 Found 56 hits with Last Name = 'ferrandis' and Initial = 'e'
Found 56 hits with Last Name = 'ferrandis' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21283

(3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cc(ccc2I)[N+]([O-])=O)c2ccccc12 Show InChI InChI=1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089965
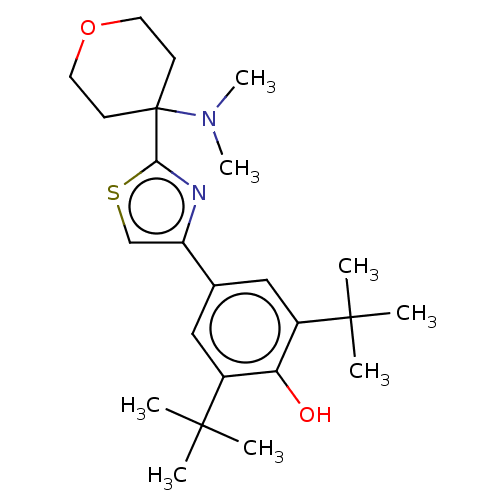
(CHEMBL3581222)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O2S/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089958
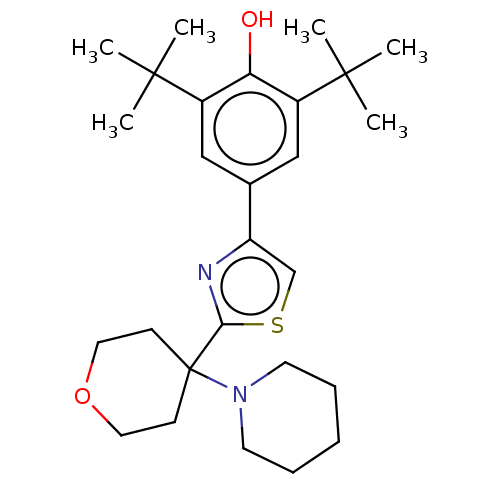
(CHEMBL3581226)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCCCC1 Show InChI InChI=1S/C27H40N2O2S/c1-25(2,3)20-16-19(17-21(23(20)30)26(4,5)6)22-18-32-24(28-22)27(10-14-31-15-11-27)29-12-8-7-9-13-29/h16-18,30H,7-15H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089956
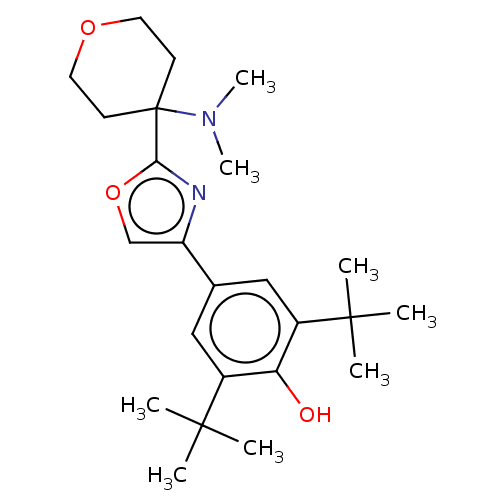
(CHEMBL3581228)Show SMILES CN(C)C1(CCOCC1)c1nc(co1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O3/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089959
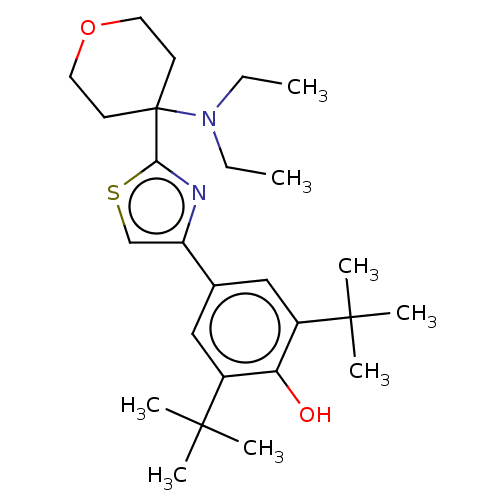
(CHEMBL3581225)Show SMILES CCN(CC)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H40N2O2S/c1-9-28(10-2)26(11-13-30-14-12-26)23-27-21(17-31-23)18-15-19(24(3,4)5)22(29)20(16-18)25(6,7)8/h15-17,29H,9-14H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089968
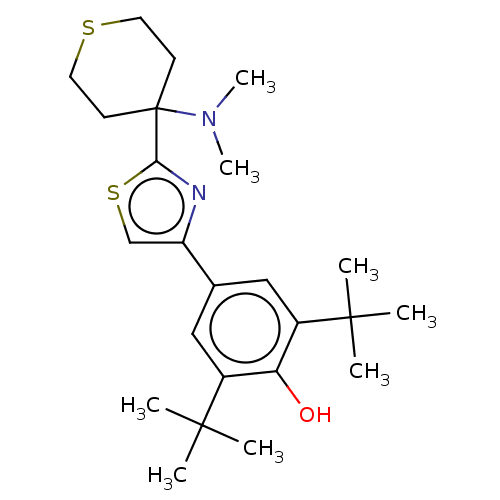
(CHEMBL3581220)Show SMILES CN(C)C1(CCSCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS2/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089970
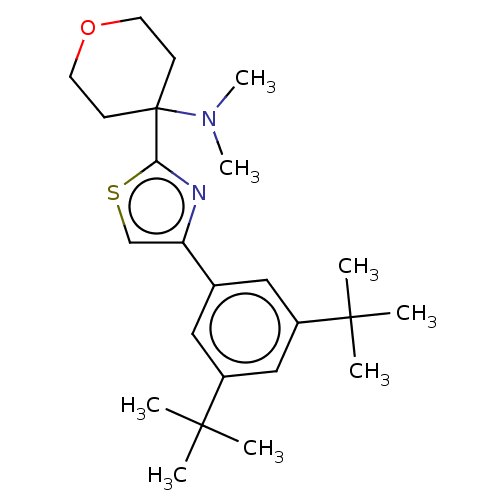
(CHEMBL3581229)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(cc(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS/c1-22(2,3)18-13-17(14-19(15-18)23(4,5)6)20-16-28-21(25-20)24(26(7)8)9-11-27-12-10-24/h13-16H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089963
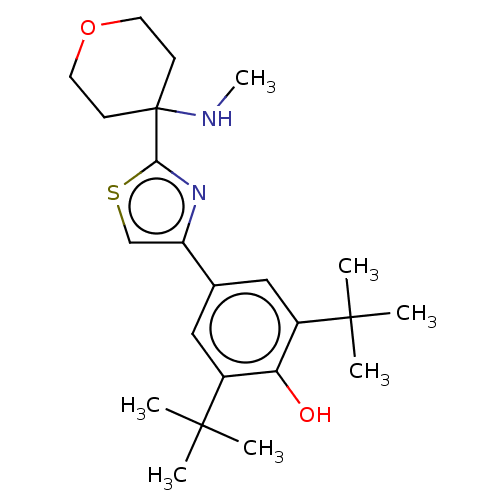
(CHEMBL3581224)Show SMILES CNC1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C23H34N2O2S/c1-21(2,3)16-12-15(13-17(19(16)26)22(4,5)6)18-14-28-20(25-18)23(24-7)8-10-27-11-9-23/h12-14,24,26H,8-11H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089957
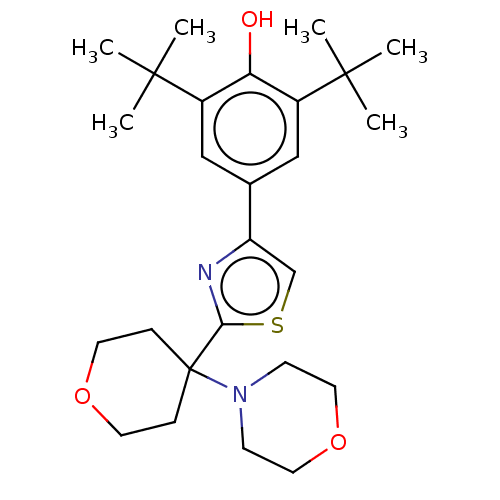
(CHEMBL3581227)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCOCC1 Show InChI InChI=1S/C26H38N2O3S/c1-24(2,3)19-15-18(16-20(22(19)29)25(4,5)6)21-17-32-23(27-21)26(7-11-30-12-8-26)28-9-13-31-14-10-28/h15-17,29H,7-14H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089956

