 Found 159 hits with Last Name = 'ferone' and Initial = 'r'
Found 159 hits with Last Name = 'ferone' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026308
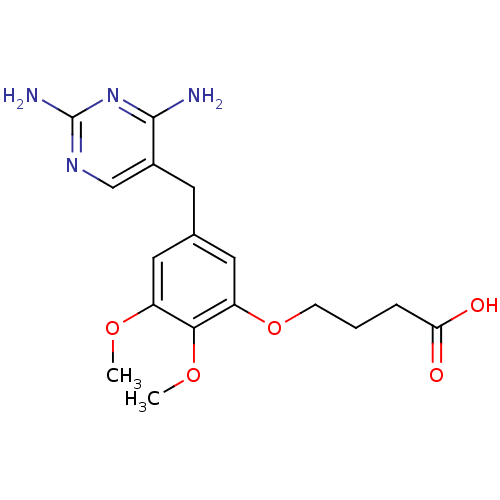
(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C17H22N4O5/c1-24-12-7-10(6-11-9-20-17(19)21-16(11)18)8-13(15(12)25-2)26-5-3-4-14(22)23/h7-9H,3-6H2,1-2H3,(H,22,23)(H4,18,19,20,21) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026308

(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C17H22N4O5/c1-24-12-7-10(6-11-9-20-17(19)21-16(11)18)8-13(15(12)25-2)26-5-3-4-14(22)23/h7-9H,3-6H2,1-2H3,(H,22,23)(H4,18,19,20,21) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026318
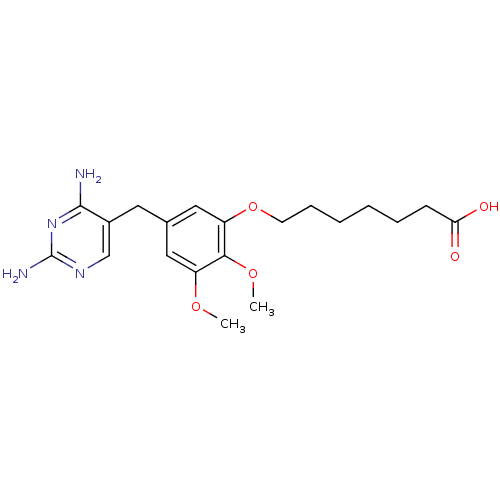
(7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-27-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-2)29-8-6-4-3-5-7-17(25)26/h10-12H,3-9H2,1-2H3,(H,25,26)(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026318

(7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-27-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-2)29-8-6-4-3-5-7-17(25)26/h10-12H,3-9H2,1-2H3,(H,25,26)(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026314
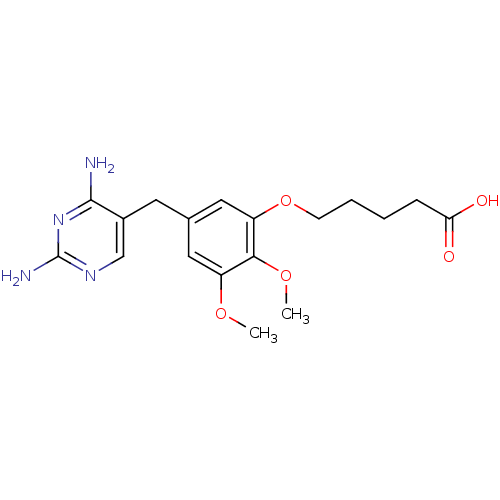
(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C18H24N4O5/c1-25-13-8-11(7-12-10-21-18(20)22-17(12)19)9-14(16(13)26-2)27-6-4-3-5-15(23)24/h8-10H,3-7H2,1-2H3,(H,23,24)(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026314

