

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50283607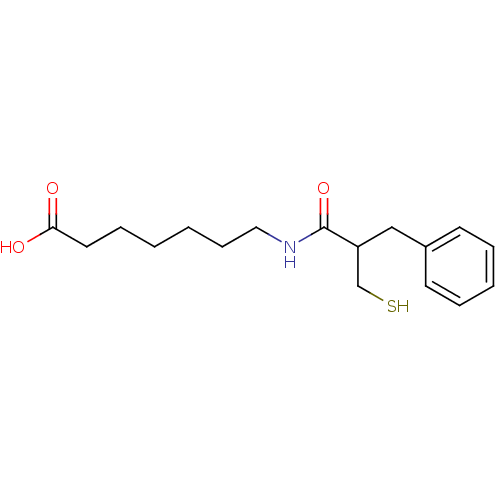 (7-(2-Mercaptomethyl-3-phenyl-propionylamino)-hepta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against neutral endopeptidase 24.11 (NEP) in rat kidney cortex membrane | Bioorg Med Chem Lett 4: 2673-2676 (1994) Article DOI: 10.1016/S0960-894X(01)80694-6 BindingDB Entry DOI: 10.7270/Q2X34XXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50017129 ((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against angiotensin converting enzyme (ACE) | Bioorg Med Chem Lett 4: 2673-2676 (1994) Article DOI: 10.1016/S0960-894X(01)80694-6 BindingDB Entry DOI: 10.7270/Q2X34XXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048318 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048312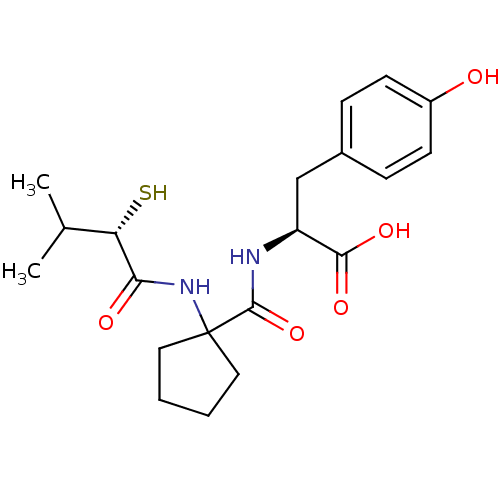 ((S)-3-(4-Hydroxy-phenyl)-2-{[1-((S)-2-mercapto-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048320 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048304 ((S)-3-(4-Fluoro-phenyl)-2-{[1-((S)-2-mercapto-3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048303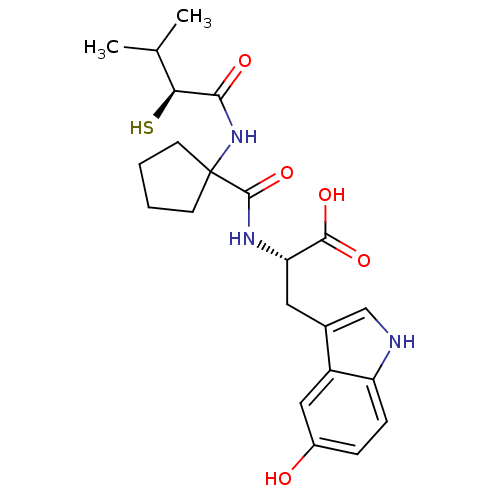 ((S)-3-(5-Hydroxy-1H-indol-3-yl)-2-{[1-((S)-2-merca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048314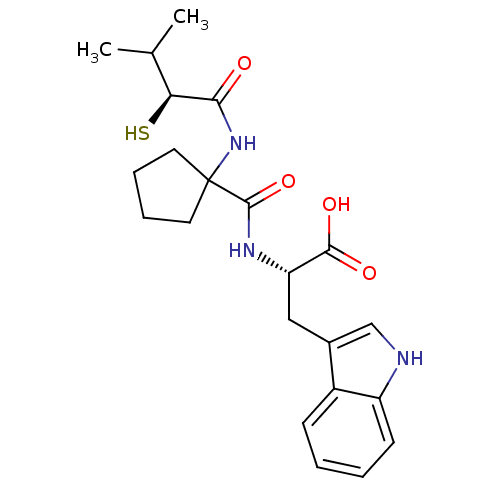 ((S)-3-(1H-Indol-3-yl)-2-{[1-((S)-2-mercapto-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048315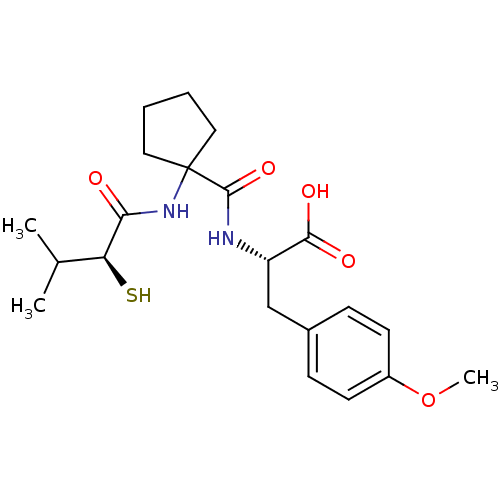 