(CHEMBL3581228)Show SMILES CN(C)C1(CCOCC1)c1nc(co1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O3/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 817 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089965

(CHEMBL3581222)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O2S/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 857 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21283

(3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cc(ccc2I)[N+]([O-])=O)c2ccccc12 Show InChI InChI=1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089958

(CHEMBL3581226)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCCCC1 Show InChI InChI=1S/C27H40N2O2S/c1-25(2,3)20-16-19(17-21(23(20)30)26(4,5)6)22-18-32-24(28-22)27(10-14-31-15-11-27)29-12-8-7-9-13-29/h16-18,30H,7-15H2,1-6H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089963

(CHEMBL3581224)Show SMILES CNC1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C23H34N2O2S/c1-21(2,3)16-12-15(13-17(19(16)26)22(4,5)6)18-14-28-20(25-18)23(24-7)8-10-27-11-9-23/h12-14,24,26H,8-11H2,1-7H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089968

(CHEMBL3581220)Show SMILES CN(C)C1(CCSCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS2/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089964
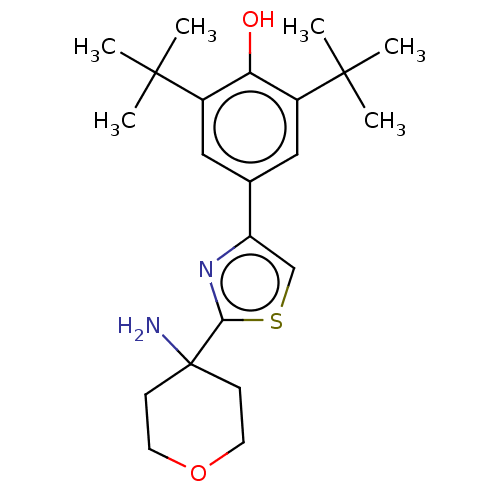
(CHEMBL3581223)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(N)CCOCC1 Show InChI InChI=1S/C22H32N2O2S/c1-20(2,3)15-11-14(12-16(18(15)25)21(4,5)6)17-13-27-19(24-17)22(23)7-9-26-10-8-22/h11-13,25H,7-10,23H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089959

(CHEMBL3581225)Show SMILES CCN(CC)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H40N2O2S/c1-9-28(10-2)26(11-13-30-14-12-26)23-27-21(17-31-23)18-15-19(24(3,4)5)22(29)20(16-18)25(6,7)8/h15-17,29H,9-14H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089970

(CHEMBL3581229)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(cc(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS/c1-22(2,3)18-13-17(14-19(15-18)23(4,5)6)20-16-28-21(25-20)24(26(7)8)9-11-27-12-10-24/h13-16H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089966
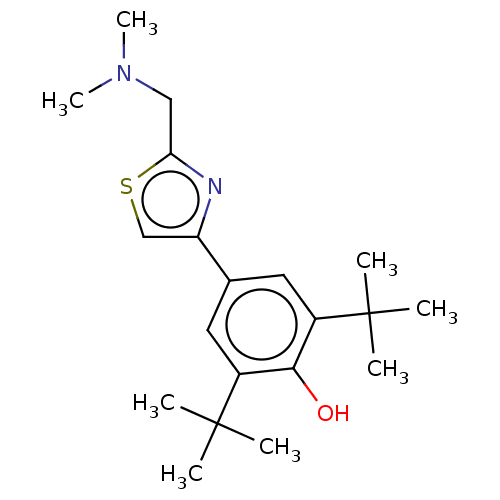
(CHEMBL3581221)Show SMILES CN(C)Cc1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C20H30N2OS/c1-19(2,3)14-9-13(10-15(18(14)23)20(4,5)6)16-12-24-17(21-16)11-22(7)8/h9-10,12,23H,11H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089964