(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C18H24N4O5/c1-25-13-8-11(7-12-10-21-18(20)22-17(12)19)9-14(16(13)26-2)27-6-4-3-5-15(23)24/h8-10H,3-7H2,1-2H3,(H,23,24)(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50040861
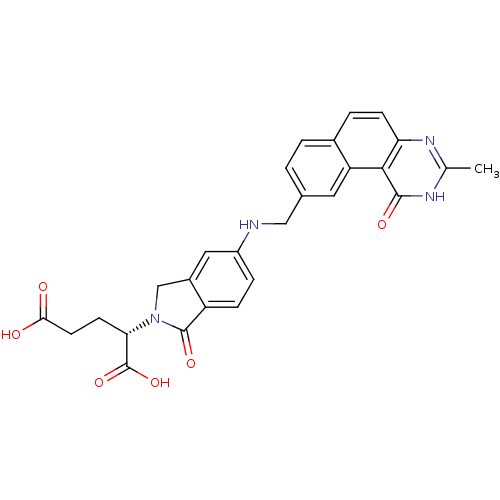
((S)-2-(5-(((1,2-DIHYDRO-3-METHYL-1-OXOBENZO(F)QUIN...)Show SMILES Cc1nc2ccc3ccc(CNc4ccc5C(=O)N(Cc5c4)[C@@H](CCC(O)=O)C(O)=O)cc3c2c(=O)[nH]1 Show InChI InChI=1S/C27H24N4O6/c1-14-29-21-7-4-16-3-2-15(10-20(16)24(21)25(34)30-14)12-28-18-5-6-19-17(11-18)13-31(26(19)35)22(27(36)37)8-9-23(32)33/h2-7,10-11,22,28H,8-9,12-13H2,1H3,(H,32,33)(H,36,37)(H,29,30,34)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli |
J Med Chem 37: 838-44 (1994)
BindingDB Entry DOI: 10.7270/Q2VM4B98 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50040857
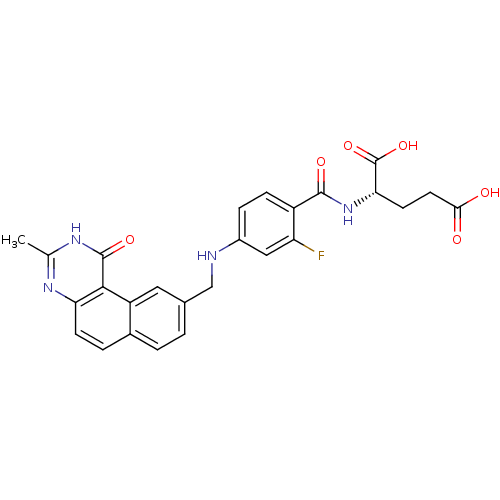
((S)-2-{2-Fluoro-4-[(3-methyl-1-oxo-1,2-dihydro-ben...)Show SMILES Cc1nc2ccc3ccc(CNc4ccc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(F)c4)cc3c2c(=O)[nH]1 Show InChI InChI=1S/C26H23FN4O6/c1-13-29-20-7-4-15-3-2-14(10-18(15)23(20)25(35)30-13)12-28-16-5-6-17(19(27)11-16)24(34)31-21(26(36)37)8-9-22(32)33/h2-7,10-11,21,28H,8-9,12H2,1H3,(H,31,34)(H,32,33)(H,36,37)(H,29,30,35)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli |
J Med Chem 37: 838-44 (1994)
BindingDB Entry DOI: 10.7270/Q2VM4B98 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026307
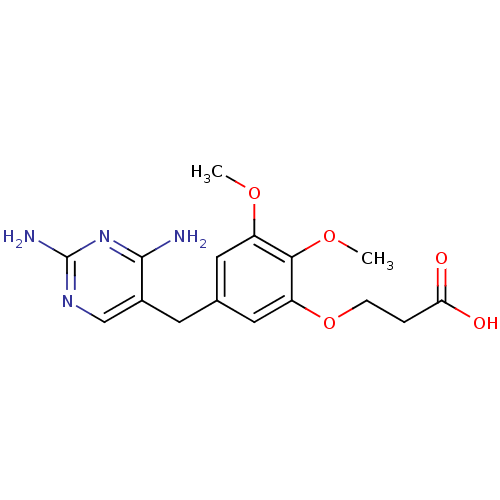
(3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C16H20N4O5/c1-23-11-6-9(5-10-8-19-16(18)20-15(10)17)7-12(14(11)24-2)25-4-3-13(21)22/h6-8H,3-5H2,1-2H3,(H,21,22)(H4,17,18,19,20) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026307

