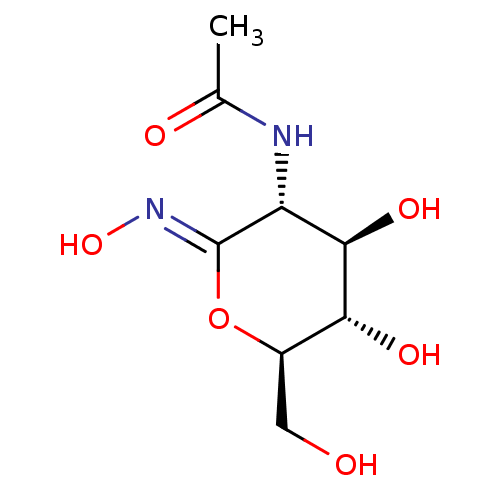 (CHEMBL402605) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of OGA (unknown origin) using pNP-O-GlcNAc as substrate assessed as inhibition constant incubated for 30 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50577841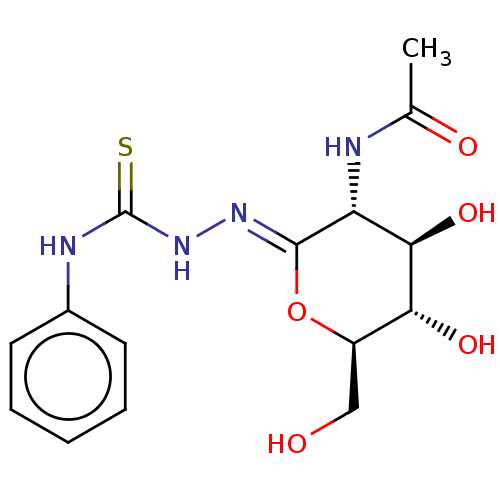 (CHEMBL4845911) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of N-terminal His-tagged human OGA using pNP-GlcNAc as chromogenic substrate assessed as inhibition constant by measuring libe... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase (Bos taurus) | BDBM50577841 (CHEMBL4845911) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of cow HexA/HexB (unknown origin) using pNP-GlcNAc as substrate assessed as inhibition constant by measuring liberation of nit... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113649 BindingDB Entry DOI: 10.7270/Q2PV6Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101929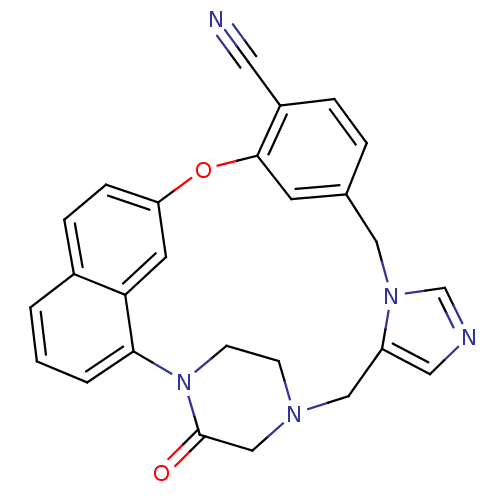 (3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50139186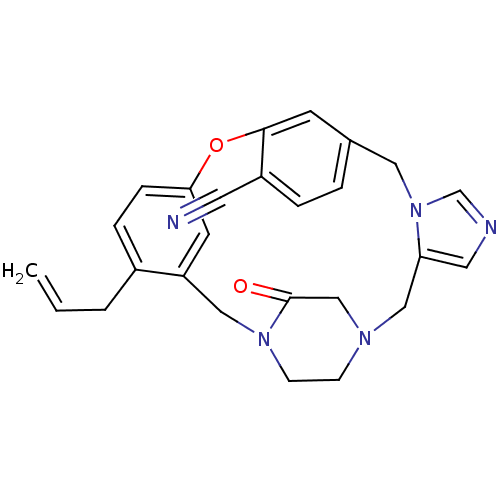 (4-allyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101929 (3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50130373 (4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. | J Med Chem 46: 2973-84 (2003) Article DOI: 10.1021/jm020587n BindingDB Entry DOI: 10.7270/Q26Q1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50115916 (4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP | Bioorg Med Chem Lett 12: 2027-30 (2002) BindingDB Entry DOI: 10.7270/Q2TQ60VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50112379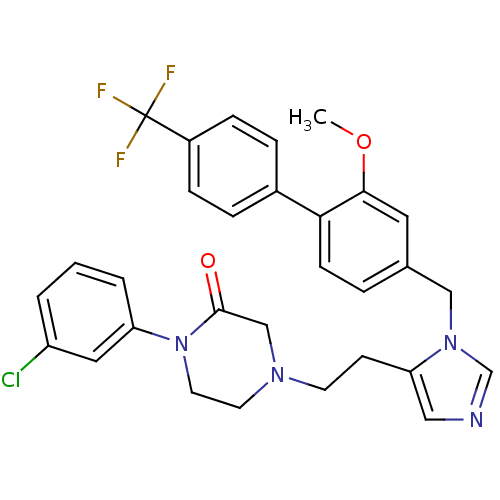 (1-(3-Chloro-phenyl)-4-{2-[3-(2-methoxy-4'-trifluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the human Geranylgeranyl transferase type I catalyzed incorporation of [3H]-GGPP into a biotinylated peptide corresponding to the C-ter... | Bioorg Med Chem Lett 12: 1269-73 (2002) BindingDB Entry DOI: 10.7270/Q2T72GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50130374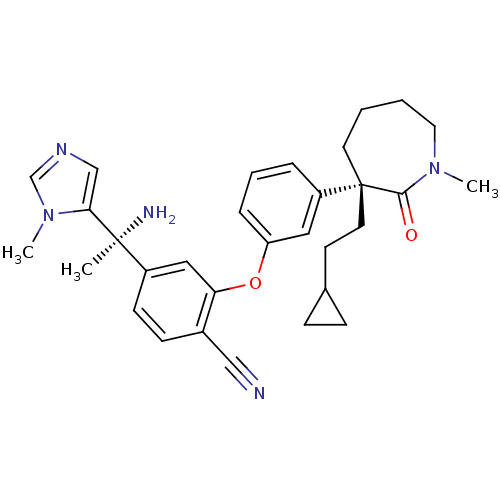 (4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. | J Med Chem 46: 2973-84 (2003) Article DOI: 10.1021/jm020587n BindingDB Entry DOI: 10.7270/Q26Q1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50130381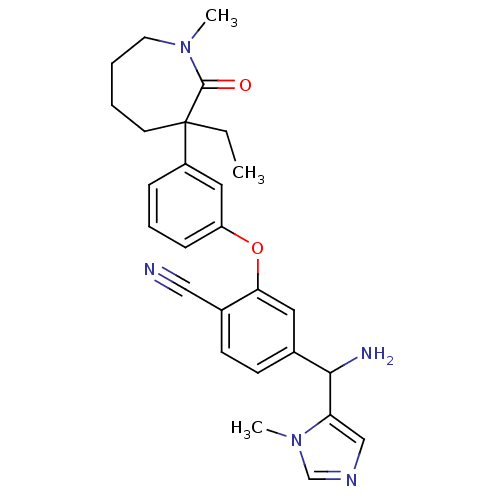 (4-[Amino-(3-methyl-3H-imidazol-4-yl)-methyl]-2-[3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. | J Med Chem 46: 2973-84 (2003) Article DOI: 10.1021/jm020587n BindingDB Entry DOI: 10.7270/Q26Q1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50130365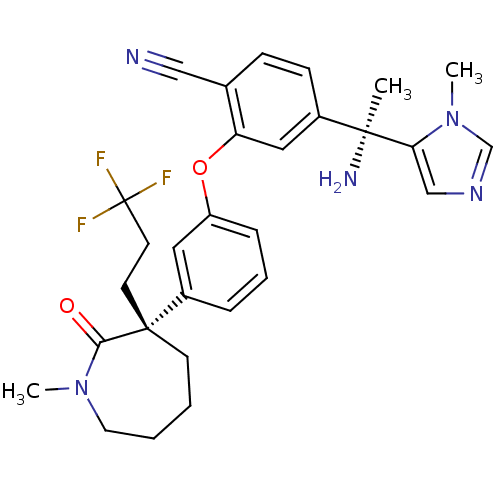 (4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. | J Med Chem 46: 2973-84 (2003) Article DOI: 10.1021/jm020587n BindingDB Entry DOI: 10.7270/Q26Q1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50112387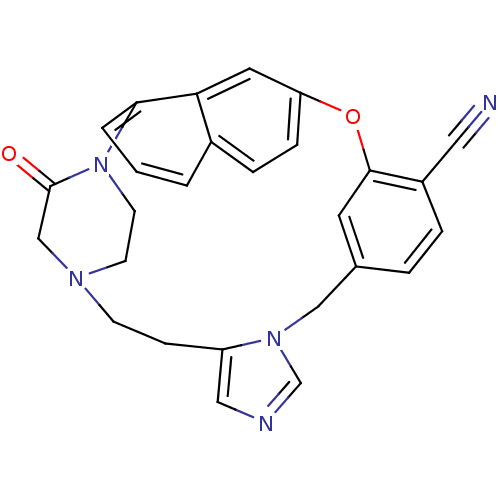 (3-oxo-19-oxa-2,5,10,12-tetraazahexacyclo[18.6.2.22...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the human Geranylgeranyl transferase type I catalyzed incorporation of [3H]-GGPP into a biotinylated peptide corresponding to the C-ter... | Bioorg Med Chem Lett 12: 1269-73 (2002) BindingDB Entry DOI: 10.7270/Q2T72GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50112387 (3-oxo-19-oxa-2,5,10,12-tetraazahexacyclo[18.6.2.22...