(CHEMBL3581223)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(N)CCOCC1 Show InChI InChI=1S/C22H32N2O2S/c1-20(2,3)15-11-14(12-16(18(15)25)21(4,5)6)17-13-27-19(24-17)22(23)7-9-26-10-8-22/h11-13,25H,7-10,23H2,1-6H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50171448
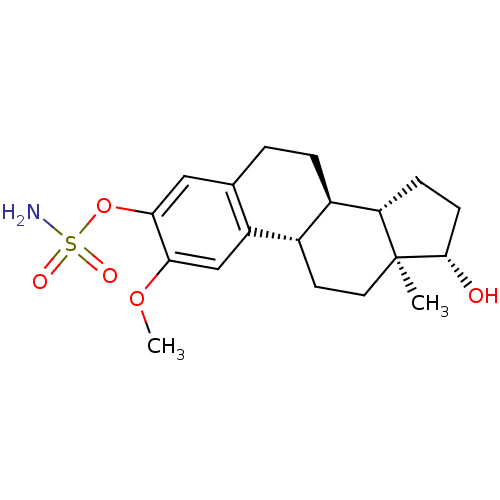
((9BETA,14BETA,17BETA)-17-HYDROXY-2-METHOXYESTRA-1,...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@@H](O)CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O Show InChI InChI=1S/C19H27NO5S/c1-19-8-7-12-13(15(19)5-6-18(19)21)4-3-11-9-17(25-26(20,22)23)16(24-2)10-14(11)12/h9-10,12-13,15,18,21H,3-8H2,1-2H3,(H2,20,22,23)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50237104
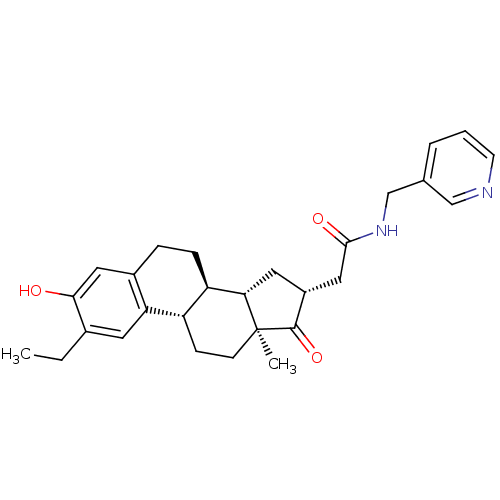
(2-((8R,9S,13S,14S,16R)-2-ethyl-3-hydroxy-13-methyl...)Show SMILES CCc1cc2[C@H]3CC[C@@]4(C)[C@@H](C[C@H](CC(=O)NCc5cccnc5)C4=O)[C@@H]3CCc2cc1O |r| Show InChI InChI=1S/C28H34N2O3/c1-3-18-11-23-19(13-25(18)31)6-7-22-21(23)8-9-28(2)24(22)12-20(27(28)33)14-26(32)30-16-17-5-4-10-29-15-17/h4-5,10-11,13,15,20-22,24,31H,3,6-9,12,14,16H2,1-2H3,(H,30,32)/t20-,21+,22-,24+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50200936
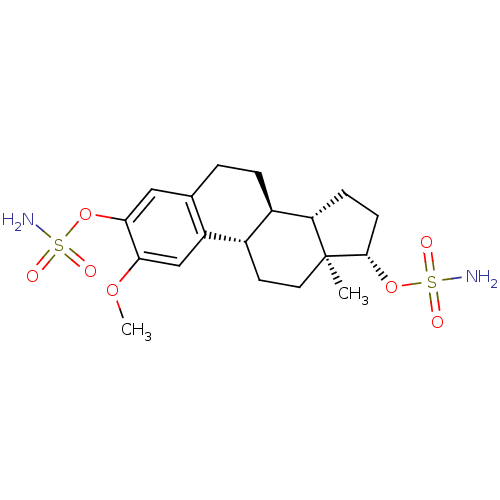
((9BETA,13ALPHA,14BETA,17ALPHA)-2-METHOXYESTRA-1,3,...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O)OS(N)(=O)=O |r| Show InChI InChI=1S/C19H28N2O7S2/c1-19-8-7-12-13(15(19)5-6-18(19)28-30(21,24)25)4-3-11-9-17(27-29(20,22)23)16(26-2)10-14(11)12/h9-10,12-13,15,18H,3-8H2,1-2H3,(H2,20,22,23)(H2,21,24,25)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50219531