(3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C16H20N4O5/c1-23-11-6-9(5-10-8-19-16(18)20-15(10)17)7-12(14(11)24-2)25-4-3-13(21)22/h6-8H,3-5H2,1-2H3,(H,21,22)(H4,17,18,19,20) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50018477
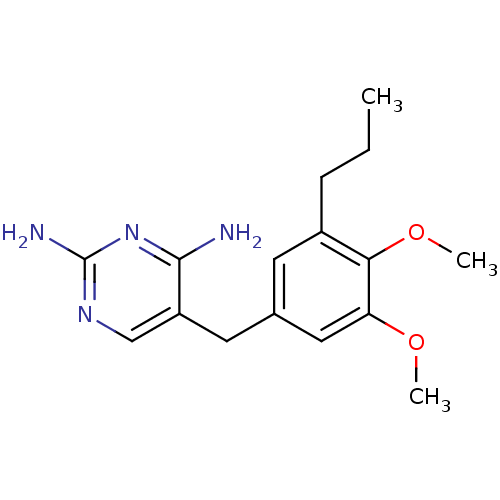
(5-(3,4-Dimethoxy-5-propyl-benzyl)-pyrimidine-2,4-d...)Show InChI InChI=1S/C16H22N4O2/c1-4-5-11-6-10(8-13(21-2)14(11)22-3)7-12-9-19-16(18)20-15(12)17/h6,8-9H,4-5,7H2,1-3H3,(H4,17,18,19,20) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Escherichia coli dihydrofolate reductase. |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50040865

((S)-2-{4-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quin...)Show SMILES Cc1nc2ccc3ccc(CNc4ccc(cc4)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc3c2c(=O)[nH]1 Show InChI InChI=1S/C26H24N4O6/c1-14-28-20-9-6-16-3-2-15(12-19(16)23(20)25(34)29-14)13-27-18-7-4-17(5-8-18)24(33)30-21(26(35)36)10-11-22(31)32/h2-9,12,21,27H,10-11,13H2,1H3,(H,30,33)(H,31,32)(H,35,36)(H,28,29,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. |
J Med Chem 37: 838-44 (1994)
BindingDB Entry DOI: 10.7270/Q2VM4B98 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026317
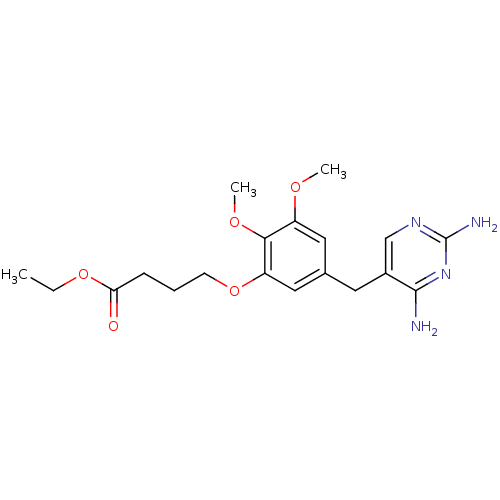
(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-4-27-16(24)6-5-7-28-15-10-12(9-14(25-2)17(15)26-3)8-13-11-22-19(21)23-18(13)20/h9-11H,4-8H2,1-3H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026317

(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-4-27-16(24)6-5-7-28-15-10-12(9-14(25-2)17(15)26-3)8-13-11-22-19(21)23-18(13)20/h9-11H,4-8H2,1-3H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50018478

