

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM16127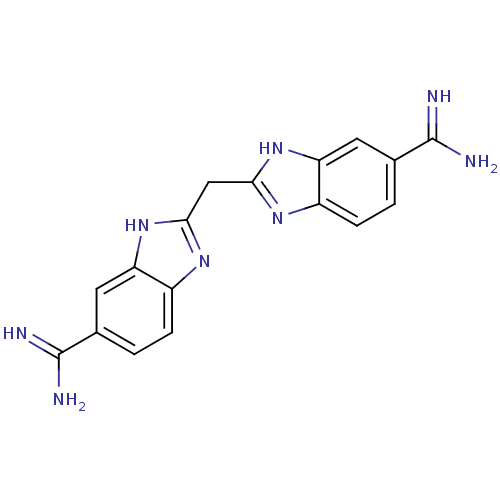 (2,2 -methanediylbis(1H-benzimidazole-6-carboximida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16127 (2,2 -methanediylbis(1H-benzimidazole-6-carboximida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5 | -46.9 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16304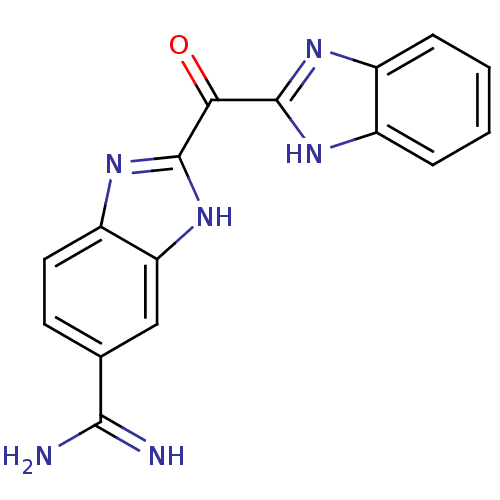 (2-(1H-1,3-benzodiazol-2-ylcarbonyl)-1H-1,3-benzodi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.20 | -46.8 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16303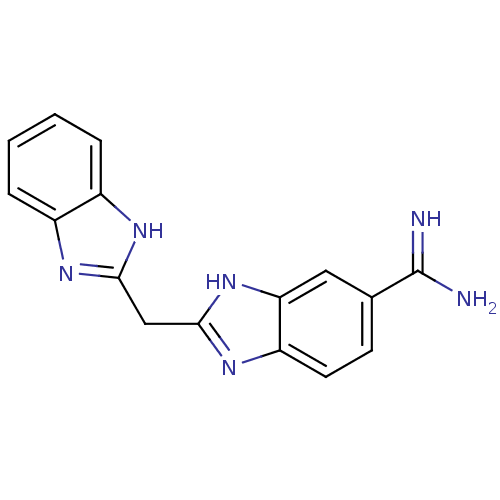 (2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.60 | -46.6 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16127 (2,2 -methanediylbis(1H-benzimidazole-6-carboximida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 23 | -43.2 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16305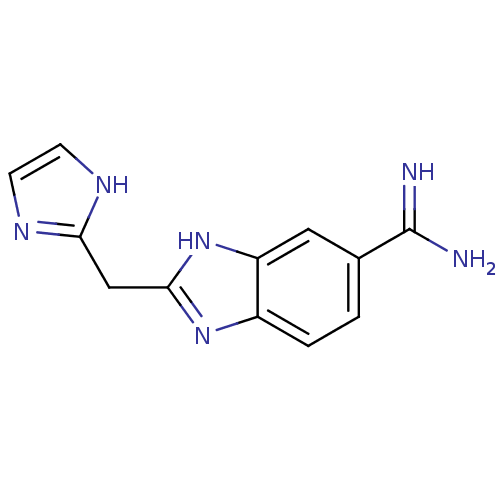 (2-(1H-imidazol-2-ylmethyl)-1H-1,3-benzodiazole-6-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 23.1 | -43.1 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16303 (2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 23.5 | -43.1 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16308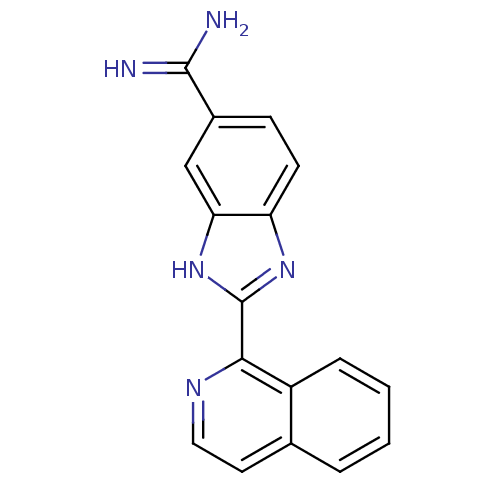 (2-(isoquinolin-1-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | -41.7 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16304 (2-(1H-1,3-benzodiazol-2-ylcarbonyl)-1H-1,3-benzodi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.3 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16303 (2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 69.5 | -40.4 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16127 (2,2 -methanediylbis(1H-benzimidazole-6-carboximida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 90 | -39.8 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16304 (2-(1H-1,3-benzodiazol-2-ylcarbonyl)-1H-1,3-benzodi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 101 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16307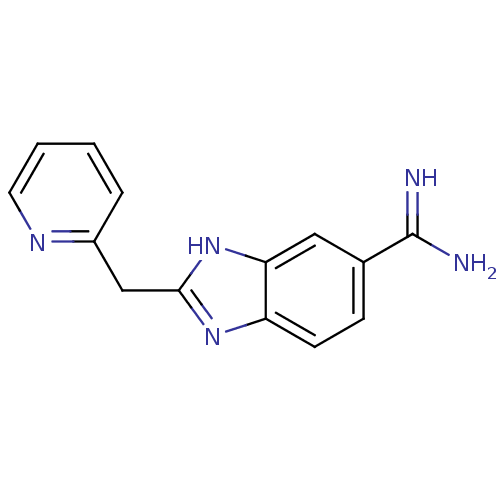 (2-(pyridin-2-ylmethyl)-1H-1,3-benzodiazole-6-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 310 | -36.8 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16309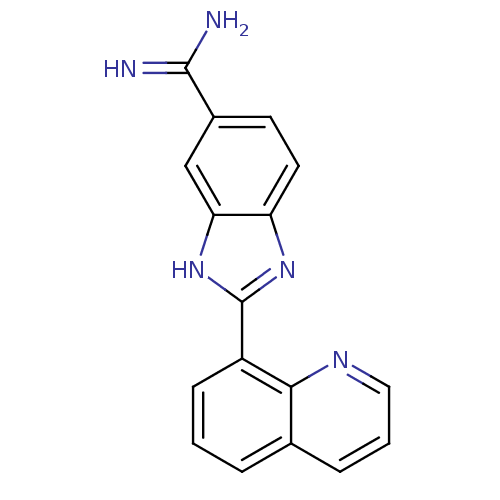 (2-(quinolin-8-yl)-1H-1,3-benzodiazole-6-carboximid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 321 | -36.7 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16305 (2-(1H-imidazol-2-ylmethyl)-1H-1,3-benzodiazole-6-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 666 | -34.9 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16306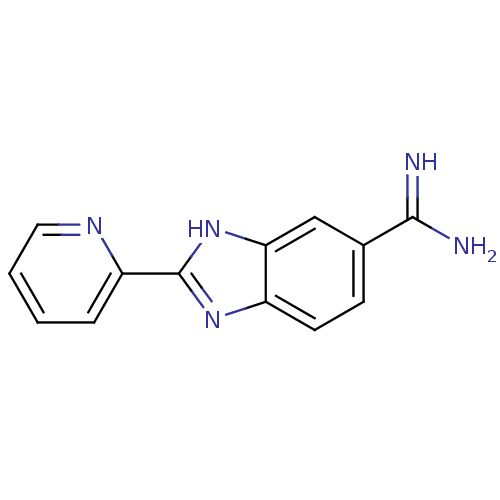 (2-(pyridin-2-yl)-1H-1,3-benzodiazole-6-carboximida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 721 | -34.7 