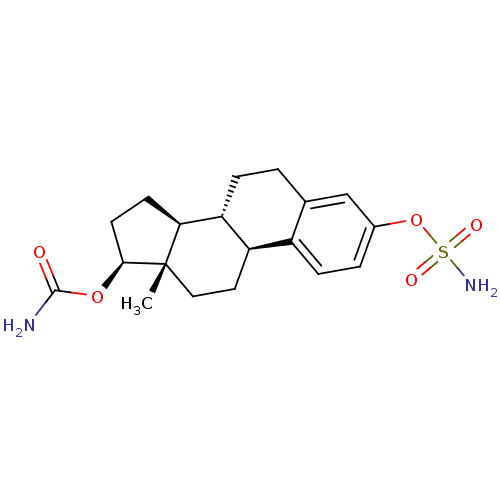
(17beta-carbamoyloxy-3-sulfamoyloxyestra-1,3,5(10)-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CC[C@@H]2OC(N)=O Show InChI InChI=1S/C19H26N2O5S/c1-19-9-8-14-13-5-3-12(26-27(21,23)24)10-11(13)2-4-15(14)16(19)6-7-17(19)25-18(20)22/h3,5,10,14-17H,2,4,6-9H2,1H3,(H2,20,22)(H2,21,23,24)/t14-,15-,16+,17+,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in placental microsomes |
J Med Chem 50: 4431-43 (2007)
Article DOI: 10.1021/jm070405v
BindingDB Entry DOI: 10.7270/Q2765F21 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50372895
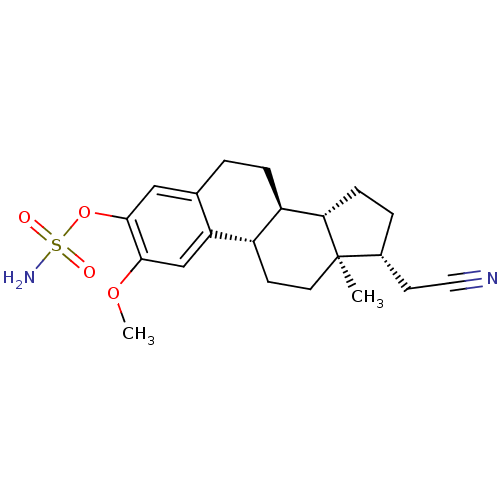
(CHEMBL408967)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@@H](CC#N)CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O |r| Show InChI InChI=1S/C21H28N2O4S/c1-21-9-7-15-16(18(21)6-4-14(21)8-10-22)5-3-13-11-20(27-28(23,24)25)19(26-2)12-17(13)15/h11-12,14-16,18H,3-9H2,1-2H3,(H2,23,24,25)/t14-,15+,16-,18+,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171450
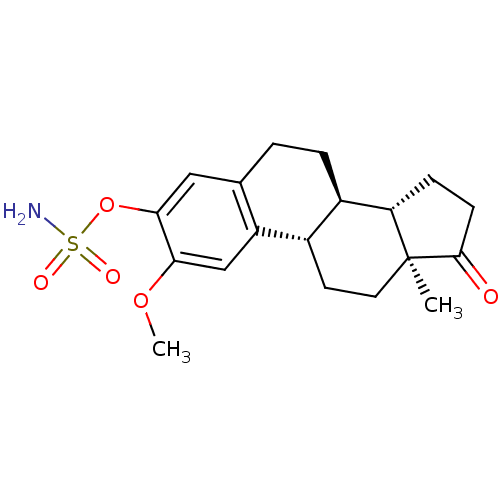
((8R,9S,13S,14S)-2-Methoxy-13-methyl-17-oxo-7,8,9,1...)Show SMILES COc1cc2[C@H]3CC[C@@]4(C)[C@@H](CCC4=O)[C@@H]3CCc2cc1OS(N)(=O)=O Show InChI InChI=1S/C19H25NO5S/c1-19-8-7-12-13(15(19)5-6-18(19)21)4-3-11-9-17(25-26(20,22)23)16(24-2)10-14(11)12/h9-10,12-13,15H,3-8H2,1-2H3,(H2,20,22,23)/t12-,13+,15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 376 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50200936

