

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50071484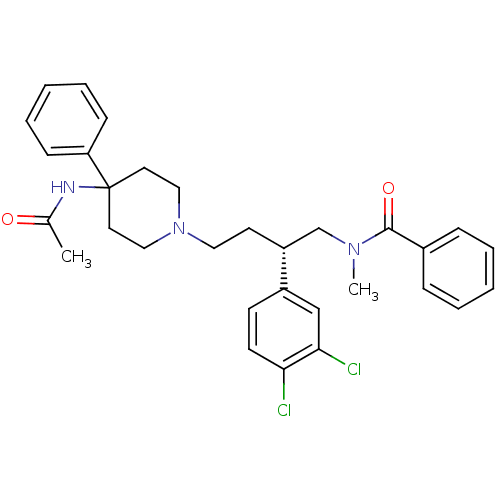 (CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro to inhibit the binding of [125I]-NKA to its receptor in rat duodenum membrane | Bioorg Med Chem Lett 3: 319-322 (1993) Article DOI: 10.1016/S0960-894X(01)80901-X BindingDB Entry DOI: 10.7270/Q2CR5T9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against specific binding of [125I]-MIP-1 alpha to human CCR5 receptor | Bioorg Med Chem Lett 11: 265-70 (2001) BindingDB Entry DOI: 10.7270/Q2668CFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015931 (3-Benzenesulfonylmethyl-7-methoxy-5,5,8-trioxo-5la...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase (HLE) | J Med Chem 33: 2529-35 (1990) BindingDB Entry DOI: 10.7270/Q2FQ9VK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50003164 (1-[3-(6-Hydroxy-2-methyl-5-oxo-2,5-dihydro-[1,2,4]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 3731-44 (1992) BindingDB Entry DOI: 10.7270/Q2BP03D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50003161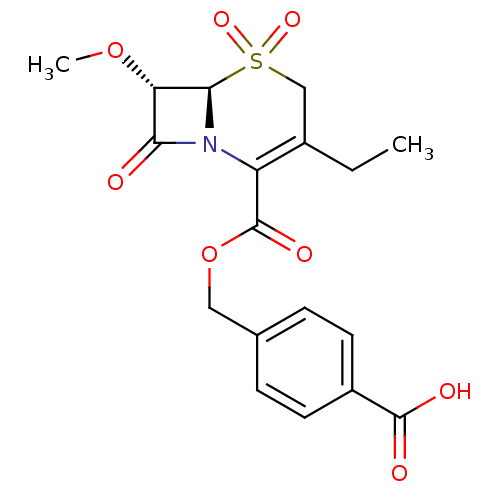 (3-Ethyl-7-methoxy-5,5,8-trioxo-5lambda*6*-thia-1-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Second order rate constant for the in vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 3731-44 (1992) BindingDB Entry DOI: 10.7270/Q2BP03D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281051 ((2S,5R,6S)-6-Ethoxy-3,3-dimethyl-4,4,7-trioxo-4lam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate | Bioorg Med Chem Lett 3: 2289-2294 (1993) Article DOI: 10.1016/S0960-894X(01)80941-0 BindingDB Entry DOI: 10.7270/Q2NC614S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281052 ((2S,5R,6S)-3,3-Dimethyl-4,7-dioxo-6-(2,2,2-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate | Bioorg Med Chem Lett 3: 2289-2294 (1993) Article DOI: 10.1016/S0960-894X(01)80941-0 BindingDB Entry DOI: 10.7270/Q2NC614S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015926 (7-Methoxy-3-methoxymethyl-5,5,8-trioxo-5lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase (HLE) | J Med Chem 33: 2529-35 (1990) BindingDB Entry DOI: 10.7270/Q2FQ9VK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50368407 (CHEMBL446371 | L-658758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity agaiinst HLE at 10 min (48 mM conc) | J Med Chem 35: 3731-44 (1992) BindingDB Entry DOI: 10.7270/Q2BP03D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406689 (CHEMBL2114137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 3731-44 (1992) BindingDB Entry DOI: 10.7270/Q2BP03D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281012 ((R)-7-Oxo-3-phenyl-4-thia-1-aza-bicyclo[3.2.0]hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory effect against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2277-2282 (1993) Article DOI: 10.1016/S0960-894X(01)80939-2 BindingDB Entry DOI: 10.7270/Q2WW7HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50003160 (CHEMBL435611 | [(3-Acetoxymethyl-7-methoxy-5,5,8-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 3731-44 (1992) BindingDB Entry DOI: 10.7270/Q2BP03D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281011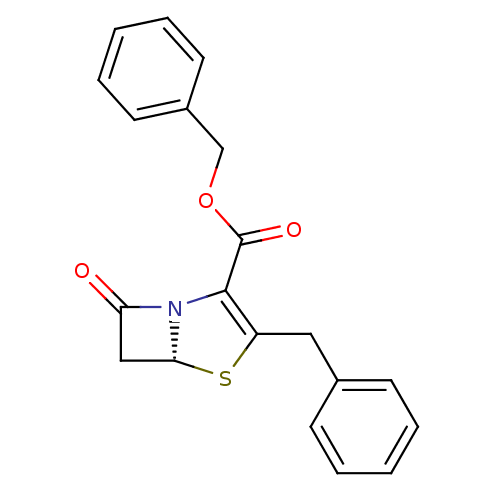 ((R)-3-Benzyl-7-oxo-4-thia-1-aza-bicyclo[3.2.0]hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human leukocyte elastase | Bioorg Med Chem Lett 3: 2277-2282 (1993) Article DOI: 10.1016/S0960-894X(01)80939-2 BindingDB Entry DOI: 