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048305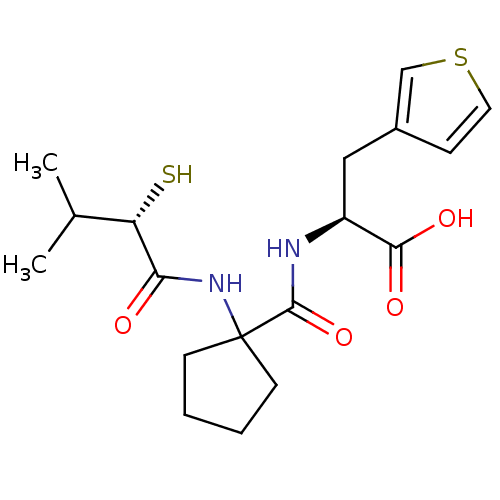 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048310 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048317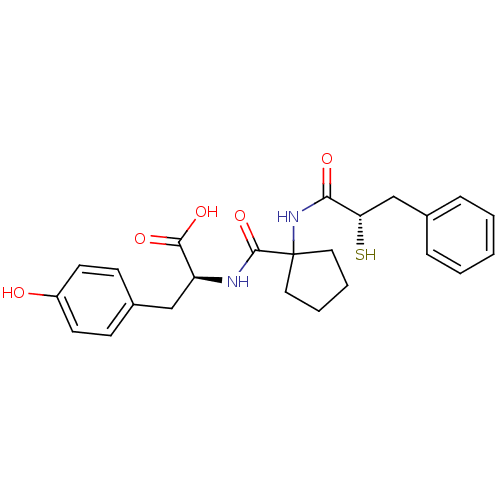 ((S)-3-(4-Hydroxy-phenyl)-2-{[1-((S)-2-mercapto-3-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048316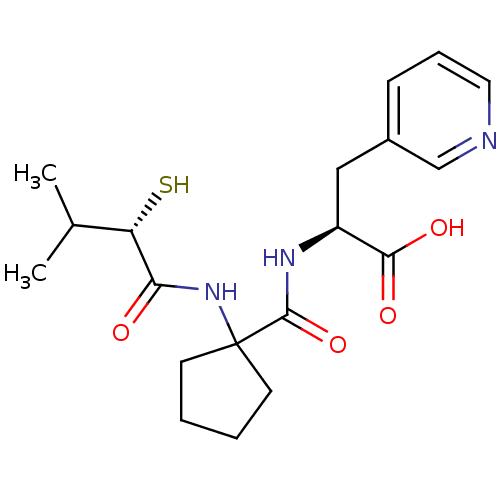 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048308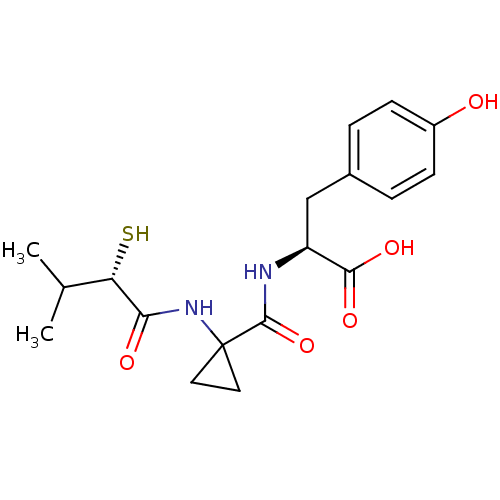 ((S)-3-(4-Hydroxy-phenyl)-2-{[1-((S)-2-mercapto-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048311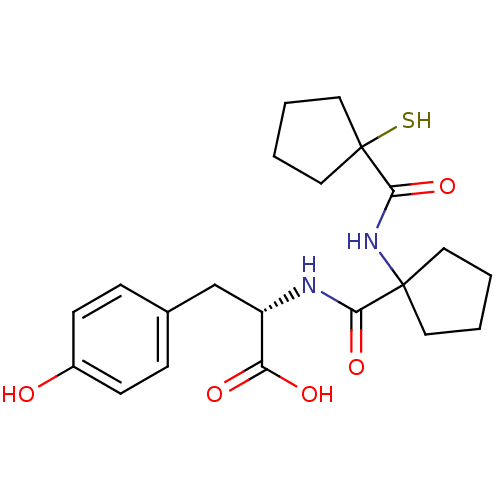 ((S)-3-(4-Hydroxy-phenyl)-2-({1-[(1-mercapto-cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against neutral endopeptidase 24.11 (NEP) in rat kidney cortex membrane | Bioorg Med Chem Lett 4: 2673-2676 (1994) Article DOI: 10.1016/S0960-894X(01)80694-6 BindingDB Entry DOI: 10.7270/Q2X34XXT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048322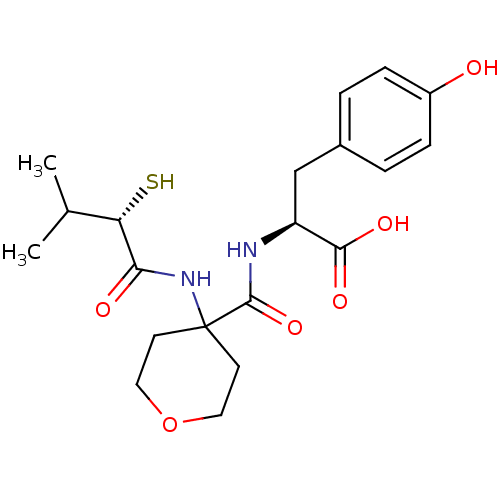 ((S)-3-(4-Hydroxy-phenyl)-2-{[4-((S)-2-mercapto-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048312 ((S)-3-(4-Hydroxy-phenyl)-2-{[1-((S)-2-mercapto-3-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048313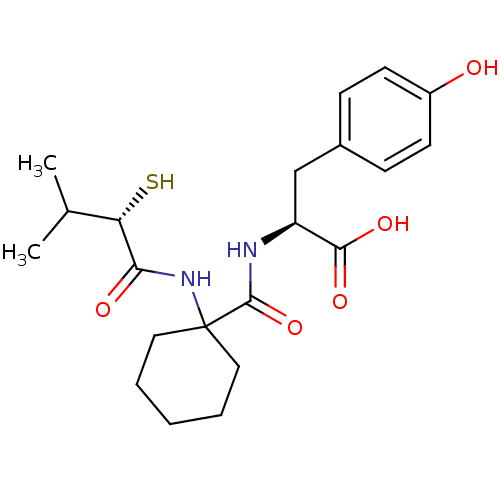 ((S)-3-(4-Hydroxy-phenyl)-2-{[1-((S)-2-mercapto-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048324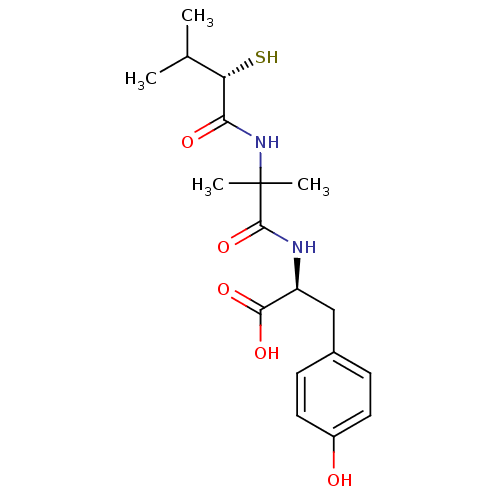 ((S)-3-(4-Hydroxy-phenyl)-2-[2-((S)-2-mercapto-3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50119751 (2-{[1-(2-Mercapto-4-methyl-pentanoylamino)-cyclope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of Endothelin-converting enzyme 1. | Bioorg Med Chem Lett 12: 3059-62 (2002) BindingDB Entry DOI: 10.7270/Q26D5SCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50119742 (2-{[1-(2-Mercapto-4-methyl-pentanoylamino)-cyclope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of Endothelin-converting enzyme 1. | Bioorg Med Chem Lett 12: 3059-62 (2002) BindingDB Entry DOI: 10.7270/Q26D5SCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048314 ((S)-3-(1H-Indol-3-yl)-2-{[1-((S)-2-mercapto-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048322 ((S)-3-(4-Hydroxy-phenyl)-2-{[4-((S)-2-mercapto-3-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048305 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091878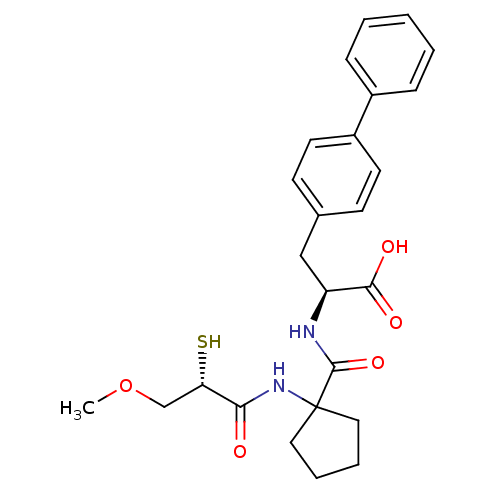 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-3-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of human Endothelin converting Enzyme-1 activity | Bioorg Med Chem Lett 10: 2037-9 (2001) BindingDB Entry DOI: 10.7270/Q2K35SW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091882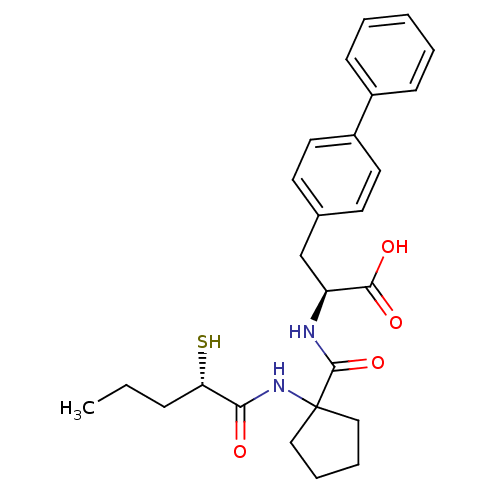 