(5-(3-Ethoxy-4,5-dimethoxy-benzyl)-pyrimidine-2,4-d...)Show InChI InChI=1S/C15H20N4O3/c1-4-22-12-7-9(6-11(20-2)13(12)21-3)5-10-8-18-15(17)19-14(10)16/h6-8H,4-5H2,1-3H3,(H4,16,17,18,19) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026316
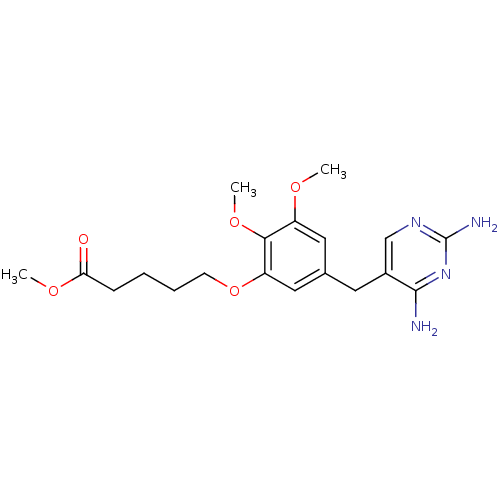
(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-25-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-3)28-7-5-4-6-16(24)26-2/h9-11H,4-8H2,1-3H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026316

(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-25-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-3)28-7-5-4-6-16(24)26-2/h9-11H,4-8H2,1-3H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50018473

(2-Allyl-4-(2,4-diamino-pyrimidin-5-ylmethyl)-6-met...)Show InChI InChI=1S/C15H18N4O2/c1-3-4-10-5-9(7-12(21-2)13(10)20)6-11-8-18-15(17)19-14(11)16/h3,5,7-8,20H,1,4,6H2,2H3,(H4,16,17,18,19) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50018469
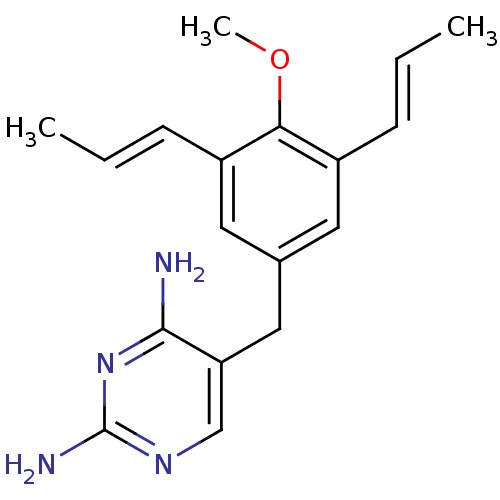
(5-(4-Methoxy-3,5-dipropenyl-benzyl)-pyrimidine-2,4...)Show InChI InChI=1S/C18H22N4O/c1-4-6-13-8-12(9-14(7-5-2)16(13)23-3)10-15-11-21-18(20)22-17(15)19/h4-9,11H,10H2,1-3H3,(H4,19,20,21,22)/b6-4+,7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026306
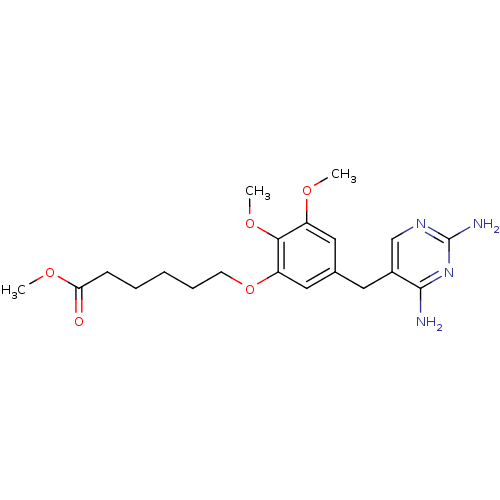
(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-26-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-3)29-8-6-4-5-7-17(25)27-2/h10-12H,4-9H2,1-3H3,(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase Inhibitor of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026306