10.7270/Q2WW7HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281055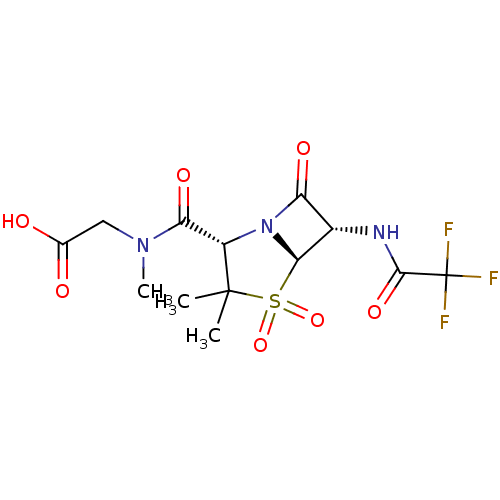 (CHEMBL73926 | {[(2S,5R,6S)-3,3-Dimethyl-4,4,7-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate | Bioorg Med Chem Lett 3: 2289-2294 (1993) Article DOI: 10.1016/S0960-894X(01)80941-0 BindingDB Entry DOI: 10.7270/Q2NC614S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280495 (4-((2S,3S)-3-Ethyl-1-methylcarbamoyl-4-oxo-azetidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of human leukocyte elastase (HLE) | Bioorg Med Chem Lett 2: 681-684 (1992) Article DOI: 10.1016/S0960-894X(00)80390-X BindingDB Entry DOI: 10.7270/Q2FX79C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281054 ((R)-1-[(2S,5R,6R)-3,3-Dimethyl-4,4,7-trioxo-6-(2,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate | Bioorg Med Chem Lett 3: 2289-2294 (1993) Article DOI: 10.1016/S0960-894X(01)80941-0 BindingDB Entry DOI: 10.7270/Q2NC614S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50403117 (CHEMBL2115522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory effect against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2277-2282 (1993) Article DOI: 10.1016/S0960-894X(01)80939-2 BindingDB Entry DOI: 10.7270/Q2WW7HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50003162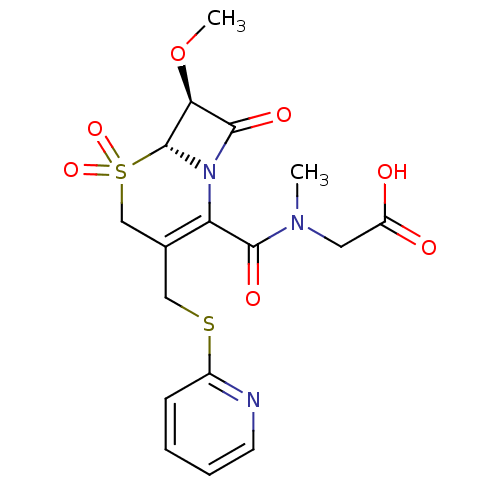 (CHEMBL123475 | {[7-Methoxy-5,5,8-trioxo-3-(pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 3731-44 (1992) BindingDB Entry DOI: 10.7270/Q2BP03D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281056 (CHEMBL307622 | {[(2S,5R,6R)-3,3-Dimethyl-4,4,7-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate | Bioorg Med Chem Lett 3: 2289-2294 (1993) Article DOI: 10.1016/S0960-894X(01)80941-0 BindingDB Entry DOI: 