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM18753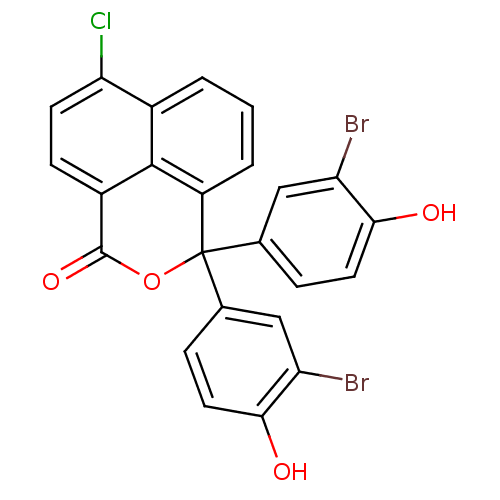 (4,4-bis(3-bromo-4-hydroxyphenyl)-10-chloro-3-oxatr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 800 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California at San Francisco | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | Acta Crystallogr D Biol Crystallogr 61: 1320-34 (2005) Article DOI: 10.1107/S0907444905022638 BindingDB Entry DOI: 10.7270/Q2WS8RHG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16306 (2-(pyridin-2-yl)-1H-1,3-benzodiazole-6-carboximida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16305 (2-(1H-imidazol-2-ylmethyl)-1H-1,3-benzodiazole-6-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.65E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16310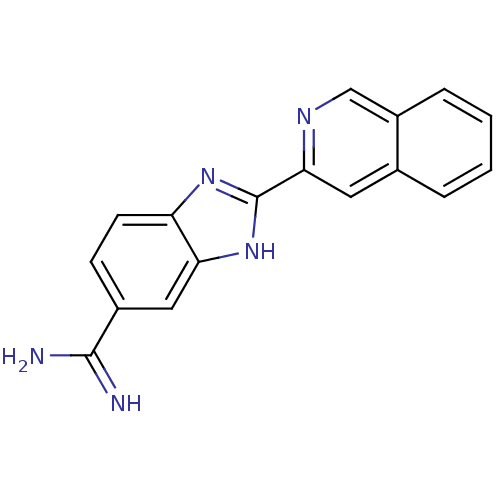 (2-(isoquinolin-3-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.85E+3 | -32.4 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16303 (2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16127 (2,2 -methanediylbis(1H-benzimidazole-6-carboximida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16127 (2,2 -methanediylbis(1H-benzimidazole-6-carboximida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM18753 (4,4-bis(3-bromo-4-hydroxyphenyl)-10-chloro-3-oxatr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 4.10E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California at San Francisco | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | Acta Crystallogr D Biol Crystallogr 61: 1320-34 (2005) Article DOI: 10.1107/S0907444905022638 BindingDB Entry DOI: 10.7270/Q2WS8RHG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Cryptococcus neoformans) | BDBM18753 (4,4-bis(3-bromo-4-hydroxyphenyl)-10-chloro-3-oxatr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.10E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California at San Francisco | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | Acta Crystallogr D Biol Crystallogr 61: 1320-34 (2005) Article DOI: 10.1107/S0907444905022638 BindingDB Entry DOI: 10.7270/Q2WS8RHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16304 (2-(1H-1,3-benzodiazol-2-ylcarbonyl)-1H-1,3-benzodi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16309 (2-(quinolin-8-yl)-1H-1,3-benzodiazole-6-carboximid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 8.04E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16308 (2-(isoquinolin-1-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM16127 (2,2 -methanediylbis(1H-benzimidazole-6-carboximida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16307 (2-(pyridin-2-ylmethyl)-1H-1,3-benzodiazole-6-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.05E+4 | -28.1 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16309 (2-(quinolin-8-yl)-1H-1,3-benzodiazole-6-carboximid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.10E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16309 (2-(quinolin-8-yl)-1H-1,3-benzodiazole-6-carboximid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16303 (2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16305 (2-(1H-imidazol-2-ylmethyl)-1H-1,3-benzodiazole-6-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16310 (2-(isoquinolin-3-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.64E+4 | -27.0 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16127 (2,2 -methanediylbis(1H-benzimidazole-6-carboximida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16306 (2-(pyridin-2-yl)-1H-1,3-benzodiazole-6-carboximida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16309 (2-(quinolin-8-yl)-1H-1,3-benzodiazole-6-carboximid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16306 (2-(pyridin-2-yl)-1H-1,3-benzodiazole-6-carboximida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.09E+4 | -26.4 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16308 (2-(isoquinolin-1-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.25E+4 | -26.3 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16310 (2-(isoquinolin-3-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16310 (2-(isoquinolin-3-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Pneumocystis carinii) | BDBM18753 (4,4-bis(3-bromo-4-hydroxyphenyl)-10-chloro-3-oxatr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.80E+4 | -25.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
University of California at San Francisco | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | Acta Crystallogr D Biol Crystallogr 61: 1320-34 (2005) Article DOI: 10.1107/S0907444905022638 BindingDB Entry DOI: 10.7270/Q2WS8RHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16305 (2-(1H-imidazol-2-ylmethyl)-1H-1,3-benzodiazole-6-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM16308 (2-(isoquinolin-1-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16308 (2-(isoquinolin-1-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16309 (2-(quinolin-8-yl)-1H-1,3-benzodiazole-6-carboximid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16308 (2-(isoquinolin-1-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.45E+4 | -24.1 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM16310 (2-(isoquinolin-3-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM16310 (2-(isoquinolin-3-yl)-1H-1,3-benzodiazole-6-carboxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.33E+4 | -23.7 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris | Assay Description Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... | Nature 391: 608-12 (1998) Article DOI: 10.1038/35422 BindingDB Entry DOI: 10.7270/Q29P2ZW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 78 total ) | Next | Last >> |