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-26-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-3)29-8-6-4-5-7-17(25)27-2/h10-12H,4-9H2,1-3H3,(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50018472

(5-(3,4-Dimethoxy-5-propenyl-benzyl)-pyrimidine-2,4...)Show InChI InChI=1S/C16H20N4O2/c1-4-5-11-6-10(8-13(21-2)14(11)22-3)7-12-9-19-16(18)20-15(12)17/h4-6,8-9H,7H2,1-3H3,(H4,17,18,19,20)/b5-4+ | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50018475

(5-(3,4-Dimethoxy-5-propoxy-benzyl)-pyrimidine-2,4-...)Show InChI InChI=1S/C16H22N4O3/c1-4-5-23-13-8-10(7-12(21-2)14(13)22-3)6-11-9-19-16(18)20-15(11)17/h7-9H,4-6H2,1-3H3,(H4,17,18,19,20) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50040860
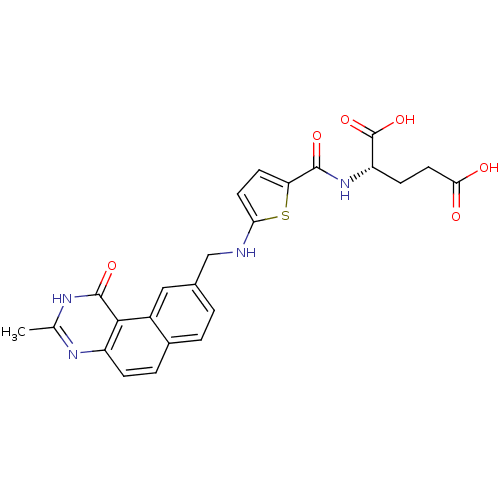
((S)-2-({5-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]qui...)Show SMILES Cc1nc2ccc3ccc(CNc4ccc(s4)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc3c2c(=O)[nH]1 Show InChI InChI=1S/C24H22N4O6S/c1-12-26-16-5-4-14-3-2-13(10-15(14)21(16)23(32)27-12)11-25-19-8-7-18(35-19)22(31)28-17(24(33)34)6-9-20(29)30/h2-5,7-8,10,17,25H,6,9,11H2,1H3,(H,28,31)(H,29,30)(H,33,34)(H,26,27,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli |
J Med Chem 37: 838-44 (1994)
BindingDB Entry DOI: 10.7270/Q2VM4B98 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50040863
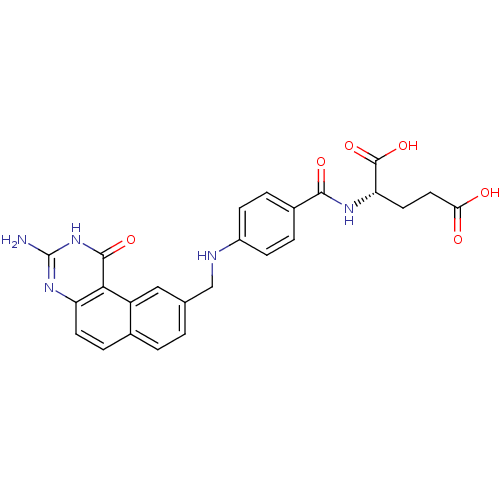
((S)-2-{4-[(3-Amino-1-oxo-1,2-dihydro-benzo[f]quina...)Show SMILES Nc1nc2ccc3ccc(CNc4ccc(cc4)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc3c2c(=O)[nH]1 Show InChI InChI=1S/C25H23N5O6/c26-25-29-18-8-5-14-2-1-13(11-17(14)21(18)23(34)30-25)12-27-16-6-3-15(4-7-16)22(33)28-19(24(35)36)9-10-20(31)32/h1-8,11,19,27H,9-10,12H2,(H,28,33)(H,31,32)(H,35,36)(H3,26,29,30,34)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. |
J Med Chem 37: 838-44 (1994)
BindingDB Entry DOI: 10.7270/Q2VM4B98 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50018472

