 Found 13 hits with Last Name = 'filocamo' and Initial = 'l'
Found 13 hits with Last Name = 'filocamo' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285179

((11-Morpholin-4-yl-undecyl)-carbamic acid (3aS,8aR...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCCCCCCCCCN3CCOCC3)cc21 Show InChI InChI=1S/C29H48N4O3/c1-29-15-18-31(2)27(29)32(3)26-14-13-24(23-25(26)29)36-28(34)30-16-11-9-7-5-4-6-8-10-12-17-33-19-21-35-22-20-33/h13-14,23,27H,4-12,15-22H2,1-3H3,(H,30,34)/t27-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285184
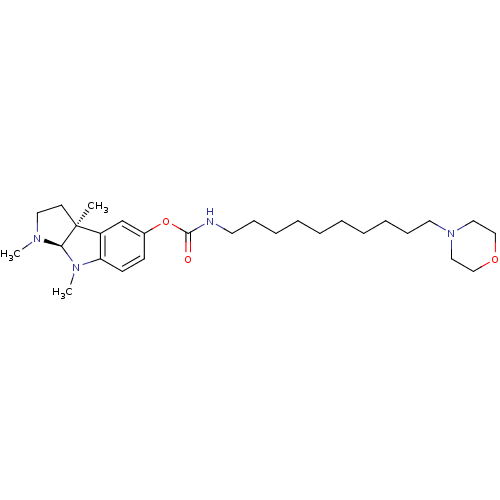
((10-Morpholin-4-yl-decyl)-carbamic acid (3aS,8aR)-...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCCCCCCCCN3CCOCC3)cc21 Show InChI InChI=1S/C28H46N4O3/c1-28-14-17-30(2)26(28)31(3)25-13-12-23(22-24(25)28)35-27(33)29-15-10-8-6-4-5-7-9-11-16-32-18-20-34-21-19-32/h12-13,22,26H,4-11,14-21H2,1-3H3,(H,29,33)/t26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285188
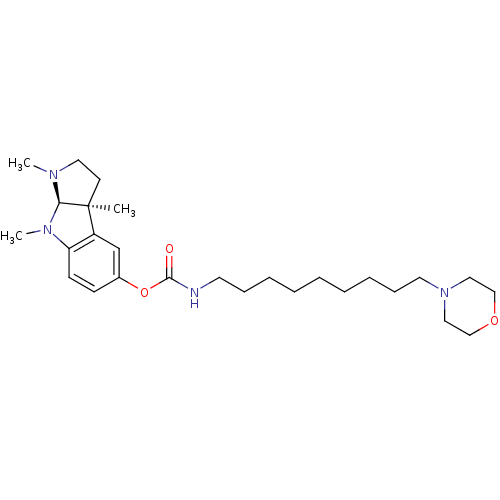
((9-Morpholin-4-yl-nonyl)-carbamic acid (3aS,8aR)-1...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCCCCCCCN3CCOCC3)cc21 Show InChI InChI=1S/C27H44N4O3/c1-27-13-16-29(2)25(27)30(3)24-12-11-22(21-23(24)27)34-26(32)28-14-9-7-5-4-6-8-10-15-31-17-19-33-20-18-31/h11-12,21,25H,4-10,13-20H2,1-3H3,(H,28,32)/t25-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285180
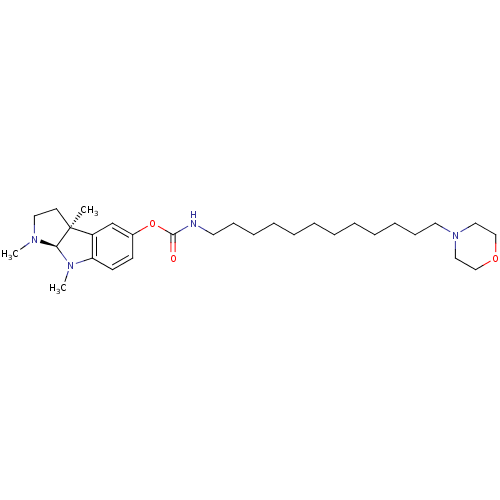
((12-Morpholin-4-yl-dodecyl)-carbamic acid (3aS,8aR...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCCCCCCCCCCN3CCOCC3)cc21 Show InChI InChI=1S/C30H50N4O3/c1-30-16-19-32(2)28(30)33(3)27-15-14-25(24-26(27)30)37-29(35)31-17-12-10-8-6-4-5-7-9-11-13-18-34-20-22-36-23-21-34/h14-15,24,28H,4-13,16-23H2,1-3H3,(H,31,35)/t28-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285182