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. | Bioorg Med Chem Lett 12: 1269-73 (2002) BindingDB Entry DOI: 10.7270/Q2T72GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50139202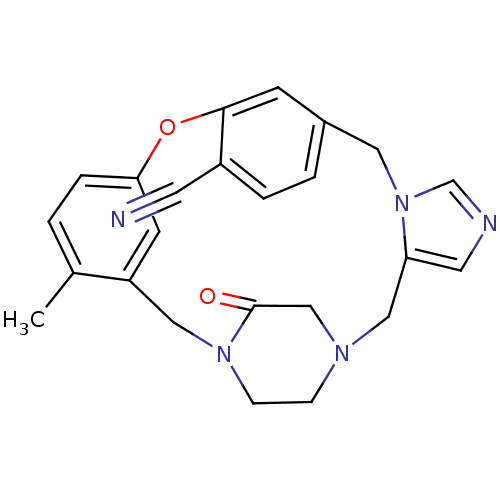 (4-methyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101929 (3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50139196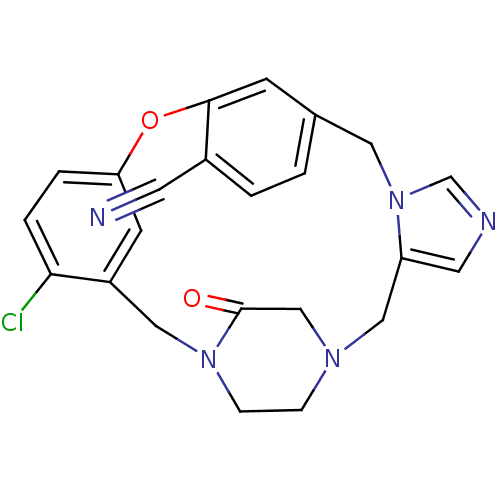 (4-chloro-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50130372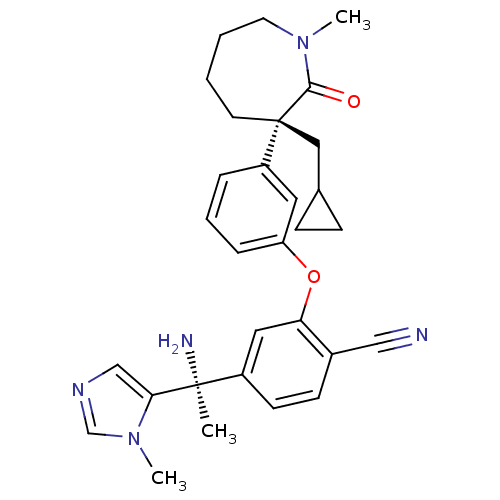 (4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. | J Med Chem 46: 2973-84 (2003) Article DOI: 10.1021/jm020587n BindingDB Entry DOI: 10.7270/Q26Q1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50139190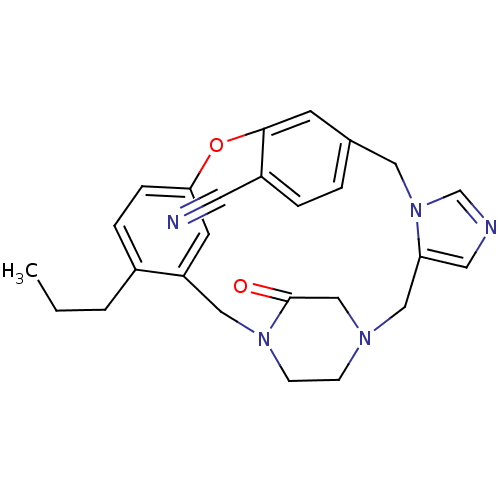 (4-propyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50115919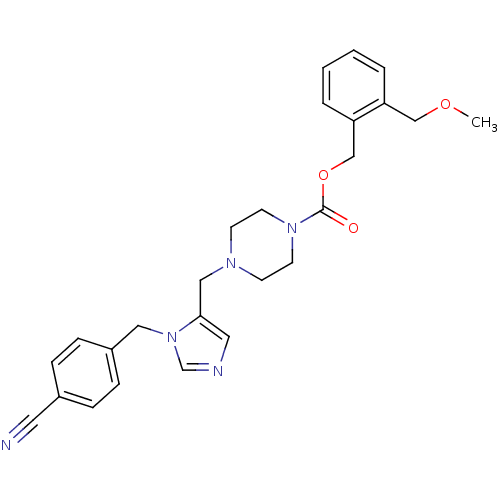 (4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP | Bioorg Med Chem Lett 12: 2027-30 (2002) BindingDB Entry DOI: 10.7270/Q2TQ60VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50115911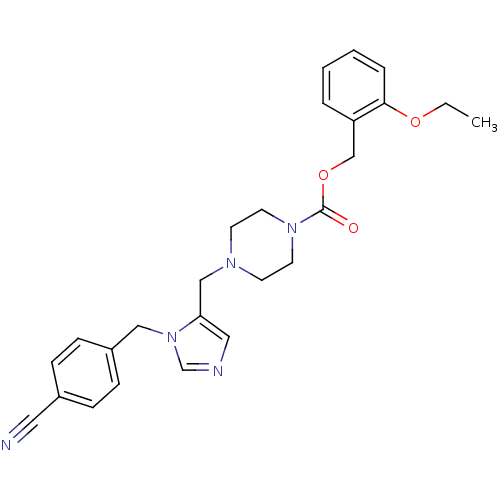 (4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP | Bioorg Med Chem Lett 12: 2027-30 (2002) BindingDB Entry DOI: 10.7270/Q2TQ60VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50139202 (4-methyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. | Bioorg Med Chem Lett 14: 639-43 (2004) BindingDB Entry DOI: 10.7270/Q2WM1CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50115927 (4-[3-(4-Cyano-benzyl)-2-methyl-3H-imidazol-4-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Farnesyl protein transferase radiolabel [1-3H] incorporation | Bioorg Med Chem Lett 12: 2027-30 (2002) BindingDB Entry DOI: 