(5-(3,4-Dimethoxy-5-propenyl-benzyl)-pyrimidine-2,4...)Show InChI InChI=1S/C16H20N4O2/c1-4-5-11-6-10(8-13(21-2)14(11)22-3)7-12-9-19-16(18)20-15(12)17/h4-6,8-9H,7H2,1-3H3,(H4,17,18,19,20)/b5-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Staphylococcus aureus |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50018475

(5-(3,4-Dimethoxy-5-propoxy-benzyl)-pyrimidine-2,4-...)Show InChI InChI=1S/C16H22N4O3/c1-4-5-23-13-8-10(7-12(21-2)14(13)22-3)6-11-9-19-16(18)20-15(11)17/h7-9H,4-6H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Staphylococcus aureus |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50018479

(5-(3-Allyl-4-methoxy-5-propyl-benzyl)-pyrimidine-2...)Show InChI InChI=1S/C18H24N4O/c1-4-6-13-8-12(9-14(7-5-2)16(13)23-3)10-15-11-21-18(20)22-17(15)19/h4,8-9,11H,1,5-7,10H2,2-3H3,(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50018474
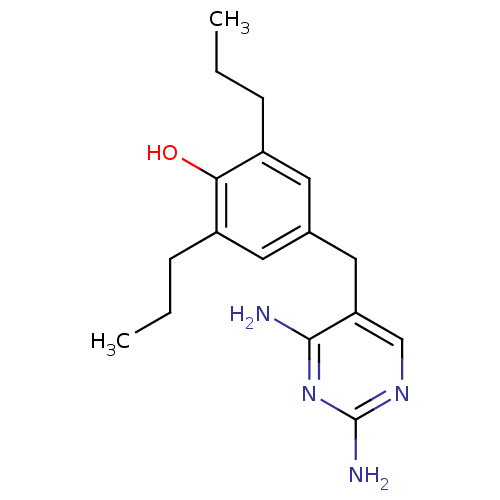
(4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dipropyl-...)Show InChI InChI=1S/C17H24N4O/c1-3-5-12-7-11(8-13(6-4-2)15(12)22)9-14-10-20-17(19)21-16(14)18/h7-8,10,22H,3-6,9H2,1-2H3,(H4,18,19,20,21) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli DHFR |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50018470
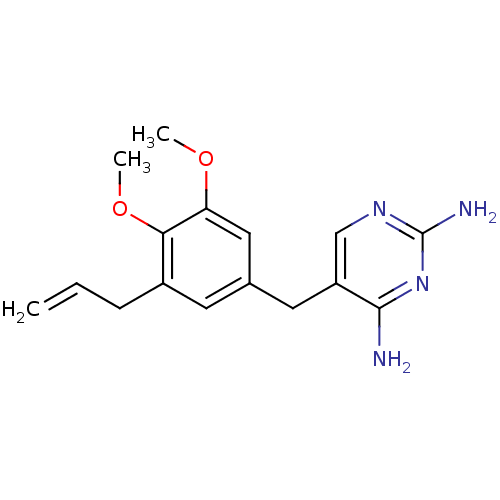
(5-(3-Allyl-4,5-dimethoxy-benzyl)-pyrimidine-2,4-di...)Show InChI InChI=1S/C16H20N4O2/c1-4-5-11-6-10(8-13(21-2)14(11)22-3)7-12-9-19-16(18)20-15(12)17/h4,6,8-9H,1,5,7H2,2-3H3,(H4,17,18,19,20) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026304