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-1-oxo-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of human Endothelin converting Enzyme-1 activity | Bioorg Med Chem Lett 10: 2037-9 (2001) BindingDB Entry DOI: 10.7270/Q2K35SW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091882 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-1-oxo-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of Endothelin-converting enzyme 1. | Bioorg Med Chem Lett 12: 3059-62 (2002) BindingDB Entry DOI: 10.7270/Q26D5SCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091882 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-1-oxo-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against Endothelin converting enzyme 1 | Bioorg Med Chem Lett 11: 375-8 (2001) BindingDB Entry DOI: 10.7270/Q2HH6JC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048303 ((S)-3-(5-Hydroxy-1H-indol-3-yl)-2-{[1-((S)-2-merca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM50073885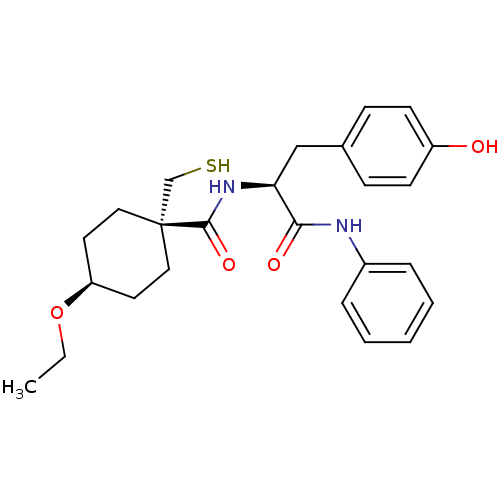 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-9 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50286721 ((S)-1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of angiotensin converting enzyme (ACE) | Bioorg Med Chem Lett 5: 735-738 (1995) Article DOI: 10.1016/0960-894X(95)00105-3 BindingDB Entry DOI: 10.7270/Q2611097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50283610 ((3S,5S)-4-(2-Mercaptomethyl-3-phenyl-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against neutral endopeptidase 24.11 (NEP) in rat kidney cortex membrane | Bioorg Med Chem Lett 4: 2673-2676 (1994) Article DOI: 10.1016/S0960-894X(01)80694-6 BindingDB Entry DOI: 10.7270/Q2X34XXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048308 ((S)-3-(4-Hydroxy-phenyl)-2-{[1-((S)-2-mercapto-3-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048324 ((S)-3-(4-Hydroxy-phenyl)-2-[2-((S)-2-mercapto-3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048323 ((S)-3-(4-Hydroxy-phenyl)-2-{[2-((S)-2-mercapto-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048309 ((S)-3-(4-Hydroxy-phenyl)-2-{[1-(2-mercapto-acetyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048319 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048311 ((S)-3-(4-Hydroxy-phenyl)-2-({1-[(1-mercapto-cyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM50073886 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-9 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Rattus norvegicus (Rat)) | BDBM50073892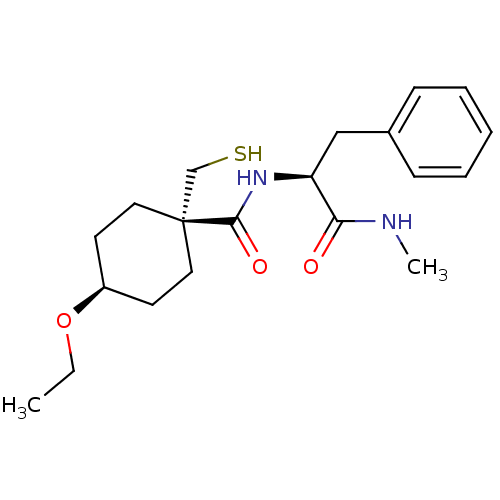 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-9 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048320 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50283612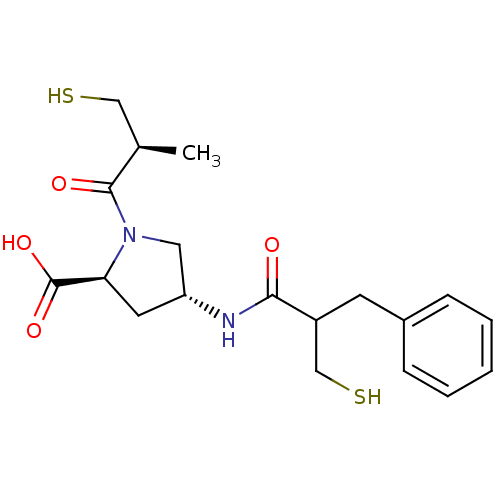 ((3S,5R)-4-(2-Mercaptomethyl-3-phenyl-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against angiotensin converting enzyme (ACE) | Bioorg Med Chem Lett 4: 2673-2676 (1994) Article DOI: 10.1016/S0960-894X(01)80694-6 BindingDB Entry DOI: 10.7270/Q2X34XXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50286722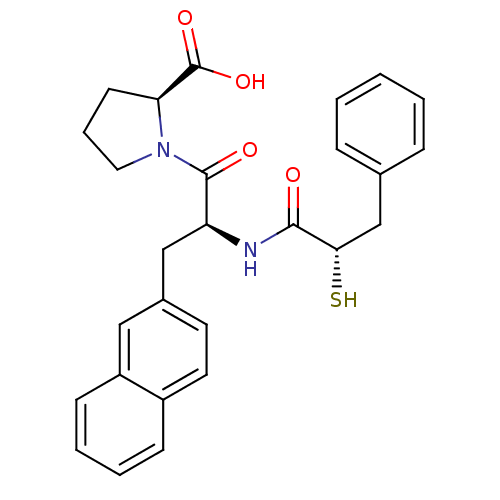 ((S)-1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of angiotensin converting enzyme (ACE) | Bioorg Med Chem Lett 5: 735-738 (1995) Article DOI: 10.1016/0960-894X(95)00105-3 BindingDB Entry DOI: 10.7270/Q2611097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048315 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50091888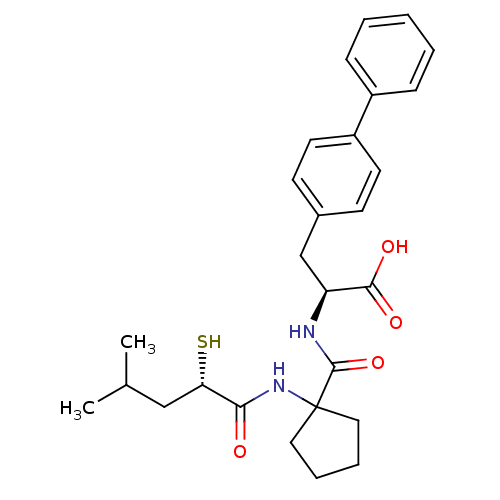 ((S)-3-Biphenyl-4-yl-2-{[1-((S)-2-mercapto-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of human Endothelin converting Enzyme-1 activity | Bioorg Med Chem Lett 10: 2037-9 (2001) BindingDB Entry DOI: 10.7270/Q2K35SW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50283609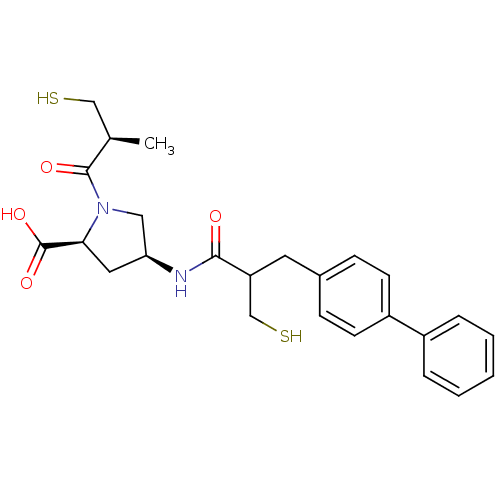 ((3S,5S)-4-(3-Biphenyl-4-yl-2-mercaptomethyl-propio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against neutral endopeptidase 24.11 (NEP) in rat kidney cortex membrane | Bioorg Med Chem Lett 4: 2673-2676 (1994) Article DOI: 10.1016/S0960-894X(01)80694-6 BindingDB Entry DOI: 10.7270/Q2X34XXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 227 total ) | Next | Last >> |