10.7270/Q2NC614S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031664 (4-((2R,3R)-1-Benzylcarbamoyl-3-methyl-4-oxo-3-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the human leukocyte elastase (HLE) inhibition, and the Kobs[I] is the second-order rate constant for the time dep... | J Med Chem 38: 2449-62 (1995) BindingDB Entry DOI: 10.7270/Q24B30BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281053 (CHEMBL430991 | [((2S,5R,6S)-6-Methoxy-3,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate | Bioorg Med Chem Lett 3: 2289-2294 (1993) Article DOI: 10.1016/S0960-894X(01)80941-0 BindingDB Entry DOI: 10.7270/Q2NC614S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280496 (4-((2S,3S)-3-Ethyl-4-oxo-azetidin-2-yloxy)-benzoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibition of human leukocyte elastase (HLE) | Bioorg Med Chem Lett 2: 681-684 (1992) Article DOI: 10.1016/S0960-894X(00)80390-X BindingDB Entry DOI: 10.7270/Q2FX79C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281010 ((R)-3-Methyl-7-oxo-4-thia-1-aza-bicyclo[3.2.0]hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory effect against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2277-2282 (1993) Article DOI: 10.1016/S0960-894X(01)80939-2 BindingDB Entry DOI: 10.7270/Q2WW7HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067933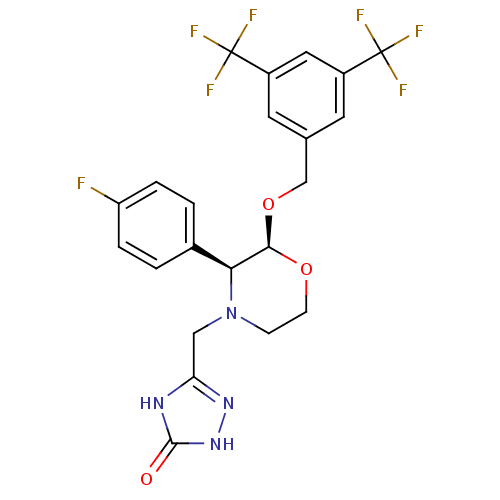 (5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50408664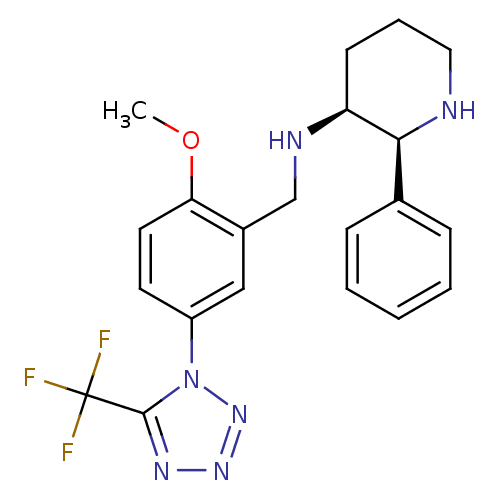 (GR-205171 | VOFOPITANT) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50049469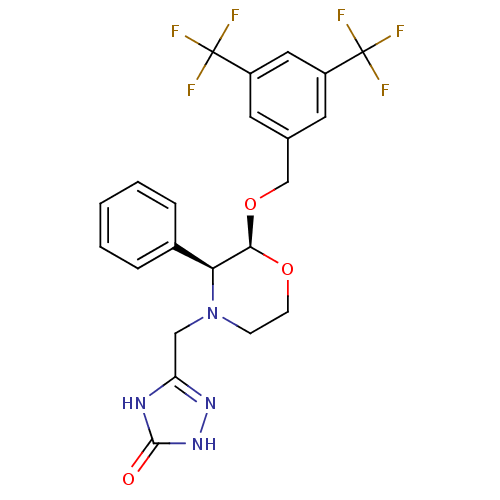 (5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067940 (5-{(2R,3S)-2-[(S)-1-(3,5-Bis-trifluoromethyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50220136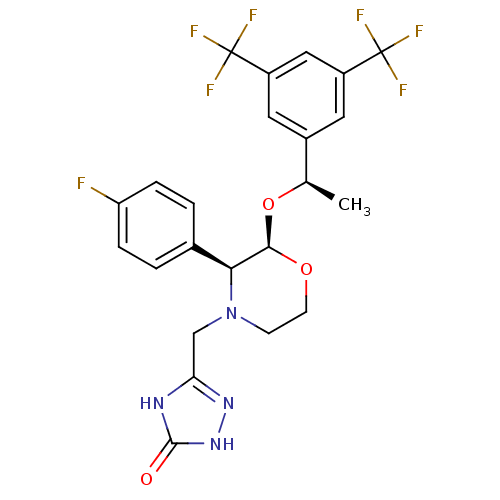 (3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description compounds were evaluated for inhibitory activity against human Tachykinin receptor 1 | J Med Chem 43: 1234-41 (2000) BindingDB Entry DOI: 10.7270/Q2G73CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50220136 (3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50220136 (3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4497-503 (2006) Article DOI: 10.1016/j.bmcl.2006.06.035 BindingDB Entry DOI: 10.7270/Q21J99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50049469 (5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4497-503 (2006) Article DOI: 10.1016/j.bmcl.2006.06.035 BindingDB Entry DOI: 10.7270/Q21J99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50220136 (3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067939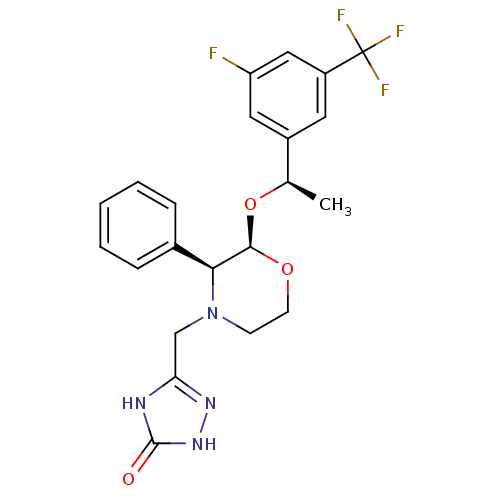 (5-{(2R,3S)-2-[(R)-1-(3-Fluoro-5-trifluoromethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50105517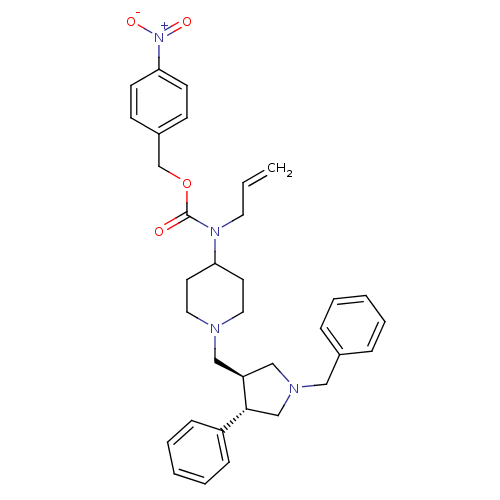 (Allyl-[1-((3S,4S)-1-benzyl-4-phenyl-pyrrolidin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells | Bioorg Med Chem Lett 11: 2741-5 (2001) BindingDB Entry DOI: 10.7270/Q2F47NFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50105510 (Allyl-{1-[(S)-4-(benzenesulfonyl-methyl-amino)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells | Bioorg Med Chem Lett 11: 2741-5 (2001) BindingDB Entry DOI: 10.7270/Q2F47NFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468144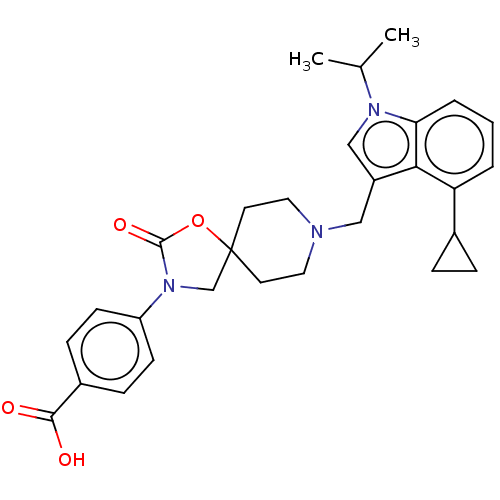 (CHEMBL4286244) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123235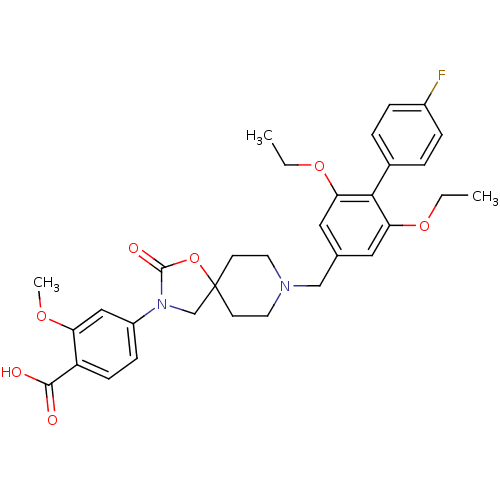 (US8742110, 3-20) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191091 ((S)-5-((((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067935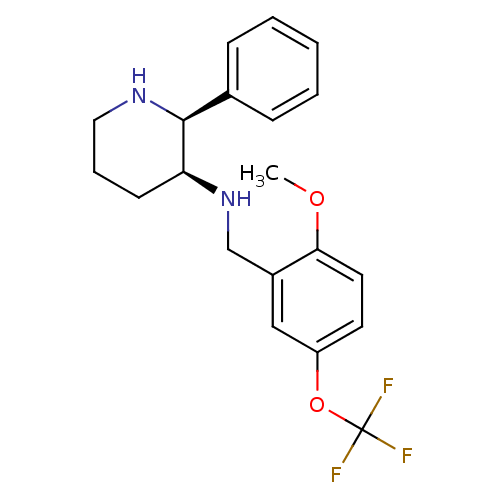 ((2-Methoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123247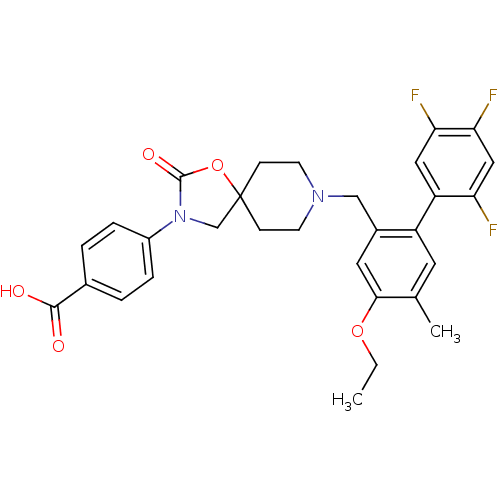 (US8742110, 4-11) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.152 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191087 ((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191092 ((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123253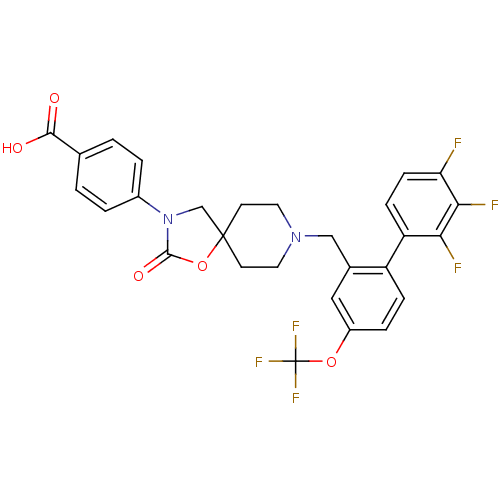 (US8742110, 4-17) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.165 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191079 (2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123270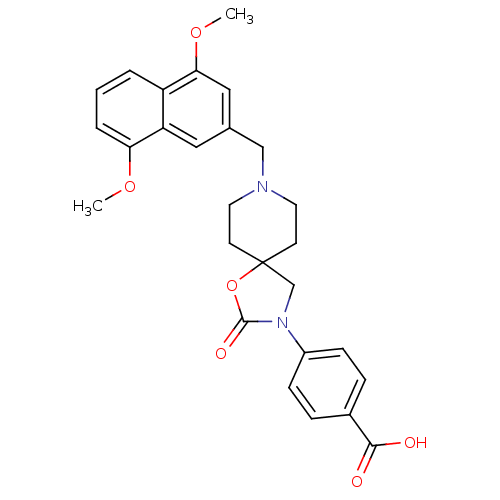 (US8742110, 5-6) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.172 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123248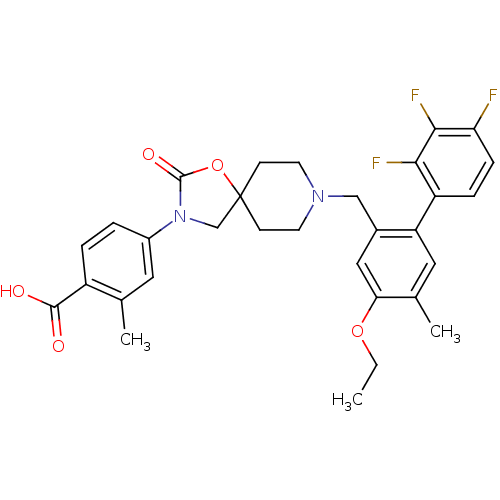 (US8742110, 4-12) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.188 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191107 (2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50191095 (2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 4504-11 (2006) Article DOI: 10.1016/j.bmcl.2006.06.044 BindingDB Entry DOI: 10.7270/Q20P0ZM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50105503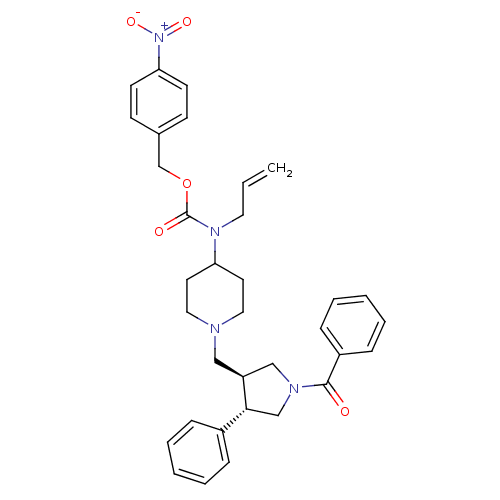 (Allyl-[1-((3S,4S)-1-benzoyl-4-phenyl-pyrrolidin-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells | Bioorg Med Chem Lett 11: 2741-5 (2001) BindingDB Entry DOI: 10.7270/Q2F47NFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468160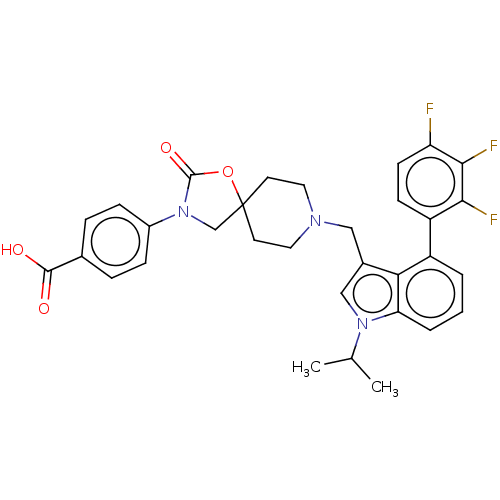 (CHEMBL4293458) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 999 total ) | Next | Last >> |