(7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show SMILES COC(=O)CCCCCCOc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC Show InChI InChI=1S/C21H30N4O5/c1-27-16-11-14(10-15-13-24-21(23)25-20(15)22)12-17(19(16)29-3)30-9-7-5-4-6-8-18(26)28-2/h11-13H,4-10H2,1-3H3,(H4,22,23,24,25) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026304

(7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show SMILES COC(=O)CCCCCCOc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC Show InChI InChI=1S/C21H30N4O5/c1-27-16-11-14(10-15-13-24-21(23)25-20(15)22)12-17(19(16)29-3)30-9-7-5-4-6-8-18(26)28-2/h11-13H,4-10H2,1-3H3,(H4,22,23,24,25) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50018471

(5-(3,5-Diallyl-4-methoxy-benzyl)-pyrimidine-2,4-di...)Show InChI InChI=1S/C18H22N4O/c1-4-6-13-8-12(9-14(7-5-2)16(13)23-3)10-15-11-21-18(20)22-17(15)19/h4-5,8-9,11H,1-2,6-7,10H2,3H3,(H4,19,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Staphylococcus aureus DHFR |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026313

(5-[4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dimeth...)Show InChI InChI=1S/C18H24N4O5/c1-25-13-8-11(7-12-10-21-18(20)22-17(12)19)9-14(26-2)16(13)27-6-4-3-5-15(23)24/h8-10H,3-7H2,1-2H3,(H,23,24)(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026309
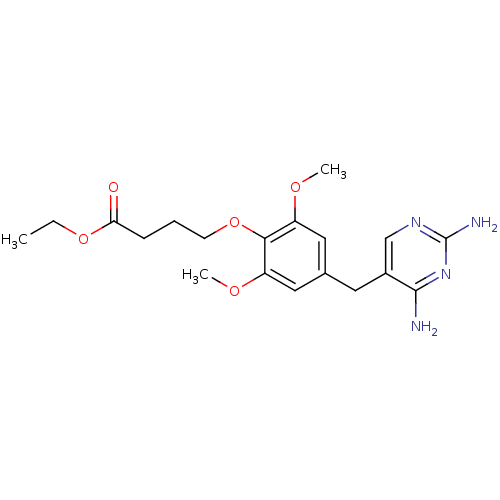
(4-[4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-4-27-16(24)6-5-7-28-17-14(25-2)9-12(10-15(17)26-3)8-13-11-22-19(21)23-18(13)20/h9-11H,4-8H2,1-3H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50040859
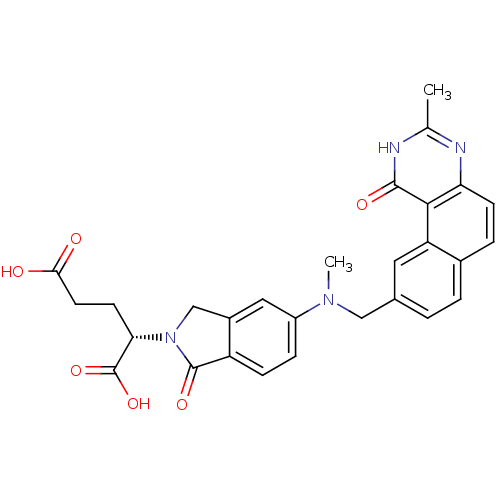
((S)-2-{5-[Methyl-(3-methyl-1-oxo-1,2-dihydro-benzo...)Show SMILES CN(Cc1ccc2ccc3nc(C)[nH]c(=O)c3c2c1)c1ccc2C(=O)N(Cc2c1)[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C28H26N4O6/c1-15-29-22-8-5-17-4-3-16(11-21(17)25(22)26(35)30-15)13-31(2)19-6-7-20-18(12-19)14-32(27(20)36)23(28(37)38)9-10-24(33)34/h3-8,11-12,23H,9-10,13-14H2,1-2H3,(H,33,34)(H,37,38)(H,29,30,35)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli |
J Med Chem 37: 838-44 (1994)
BindingDB Entry DOI: 10.7270/Q2VM4B98 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50018478