((9BETA,13ALPHA,14BETA,17ALPHA)-2-METHOXYESTRA-1,3,...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O)OS(N)(=O)=O |r| Show InChI InChI=1S/C19H28N2O7S2/c1-19-8-7-12-13(15(19)5-6-18(19)28-30(21,24)25)4-3-11-9-17(27-29(20,22)23)16(26-2)10-14(11)12/h9-10,12-13,15,18H,3-8H2,1-2H3,(H2,20,22,23)(H2,21,24,25)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50219532
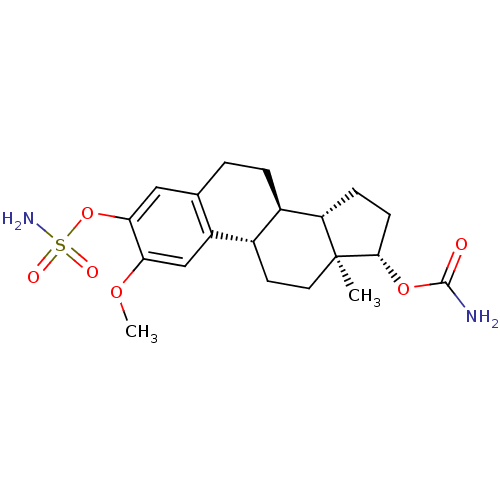
(17beta-Carbamoyloxy-2-methoxy-3-sulfamoyloxyestra-...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O)OC(N)=O Show InChI InChI=1S/C20H28N2O6S/c1-20-8-7-12-13(15(20)5-6-18(20)27-19(21)23)4-3-11-9-17(28-29(22,24)25)16(26-2)10-14(11)12/h9-10,12-13,15,18H,3-8H2,1-2H3,(H2,21,23)(H2,22,24,25)/t12-,13+,15-,18-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in placental microsomes |
J Med Chem 50: 4431-43 (2007)
Article DOI: 10.1021/jm070405v
BindingDB Entry DOI: 10.7270/Q2765F21 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50200940
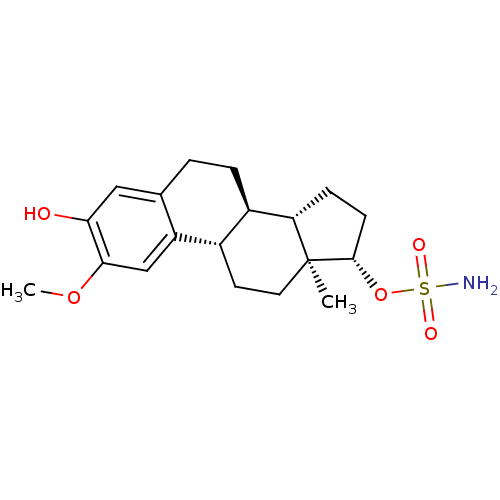
((13ALPHA,14BETA,17ALPHA)-3-HYDROXY-2-METHOXYESTRA-...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CCc2cc1O)OS(N)(=O)=O |r| Show InChI InChI=1S/C19H27NO5S/c1-19-8-7-12-13(15(19)5-6-18(19)25-26(20,22)23)4-3-11-9-16(21)17(24-2)10-14(11)12/h9-10,12-13,15,18,21H,3-8H2,1-2H3,(H2,20,22,23)/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50372894
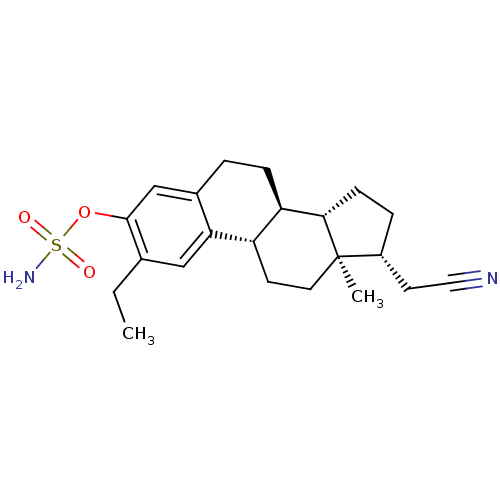
(CHEMBL407952)Show SMILES CCc1cc2[C@H]3CC[C@]4(C)[C@@H](CC#N)CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O |r| Show InChI InChI=1S/C22H30N2O3S/c1-3-14-12-19-15(13-21(14)27-28(24,25)26)4-6-18-17(19)8-10-22(2)16(9-11-23)5-7-20(18)22/h12-13,16-18,20H,3-10H2,1-2H3,(H2,24,25,26)/t16-,17+,18-,20+,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase-mediated coversion of [3H]E1S to E1 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372895

