

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448484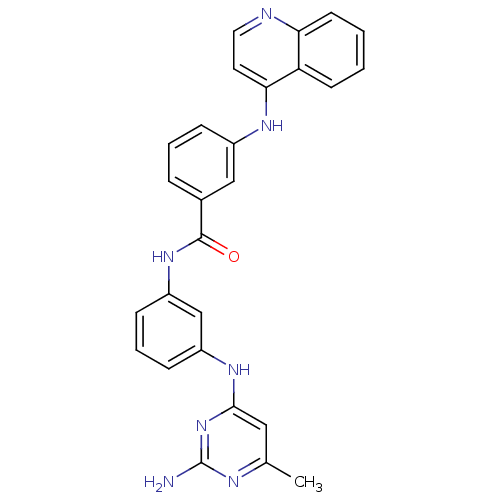 (CHEMBL3126646) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448487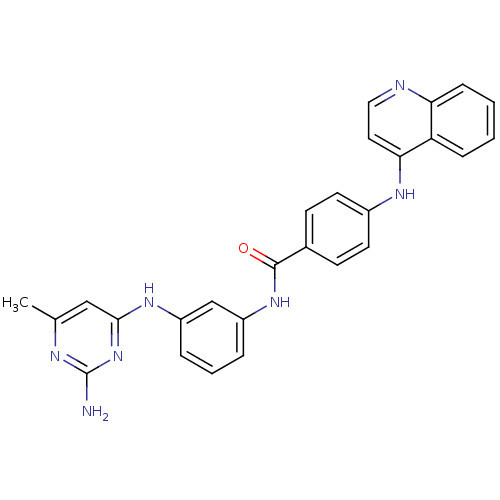 (CHEMBL2385821) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin G | J Med Chem 51: 1068-72 (2008) Article DOI: 10.1021/jm7010589 BindingDB Entry DOI: 10.7270/Q2XG9S0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448489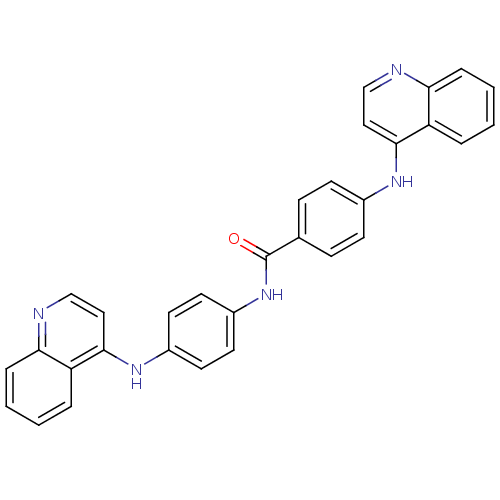 (CHEMBL3126651) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50430056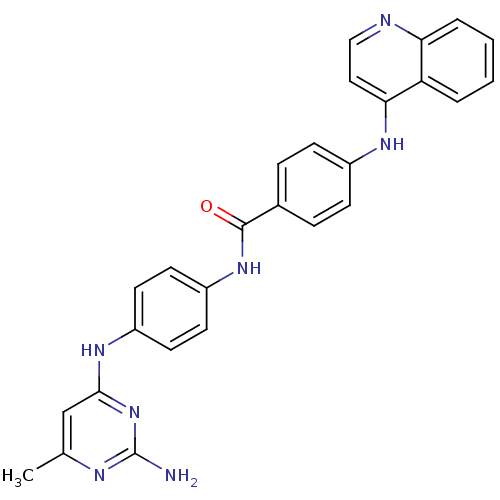 (CHEMBL2336409 | SGI-1027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Inhibition of human chymase | J Med Chem 51: 1068-72 (2008) Article DOI: 10.1021/jm7010589 BindingDB Entry DOI: 10.7270/Q2XG9S0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50329819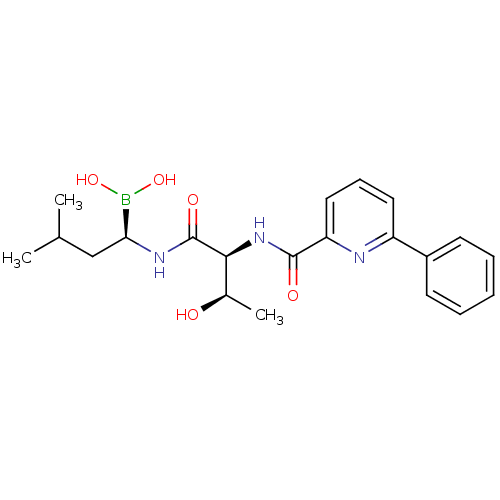 ((R)-1-((2S,3R)-3-hydroxy-2-(6-phenylpicolinamido)b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Inhibition of human cathepsin G | J Med Chem 51: 1068-72 (2008) Article DOI: 10.1021/jm7010589 BindingDB Entry DOI: 10.7270/Q2XG9S0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448488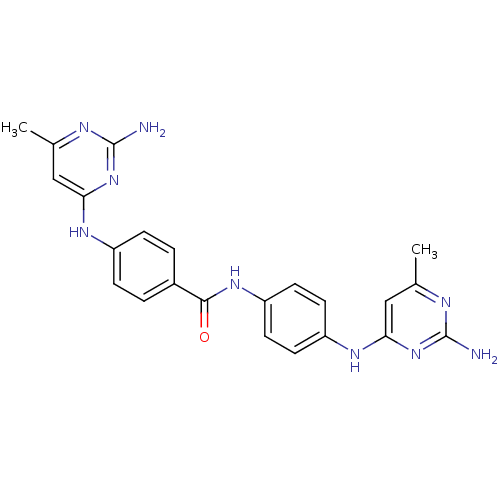 (CHEMBL3126652) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3-like (Homo sapiens (Human)) | BDBM50448484 (CHEMBL3126646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human DNMT3A2/3L using unmethylated DNA as substrate in presence of [methyl-3H]-AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50329819 ((R)-1-((2S,3R)-3-hydroxy-2-(6-phenylpicolinamido)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 | J Med Chem 51: 1068-72 (2008) Article DOI: 10.1021/jm7010589 BindingDB Entry DOI: 10.7270/Q2XG9S0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50329819 ((R)-1-((2S,3R)-3-hydroxy-2-(6-phenylpicolinamido)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Inhibition of human chymase | J Med Chem 51: 1068-72 (2008) Article DOI: 10.1021/jm7010589 BindingDB Entry DOI: 10.7270/Q2XG9S0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50329819 ((R)-1-((2S,3R)-3-hydroxy-2-(6-phenylpicolinamido)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase 2 | J Med Chem 51: 1068-72 (2008) Article DOI: 10.1021/jm7010589 BindingDB Entry DOI: 10.7270/Q2XG9S0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448485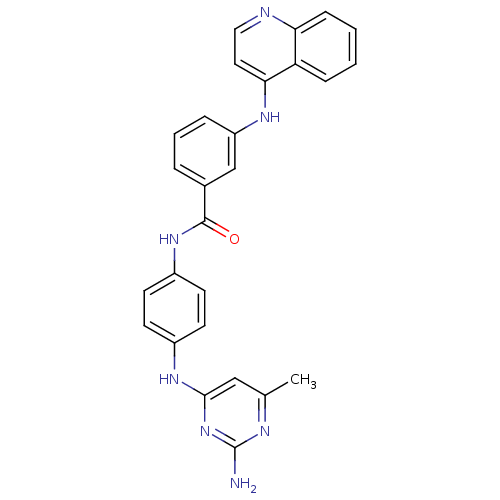 (CHEMBL3126645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448484 (CHEMBL3126646) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using hemimethylated (GAC)12 as substrate preincubated for 5 mins followed by substrate add... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3-like (Homo sapiens (Human)) | BDBM50430056 (CHEMBL2336409 | SGI-1027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human DNMT3A2/3L using unmethylated DNA as substrate in presence of [methyl-3H]-AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448493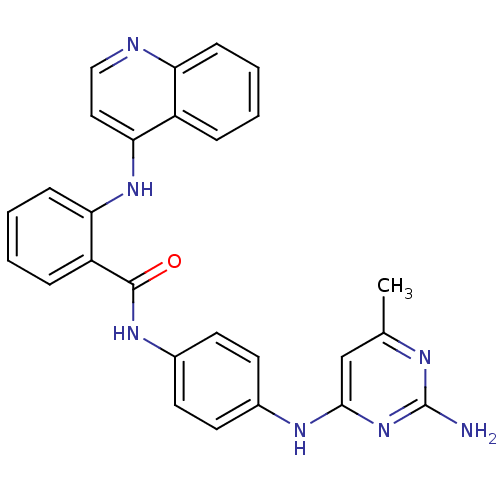 (CHEMBL3126648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3-like/3A (Homo sapiens (Human)) | BDBM50448488 (CHEMBL3126652) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human DNMT3A2/3L using unmethylated DNA as substrate in presence of [methyl-3H]-AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3-like (Homo sapiens (Human)) | BDBM50448487 (CHEMBL2385821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human DNMT3A2/3L using unmethylated DNA as substrate in presence of [methyl-3H]-AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3-like (Homo sapiens (Human)) | BDBM50448489 (CHEMBL3126651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human DNMT3A2/3L using unmethylated DNA as substrate in presence of [methyl-3H]-AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448491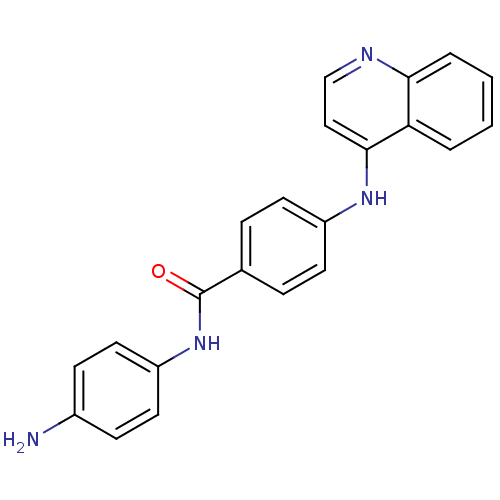 (CHEMBL3126653) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448489 (CHEMBL3126651) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using hemimethylated (GAC)12 as substrate preincubated for 5 mins followed by substrate add... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448486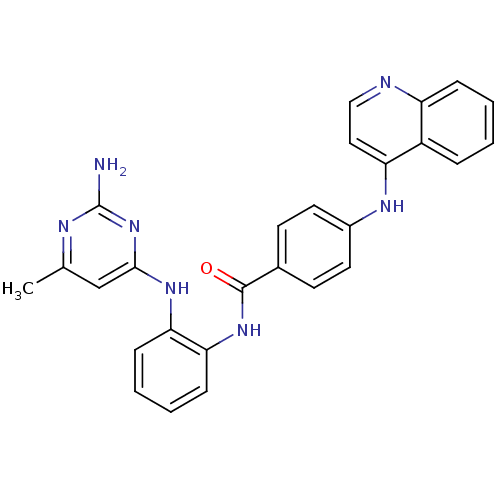 (CHEMBL3126644) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50430056 (CHEMBL2336409 | SGI-1027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using hemimethylated (GAC)12 as substrate preincubated for 5 mins followed by substrate add... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448492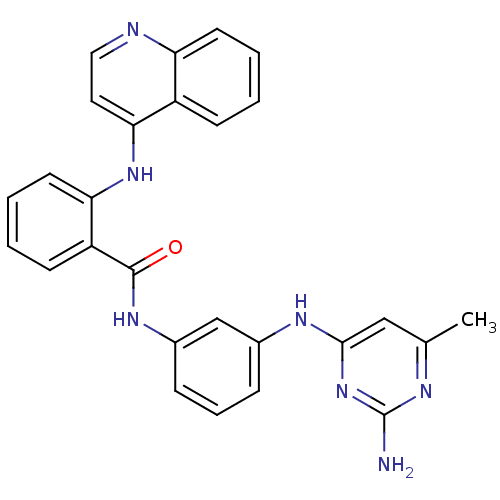 (CHEMBL3126649) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3-like (Homo sapiens (Human)) | BDBM50448485 (CHEMBL3126645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human DNMT3A2/3L using unmethylated DNA as substrate in presence of [methyl-3H]-AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448487 (CHEMBL2385821) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using hemimethylated (GAC)12 as substrate preincubated for 5 mins followed by substrate add... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT1 (Homo sapiens (Human)) | BDBM50430056 (CHEMBL2336409 | SGI-1027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human GLP (734 to 1235) using histone as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448488 (CHEMBL3126652) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using hemimethylated (GAC)12 as substrate preincubated for 5 mins followed by substrate add... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50448484 (CHEMBL3126646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of rat recombinant PRMT1 using histone as substrate preincubated for 5 mins followed by substrate addition measured after 6.5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT1 (Homo sapiens (Human)) | BDBM50448484 (CHEMBL3126646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human GLP (734 to 1235) using histone as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT1 (Homo sapiens (Human)) | BDBM50448485 (CHEMBL3126645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human GLP (734 to 1235) using histone as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50430056 (CHEMBL2336409 | SGI-1027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of rat recombinant PRMT1 using histone as substrate preincubated for 5 mins followed by substrate addition measured after 6.5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448490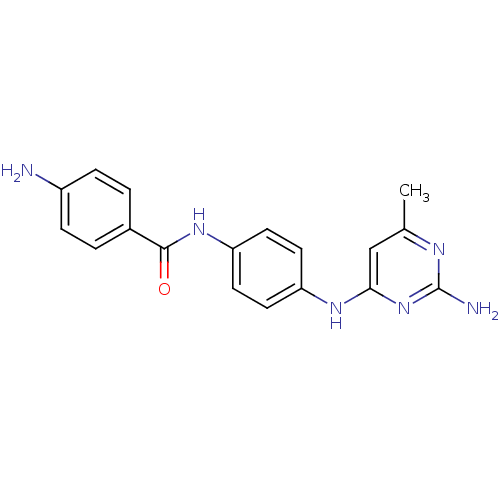 (CHEMBL3126654) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448483 (CHEMBL3126647) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using poly(dI-dC) as substrate in presence of AdoMet | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50448485 (CHEMBL3126645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human N-terminal 600 residues-deleted DNMT1 using hemimethylated (GAC)12 as substrate preincubated for 5 mins followed by substrate add... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50448486 (CHEMBL3126644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of rat recombinant PRMT1 using histone as substrate preincubated for 5 mins followed by substrate addition measured after 6.5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50448487 (CHEMBL2385821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of rat recombinant PRMT1 using histone as substrate preincubated for 5 mins followed by substrate addition measured after 6.5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50448485 (CHEMBL3126645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of rat recombinant PRMT1 using histone as substrate preincubated for 5 mins followed by substrate addition measured after 6.5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT1 (Homo sapiens (Human)) | BDBM50448486 (CHEMBL3126644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human GLP (734 to 1235) using histone as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT1 (Homo sapiens (Human)) | BDBM50448483 (CHEMBL3126647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human GLP (734 to 1235) using histone as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT1 (Homo sapiens (Human)) | BDBM50448487 (CHEMBL2385821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human GLP (734 to 1235) using histone as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50448483 (CHEMBL3126647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of rat recombinant PRMT1 using histone as substrate preincubated for 5 mins followed by substrate addition measured after 6.5 mins by liqu... | J Med Chem 57: 701-13 (2014) Article DOI: 10.1021/jm4012627 BindingDB Entry DOI: 10.7270/Q2028T1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||