((8-Morpholin-4-yl-octyl)-carbamic acid (3aS,8aR)-1...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCCCCCCN3CCOCC3)cc21 Show InChI InChI=1S/C26H42N4O3/c1-26-12-15-28(2)24(26)29(3)23-11-10-21(20-22(23)26)33-25(31)27-13-8-6-4-5-7-9-14-30-16-18-32-19-17-30/h10-11,20,24H,4-9,12-19H2,1-3H3,(H,27,31)/t24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10972
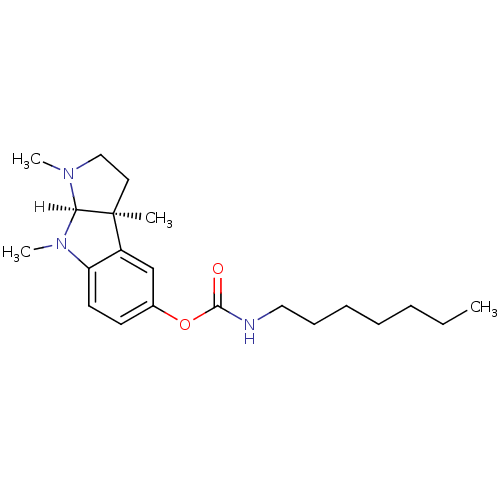
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285185

((7-Morpholin-4-yl-heptyl)-carbamic acid (3aS,8aR)-...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCCCCCN3CCOCC3)cc21 Show InChI InChI=1S/C25H40N4O3/c1-25-11-14-27(2)23(25)28(3)22-10-9-20(19-21(22)25)32-24(30)26-12-7-5-4-6-8-13-29-15-17-31-18-16-29/h9-10,19,23H,4-8,11-18H2,1-3H3,(H,26,30)/t23-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285183

((2-Morpholin-4-yl-ethyl)-carbamic acid (3aS,8aR)-1...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCN3CCOCC3)cc21 Show InChI InChI=1S/C20H30N4O3/c1-20-6-8-22(2)18(20)23(3)17-5-4-15(14-16(17)20)27-19(25)21-7-9-24-10-12-26-13-11-24/h4-5,14,18H,6-13H2,1-3H3,(H,21,25)/t18-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Acetylcholinesterase from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285178

((6-Morpholin-4-yl-hexyl)-carbamic acid (3aS,8aR)-1...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCCCCN3CCOCC3)cc21 Show InChI InChI=1S/C24H38N4O3/c1-24-10-13-26(2)22(24)27(3)21-9-8-19(18-20(21)24)31-23(29)25-11-6-4-5-7-12-28-14-16-30-17-15-28/h8-9,18,22H,4-7,10-17H2,1-3H3,(H,25,29)/t22-,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285186

((3-Morpholin-4-yl-propyl)-carbamic acid (3aS,8aR)-...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCN3CCOCC3)cc21 Show InChI InChI=1S/C21H32N4O3/c1-21-7-10-23(2)19(21)24(3)18-6-5-16(15-17(18)21)28-20(26)22-8-4-9-25-11-13-27-14-12-25/h5-6,15,19H,4,7-14H2,1-3H3,(H,22,26)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285181

((5-Morpholin-4-yl-pentyl)-carbamic acid (3aS,8aR)-...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCCCN3CCOCC3)cc21 Show InChI InChI=1S/C23H36N4O3/c1-23-9-12-25(2)21(23)26(3)20-8-7-18(17-19(20)23)30-22(28)24-10-5-4-6-11-27-13-15-29-16-14-27/h7-8,17,21H,4-6,9-16H2,1-3H3,(H,24,28)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50285187
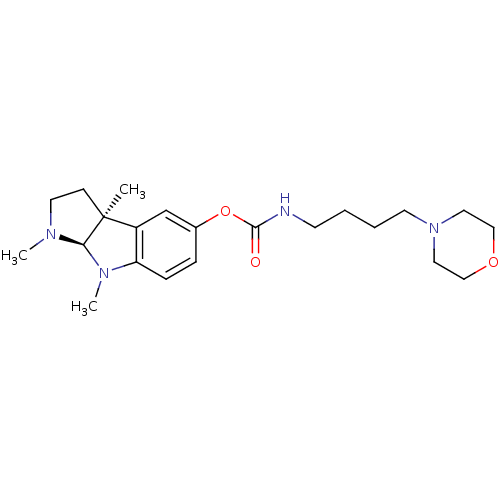
((4-Morpholin-4-yl-butyl)-carbamic acid (3aS,8aR)-1...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NCCCCN3CCOCC3)cc21 Show InChI InChI=1S/C22H34N4O3/c1-22-8-11-24(2)20(22)25(3)19-7-6-17(16-18(19)22)29-21(27)23-9-4-5-10-26-12-14-28-15-13-26/h6-7,16,20H,4-5,8-15H2,1-3H3,(H,23,27)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase(AChE) from human RBC |
Bioorg Med Chem Lett 5: 2077-2080 (1995)
Article DOI: 10.1016/0960-894X(95)00371-Y
BindingDB Entry DOI: 10.7270/Q2NP24CR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data