10.7270/Q2TQ60VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50115927 (4-[3-(4-Cyano-benzyl)-2-methyl-3H-imidazol-4-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of radiolabeled farnesyl transferase inhibitor | Bioorg Med Chem Lett 12: 2027-30 (2002) BindingDB Entry DOI: 10.7270/Q2TQ60VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50115916 (4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitor of Farnesyl protein transferase(FTPase) required to reduce radiolabel [1-3H] incorporation by 50% | Bioorg Med Chem Lett 12: 2027-30 (2002) BindingDB Entry DOI: 10.7270/Q2TQ60VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50130367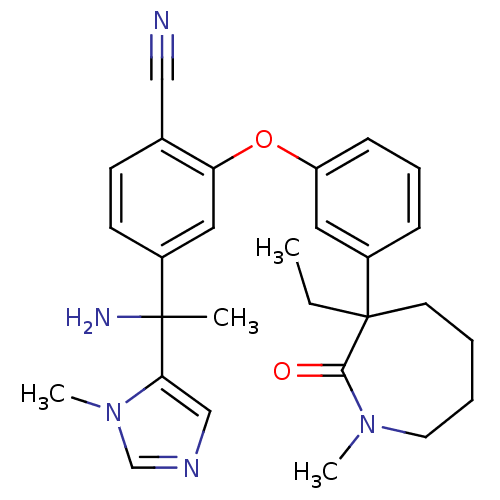 ((RR)-4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. | J Med Chem 46: 2973-84 (2003) Article DOI: 10.1021/jm020587n BindingDB Entry DOI: 10.7270/Q26Q1Z0P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50453019 (CHEMBL4206812) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human microsomal MAO-B expressed in recombinant baculovirus infected insect BTI-TN-5B1-4 cells assessed as reduction in H2O2 production... | J Med Chem 59: 5879-93 (2016) Article DOI: 10.1021/acs.jmedchem.6b00527 BindingDB Entry DOI: 10.7270/Q2X92FSJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50453019 (CHEMBL4206812) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human microsomal MAO-B expressed in recombinant baculovirus infected insect BTI-TN-5B1-4 cells assessed as reduction in H2O2 production... | J Med Chem 59: 5879-93 (2016) Article DOI: 10.1021/acs.jmedchem.6b00527 BindingDB Entry DOI: 10.7270/Q2X92FSJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 754 total ) | Next | Last >> |