(CHEMBL408967)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@@H](CC#N)CC[C@H]4[C@@H]3CCc2cc1OS(N)(=O)=O |r| Show InChI InChI=1S/C21H28N2O4S/c1-21-9-7-15-16(18(21)6-4-14(21)8-10-22)5-3-13-11-20(27-28(23,24)25)19(26-2)12-17(13)15/h11-12,14-16,18H,3-9H2,1-2H3,(H2,23,24,25)/t14-,15+,16-,18+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
J Med Chem 51: 1295-308 (2008)
Article DOI: 10.1021/jm701319c
BindingDB Entry DOI: 10.7270/Q2M0468S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376293
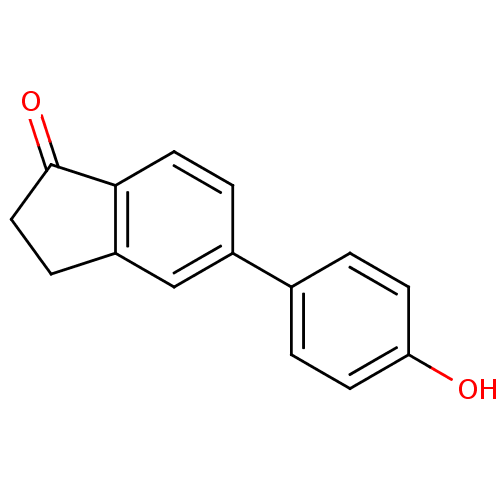
(CHEMBL408487)Show InChI InChI=1S/C15H12O2/c16-13-5-1-10(2-6-13)11-3-7-14-12(9-11)4-8-15(14)17/h1-3,5-7,9,16H,4,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376289
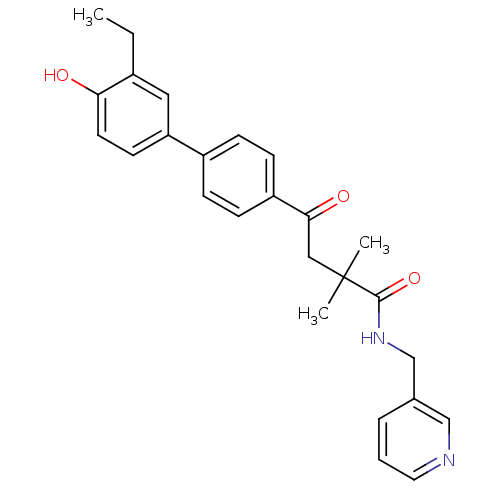
(CHEMBL410224)Show SMILES CCc1cc(ccc1O)-c1ccc(cc1)C(=O)CC(C)(C)C(=O)NCc1cccnc1 Show InChI InChI=1S/C26H28N2O3/c1-4-19-14-22(11-12-23(19)29)20-7-9-21(10-8-20)24(30)15-26(2,3)25(31)28-17-18-6-5-13-27-16-18/h5-14,16,29H,4,15,17H2,1-3H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376292
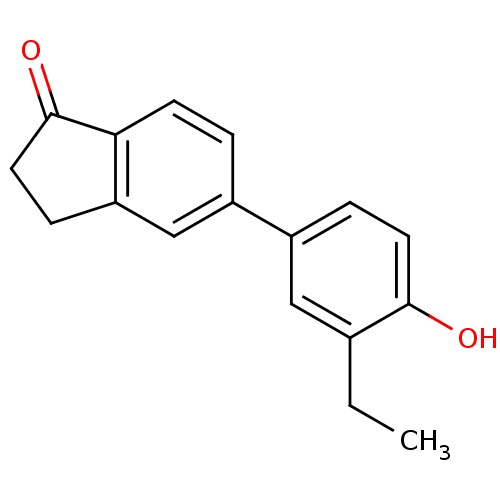
(CHEMBL259000)Show InChI InChI=1S/C17H16O2/c1-2-11-9-13(4-7-16(11)18)12-3-6-15-14(10-12)5-8-17(15)19/h3-4,6-7,9-10,18H,2,5,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376298