(5-(3-Ethoxy-4,5-dimethoxy-benzyl)-pyrimidine-2,4-d...)Show InChI InChI=1S/C15H20N4O3/c1-4-22-12-7-9(6-11(20-2)13(12)21-3)5-10-8-18-15(17)19-14(10)16/h6-8H,4-5H2,1-3H3,(H4,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Staphylococcus aureus |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50042378
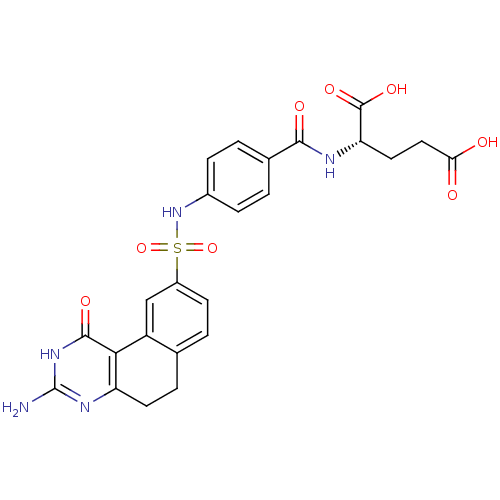
(2-[4-(3-Amino-1-oxo-1,2,5,6-tetrahydro-benzo[f]qui...)Show SMILES Nc1nc2CCc3ccc(cc3-c2c(=O)[nH]1)S(=O)(=O)Nc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C24H23N5O8S/c25-24-27-17-8-4-12-3-7-15(11-16(12)20(17)22(33)28-24)38(36,37)29-14-5-1-13(2-6-14)21(32)26-18(23(34)35)9-10-19(30)31/h1-3,5-7,11,18,29H,4,8-10H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells |
J Med Chem 36: 3464-71 (1993)
BindingDB Entry DOI: 10.7270/Q2K0739T |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026302

(CHEMBL14201 | [5-(2,4-Diamino-pyrimidin-5-ylmethyl...)Show InChI InChI=1S/C15H18N4O5/c1-22-10-4-8(3-9-6-18-15(17)19-14(9)16)5-11(13(10)23-2)24-7-12(20)21/h4-6H,3,7H2,1-2H3,(H,20,21)(H4,16,17,18,19) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026302

(CHEMBL14201 | [5-(2,4-Diamino-pyrimidin-5-ylmethyl...)Show InChI InChI=1S/C15H18N4O5/c1-22-10-4-8(3-9-6-18-15(17)19-14(9)16)5-11(13(10)23-2)24-7-12(20)21/h4-6H,3,7H2,1-2H3,(H,20,21)(H4,16,17,18,19) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026305

(CHEMBL322001 | [4-(2,4-Diamino-pyrimidin-5-ylmethy...)Show InChI InChI=1S/C17H22N4O5/c1-4-25-14(22)9-26-15-12(23-2)6-10(7-13(15)24-3)5-11-8-20-17(19)21-16(11)18/h6-8H,4-5,9H2,1-3H3,(H4,18,19,20,21) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50018470

(5-(3-Allyl-4,5-dimethoxy-benzyl)-pyrimidine-2,4-di...)Show InChI InChI=1S/C16H20N4O2/c1-4-5-11-6-10(8-13(21-2)14(11)22-3)7-12-9-19-16(18)20-15(12)17/h4,6,8-9H,1,5,7H2,2-3H3,(H4,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Staphylococcus aureus |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50018476

(5-(3-Allyloxy-4,5-dimethoxy-benzyl)-pyrimidine-2,4...)Show InChI InChI=1S/C16H20N4O3/c1-4-5-23-13-8-10(7-12(21-2)14(13)22-3)6-11-9-19-16(18)20-15(11)17/h4,7-9H,1,5-6H2,2-3H3,(H4,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Staphylococcus aureus |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026310

(6-[4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(27-2)17(14)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data