(CHEMBL408485)Show InChI InChI=1S/C15H11FO2/c16-13-8-10(2-6-15(13)18)9-1-4-12-11(7-9)3-5-14(12)17/h1-2,4,6-8,18H,3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376297
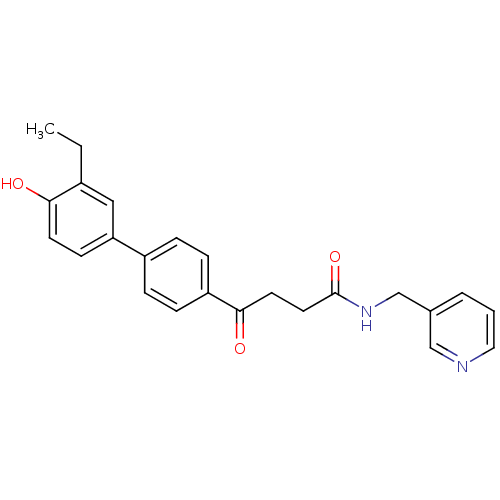
(CHEMBL408594)Show SMILES CCc1cc(ccc1O)-c1ccc(cc1)C(=O)CCC(=O)NCc1cccnc1 Show InChI InChI=1S/C24H24N2O3/c1-2-18-14-21(9-10-22(18)27)19-5-7-20(8-6-19)23(28)11-12-24(29)26-16-17-4-3-13-25-15-17/h3-10,13-15,27H,2,11-12,16H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376295
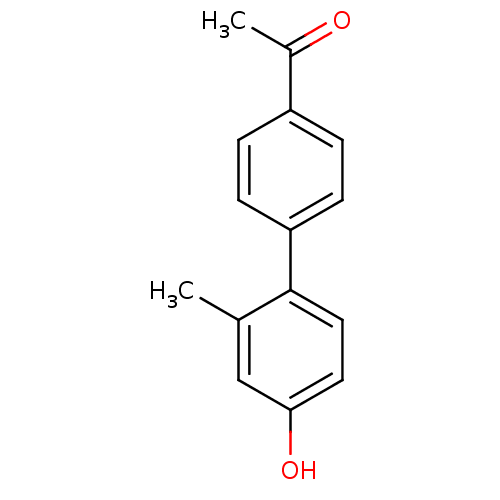
(CHEMBL260518)Show InChI InChI=1S/C15H14O2/c1-10-9-14(17)7-8-15(10)13-5-3-12(4-6-13)11(2)16/h3-9,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376287
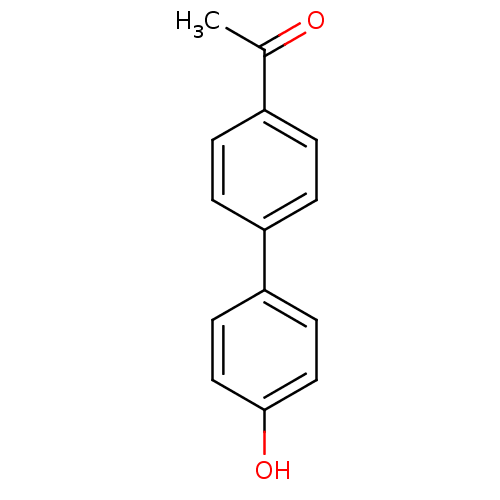
(CHEMBL261578)Show InChI InChI=1S/C14H12O2/c1-10(15)11-2-4-12(5-3-11)13-6-8-14(16)9-7-13/h2-9,16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
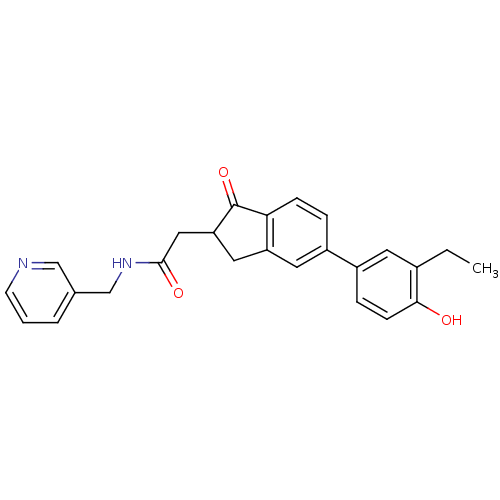
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376288
(CHEMBL260227)Show SMILES CCc1cc(ccc1O)-c1ccc2C(=O)C(CC(=O)NCc3cccnc3)Cc2c1 Show InChI InChI=1S/C25H24N2O3/c1-2-17-10-19(6-8-23(17)28)18-5-7-22-20(11-18)12-21(25(22)30)13-24(29)27-15-16-4-3-9-26-14-16/h3-11,14,21,28H,2,12-13,15H2,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376286
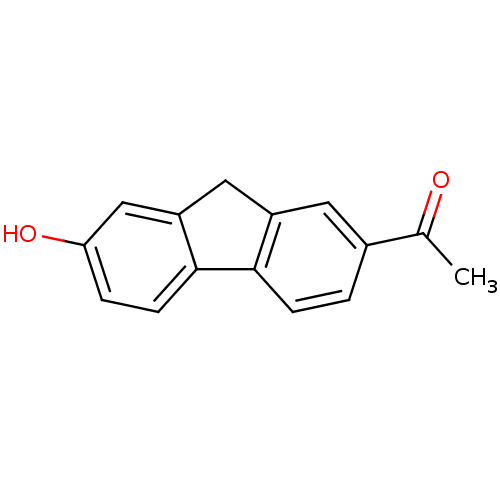
(CHEMBL260234)Show InChI InChI=1S/C15H12O2/c1-9(16)10-2-4-14-11(6-10)7-12-8-13(17)3-5-15(12)14/h2-6,8,17H,7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376296
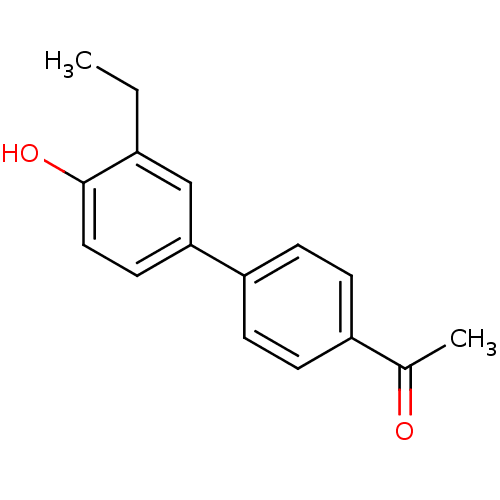
(CHEMBL260075)Show InChI InChI=1S/C16H16O2/c1-3-12-10-15(8-9-16(12)18)14-6-4-13(5-7-14)11(2)17/h4-10,18H,3H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376290
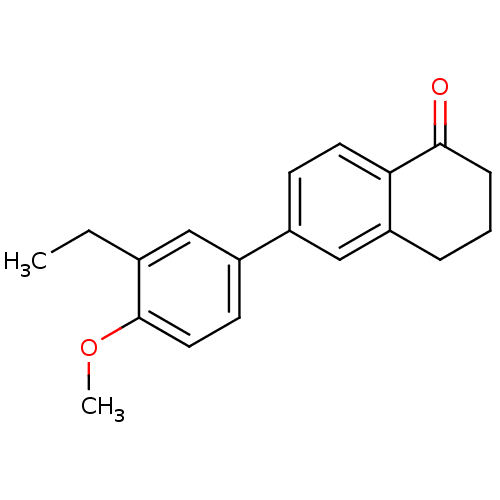
(CHEMBL259815)Show InChI InChI=1S/C19H20O2/c1-3-13-11-15(8-10-19(13)21-2)14-7-9-17-16(12-14)5-4-6-18(17)20/h7-12H,3-6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376294

(CHEMBL407865)Show InChI InChI=1S/C16H16O2/c1-3-12-10-14(6-9-16(12)11(2)17)13-4-7-15(18)8-5-13/h4-10,18H,3H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50376291

(CHEMBL259814)Show InChI InChI=1S/C16H14O2/c17-14-7-4-11(5-8-14)12-6-9-15-13(10-12)2-1-3-16(15)18/h4-10,17H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD1 expressed in intact human T47D cells after 30 mins |
Bioorg Med Chem 16: 4438-56 (2008)
Article DOI: 10.1016/j.bmc.2008.02.059
BindingDB Entry DOI: 10.7270/Q20002Z0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089959

(CHEMBL3581225)Show SMILES CCN(CC)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H40N2O2S/c1-9-28(10-2)26(11-13-30-14-12-26)23-27-21(17-31-23)18-15-19(24(3,4)5)22(29)20(16-18)25(6,7)8/h15-17,29H,9-14H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089965

(CHEMBL3581222)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O2S/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089968

(CHEMBL3581220)Show SMILES CN(C)C1(CCSCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS2/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089956

(CHEMBL3581228)Show SMILES CN(C)C1(CCOCC1)c1nc(co1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O3/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21283

(3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cc(ccc2I)[N+]([O-])=O)c2ccccc12 Show InChI InChI=1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089958

(CHEMBL3581226)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCCCC1 Show InChI InChI=1S/C27H40N2O2S/c1-25(2,3)20-16-19(17-21(23(20)30)26(4,5)6)22-18-32-24(28-22)27(10-14-31-15-11-27)29-12-8-7-9-13-29/h16-18,30H,7-15H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data